Abstract
Cryptococcus neoformans is an opportunistic fungus that can cause lethal brain infections in immunosuppressed individuals. Infection usually occurs via the inhalation of a spore or desiccated yeast which can then disseminate from the lung to the brain and other tissues. Dissemination and disease is largely influence by the production of copious amounts of cryptococcal polysaccharides, both which are secreted to the extracellular environment or assembled into a thick capsule surrounding the cell body. There are two important polysaccharides: glucuronoxylomannan (GXM) and galactoxylomannan, also called as glucuronoxylomanogalactan (GXMGal or GalXM). Although GXM is more abundant, GalXM has a more potent modulatory effect. In the present study, we show that GalXM is a potent activator of murine dendritic cells, and when co-cultured with T cells, induces a Th17 cytokine response. We also demonstrated that treating mice with GalXM prior to infection with C. neoformans protects from infection, and this phenomenon is dependent on IL-6 and IL-17. These findings help us understand the immune biology of capsular polysaccharides in fungal pathogenesis.
Similar content being viewed by others
Introduction
Cryptococcus neoformans is an environmental yeast that has a polysaccharide capsule and can cause meningoencephalitis in immunosuppressed hosts and eventually, in immunocompetent individuals1,2,3. Cryptococcosis begins when the individual inhales the sporulated form of C. neoformans present in the environment. The microorganisms from the lung spread through the bloodstream to reach different vertebrate4,5,6 host organs, after which they can invade the CNS7,8,9,10. Persistence and dissemination in the host is largely influenced by Cryptococcal polysaccharides, which are both secreted or assembled into a think polysaccharide capsule. The capsule consists primarily of 88% glucuronoxylomannan (GXM). GXM is a polymer that consists mostly of an α-(1–3)-mannan substituted with β-(1–2)-glucopyranosyluronic acid and β-(1–4)-xylopyranosyl. O-acetylation occurs on the C-6 of about half of the mannose residues11,12,13,14,15. The C. neoformans capsule also contains 10% galactoxylomannan (GalXM) and 2% mannoproteins16. Galactoxylomannan consists of an α-(1 → 6)-galactan backbone with galactomannan side chains that are further substituted with variable numbers of xylose and glucuronic acid residues16,17,18,19.
These two capsular polysaccharides can act on the immune system in different ways. GXM has already been characterized as a molecule with immunosuppressive activity on monocytes/macrophages, neutrophils, and dendritic cells. Monocytes/macrophages are involved in the capture and internalization of GXM mediated by Toll-like receptors, CD14, CD18, and the IgG receptor FcgRIIB20,21,22,23,24,25,26,27.
Retini and colleagues28 found that GXM blocked the production of interleukin (IL)-12 by monocytes and increased the secretion of IL-10 when stimulated monocytes were co-cultured with T cells28. In addition, GXM induced transforming growth factor (TGF)-β in the macrophage cell line RAW 264.729. Mice infected with encapsulated strains were unable to induce T-helper (Th) 1 cytokines such as IL-2 and interferon (IFN)-γ, inducing a significant accumulation of IL-10 that was not observed in the mice infected with an acapsular mutant. These results suggest that yeasts containing GXM on their surface limit the development of a Th1-type protective response in an inhibitory process in which IL-10 plays a critical role28,30,31. Our group recently showed that GXM does not induce the release of neutrophil extracellular traps (NETs) by human neutrophils and that in the presence of GXM, stimulated human neutrophils block NET release32. In addition to these immunomodulations, GXM can also induce apoptosis in different systems. Monari and colleagues33 demonstrated that FasL expression in murine macrophages induces apoptosis in activated T cells through processes involving intrinsic and extrinsic pathways24,33,34. It has also been shown that GXM can induce apoptosis in macrophages through a mechanism that involves an increase in Fas and FasL29.
The majority of studies on the immunomodulatory effects of capsular polysaccharides from C. neoformans have been performed with GXM, but the possible roles of GalXM as an immunomodulatory molecule remain unclear. Reports have increased in recent years suggesting this polysaccharide may also have important immunomodulatory activities. Chaka and colleagues35 showed that GalXM could induce the production of tumor necrosis factor (TNF)-α in peripheral blood mononuclear cells (PBMCs)35. The production of nitric oxide through the expression of inducible nitric oxide synthase and the release of TNF-α induced by GalXM have also been described29. Unlike the action of GXM on the production of NETs, Rocha and colleagues32 have shown that stimulation with GalXM or with acapsular fungus CAP67 (which lacks GXM in the polysaccharide capsule) is sufficient for the induction of NETs by human neutrophils32.
These observations suggest GXM and GalXM have different immunomodulatory activities. In addition, GalXM can induce apoptosis in different cells of the immune system. Pericolini and colleagues36 showed that GalXM induced apoptosis in memory T cells in rheumatoid arthritis patients36. Villena and colleagues29 also demonstrated that GalXM’s induction of apoptosis in the RAW cell line was mediated by Fas/FasL interactions, and the effect was ~50 times greater than that observed for GXM29. GalXM-mediated cell death might enhance the suppressive effect of GXM and contribute to suppression during cryptococcosis37,38,39. Furthermore, Moyrand et al. demonstrated the importance of GalXM in the virulence of C. neoformans by compromising its biosynthesis via UDP-Glc epimerase (uge1Δ) and UDP-Gal transporter (ugt1Δ)40.
In this report, we provide the first description of the in vitro modulation of dendritic cell activation and evaluate the participation of purified capsular polysaccharides. Our results showed that GalXM induced the production of IL-6, TGF-β, and IL-17 in co-cultures of stimulated dendritic cells and T lymphocytes, a characteristic profile for a Th17 response. The GalXM also induced a protective effect in vivo where the number of colony-forming units (CFUs) showed reductions in the fungal loads in different organs. Our data also show that GalXM induces a protective response in mice infected with C. neoformans, suggesting an effect mediated by Th17 lymphocytes during the early stages of infection.
Results
Capsular constituents GXM and GalXM induce the maturation of murine dendritic cells and the production of cytokines
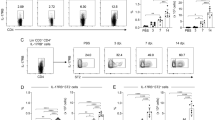
Dendritic cells are professional antigen-presenting cells in which T cells can initiate the primary and secondary responses41,42,43. Immature dendritic cells reside inside non-lymphoid organs where they actively capture and process antigens. Their maturation can be induced by contact with proinflammatory cytokines and by the recognition of microbial products migrating to the lymphoid organs, where the initiation of the adaptive immune response occurs44. To evaluate the involvement of the purified capsular constituents from C. neoformans on dendritic-cell maturation, the cells were incubated for 24 h in the presence or absence of 50-μg/mL GXM or GalXM. Both capsular constituents induced the increased expression of major histocompatibility complex class II (MHC II) and CD86 (surface structures characteristic of mature dendritic cells), with GalXM eliciting a more intense increase in marker expression than GXM (Fig. 1A,B). To eliminate potential LPS contamination of the polysaccharides, the experiments were performed with polymyxin B (Fig. 1A,B).
CD86 and MHC II expression and cytokine production in murine dendritic cells stimulated with GXM or GalXM capsular polysaccharides. Dendritic cells (2 × 105/well) were incubated for 24 h in the presence of 50 μg/mL of capsular GXM or GalXM (wild-type strain B3501) from Cryptococcus neoformans or 10-ng/mL lipopolysaccharide (LPS) in the presence or absence of polymyxin B (100 ng/mL). Cells with labeled (A) CD11c (phycoerythrin; PE) and CD86 (FITC) or (B) CD11c (PE) and MHC II (FITC) were checked by flow cytometry along with positive cells. The control corresponds to dendritic cells without stimulus. The graph represents the mean fluorescence intensity (MFI). The result represents one of three independent experiments. The asterisks indicate ***P < 0.001, **P < 0.01, and *P < 0.05 compared to the control, and the hash symbol (#) indicates P < 0.01 compared to LPS without the addition of polymyxin B. To test cytokine production after stimulation, the culture supernatants were collected, and the production of cytokines IL-12p40 and IL-10 were determined with an ELISA assay. The control corresponds to dendritic cells without stimulus. The results represent one of four independent experiments. The asterisks indicate ***P < 0.001 and **P < 0.01 in relation to the control.
We also evaluated the cytokine profile produced by dendritic cells stimulated by the capsular constituents purified from C. neoformans. The results showed that both capsular polysaccharides (GXM and GalXM) induced a significant increase in the production of IL-10 (Fig. 1C). Furthermore, both capsular polysaccharides induced increased IL-12p40 cytokine production in the dendritic cells. Figure 2 shows the production of IL-12p40 at different concentrations of GXM and GalXM. Although some authors have used higher than 50 μg/mL concentrations45,46, our data show that small concentrations of GXM or GalXM (Fig. 2) result in the same level of IL-12p40 production as when larger concentrations (up to 250 μg/mL) are used. These experiments showed that the 50 μg/mL dosage did not compromise the results obtained and promoted the increase in IL-12p40 production indicative of dendritic cell activation.
The effect of different concentrations of GXM and GalXM on the production of IL-12p40 by dendritic cells. Dendritic cells (2 × 105/well) were incubated in the presence of different concentrations (μg/mL) of (A) GXM or (B) GalXM for 24 hours. The culture supernatants were then collected, and ELISA assays were used to determine the amount of the cytokine IL-12p40. The control (100 ng/mL LPS) corresponds to dendritic cells without stimulus. The results represent one of four independent experiments. The asterisks indicate ***P < 0.001 in relation to the control.
Increased lymphoproliferative response induced by GXM or GalXM capsule constituents
Because the capsular constituents were able to induce the maturation of dendritic cells, we evaluated the effect of these GXM- or GalXM-stimulated cells on lymphocyte activation in vitro. The proliferative response of the total mesenteric lymph node (MLN) cells was significantly increased (Fig. 3B). When the MLN cells were exposed to ConA (5 μg/mL), the proliferative response was even higher (Fig. 3B).
Increased in vitro lymphoproliferative response induced by murine dendritic cells treated with GXM or GalXM. Dendritic cells (3 × 104/well) were incubated for 24 h in the presence of 50 μg/mL of the constituents’ capsule of C. neoformans. The dendritic cells were then incubated with (A) MLN cells (3 × 105/well) or with (B) MLN (3 × 105/well) in the presence of ConA (5 μg/mL). After 72 h, lymphoproliferation was analyzed by the incorporation of 3H-thymidine in the last 18 h of culture. Lipopolysaccharide (LPS) was used at a concentration of 100 ng/mL. (C) Dendritic cells were incubated with MLN cells (3 × 105/well). After 24 or 48 hours, T cells were labeled with anti-CD4 (allophycocyanin) and the percentage of apoptotic cells was quantified by flow cytometry using annexin V (FITC) and propidium iodide. Dexamethasone (1 μM) was used as a positive control for apoptosis. The results represent one of three independent experiments. The asterisks indicate ***P < 0.001 and *P < 0.05 relative to the control.
As previously demonstrated by Pericolini and colleagues37, when interacting directly with human T lymphocytes and the Jurkat lineage, GalXM induces apoptosis via the activation of caspase 8 in the cells. We performed the annexin V/propidium iodide apoptosis assay with flow cytometry to detect phosphatidylserine expression on the cell surface. Neither the GXM- nor GalXM-activated dendritic cells induced cell death by apoptosis in CD4+ T cells when co-incubated for 24 or 48 hours (Fig. 3C).
We also evaluated whether capsular GXM or GalXM interacting with MLN cells could induce an increase in the proliferative response in the absence of dendritic cells or induce apoptosis as previously published by Pericolini and colleagues37. The capsular GXM did not induce a lymphoproliferative response directly, a result in agreement with Yauch and colleagues46. Moreover, GalXM did induce an increase in the lymphoproliferative response at concentrations of 10 or 50 μg/mL (Supplementary Fig. S1), as previously described by Pericolini and colleagues37. These results show that the capsular constituents have different mechanisms of action, but acting together they may lead to a suppression of the immune response29,37.
Production of IFN-γ by CD4+ T cells in cultures with murine dendritic cells pre-treated with GalXM
As the MLN cells showed an impressive lymphoproliferative response when co-cultured with dendritic cells previously stimulated with both capsular constituents, we decided to evaluate the possible implications for the differentiation of T-cell subpopulations. CD4+ T cells were purified by negative selection to produce 92.14% CD4+-positive cells (data not shown). Dendritic cells stimulated with GXM co-cultivated with the CD4+ T cells were unable to induce differentiation to the Th1 or Th2 subpopulations, represented respectively by the cytokines IFN-γ and IL-4 (Fig. 4A,B). However, when dendritic cells stimulated with GalXM were co-cultured with T lymphocytes, a significant increase in IFN-γ production was observed (Fig. 4A). This result is in agreement with some previous reports, where it was demonstrated that during a fungal infection, patients develop a Th1-type response with CD4+ and CD8+ T-cell recruitment47,48,49,50,51.
CD4+ T cells in culture with murine dendritic cells pretreated with GalXM secrete IFN-γ. Dendritic cells (3 × 104/well) were incubated for 24 h in the presence of 50 μg/mL of the constituents’ capsule of C. neoformans. The dendritic cells were then incubated with CD4+ T cells (3 × 105/well) for 48 h. The culture supernatant was collected, and ELISA assays were used to determine the production of (A) IFN-γ and (B) IL-4. ConA was used at 5 μg/mL, and anti-IFN-γ and its IgG1a κ control were used at a concentration of 3 μg/mL. The control corresponds to dendritic cells without stimulus. The results represent one of four independent experiments. The asterisks indicate **P < 0.01 and *P < 0.05 relative to the control.
Dendritic cells stimulated with the capsular polysaccharide GalXM in co-culture with CD4+ T cells induced differentiation to the Th17 subtype
A significant question evaluated in this work was the differentiation of T cells to the Th17 subpopulation. The importance of Th17 cells has been well characterized in autoimmune-disease models, and they are important in host defense against various infections caused by bacteria and fungi, particularly on mucosal surfaces52,53,54,55,56,57. Therefore, we evaluated the role of capsular GXM and GalXM in the induction of Th17 cells. In addition to inducing the production of IFN-γ (Fig. 4A), the GalXM-activated dendritic cells surprisingly induced the production of the TGF-β, IL-6, and IL-17 cytokines in the co-cultured CD4+ T cells (Fig. 5A,C). We also found that in the presence of GalXM, dendritic cells could secrete IL-23 (Supplementary Fig. S2A), but not IL12p70 (Supplementary Fig. S2B). These results strongly suggest that dendritic cells activated by the GalXM capsular constituent induce the differentiation of CD4+ T cells into the Th17 subpopulation.
Murine dendritic cells pre-treated with capsular GalXM in co-culture with CD4+ T cells induce the production of cytokines that direct the Th17 profile. Dendritic cells (3 × 104/well) were incubated for 24 h in the presence of 50 μg/mL of capsular GXM or GalXM from C. neoformans. The dendritic cells were then incubated with CD4+ T cells (3 × 105/well) for 48 h. The culture supernatant was collected, and the cytokines (A) TGF-β, (B) IL-6, and (C) IL-17 were analyzed with ELISA assays. The αCD3 and LPS were used at 5 μg/mL and 100 ng/mL, respectively. The control corresponds to dendritic cells without stimulus. The results represent one of four independent experiments. The asterisks indicate ***P < 0.001, **P < 0.01, and *P < 0.05 relative to the control.
Capsular GalXM induces the removal of fungal cells during C. neoformans infection
The cytokines IL-17 and IL-23 have been described as necessary factors for the protective immune response in murine pulmonary histoplasmosis58, and the absence of cytokines IL-6 and granulocyte colony stimulating factor increase susceptibility to Candida albicans infection in the murine model59. These findings suggest that Th17 effector cells could be involved in immunoprotection during fungal infections. Based on this information, we next evaluated the role of the GalXM during C. neoformans infection in vivo. Our results showed that treatment with GalXM 24 h prior infection with C. neoformans significantly decreased the number of CFUs in the brain (Fig. 6A) and spleen (Fig. 6B) 14 days after infection. In the lung, which is the first site of infection, the number of CFUs was approximately 3-fold lower than that in the control (Fig. 6C). Figure 6D shows that CFUs from the GalXM-treated animals had a similar shape, size, and texture as those from the control. These results demonstrate that capsular GalXM induces the removal of fungal cells in these organs, and the effect remains 30 days after infection (Supplementary Fig. S3). To evaluate whether this decrease in the number of CFUs was a mechanism conserved in other strains of mice, we performed the same infection assay with Balb/c mice. The treatment with capsular GalXM decreased the CFUs in the lung relative to the control 14 days after infection (Supplementary Fig. S4) and in the same way as was seen in the C57BL/6 mice (Fig. 6C).
Treatment with GalXM induces the removal of fungal cells from the organs of C57BL/6 mice infected with C. neoformans. Mice were pretreated with PBS or capsular GalXM (250 μg/mL intratracheally) 24 h prior to an intratracheal injection with 106 C. neoformans cells. After 14 days, the mice were euthanized and the brain, spleen, and lungs were recovered. Viable fungal cells were quantified in petri dishes containing agar from the respective homogenized organs: (A) brain, (B) spleen, and (C) lung. (D) Illustrative picture of a lung count. The results are expressed in CFUs (dilutions: 1:2 for brain and spleen; 1:100 for lung). The results represent one of three independent experiments (n = 5 mice/group). The asterisks indicate ***P < 0.001 and **P < 0.01 relative to the control.
IL-17 cytokine is essential for the control of murine cryptococcosis
To understand the protection mechanisms used by GalXM during the early stages of C. neoformans infection in the murine model, we investigated whether the Th17 phenotype was involved in the protection mediated by this capsular constituent during experimental cryptococcosis. Cells belonging to the Th17 subtype produce IL-17, IL-17F, IL-21, and IL-22. The cytokine TGF-β is required to initiate the differentiation process, and IL-6 has been identified as a critical cofactor for Th17 cell differentiation52,60. Based on this information and the previously reported modulatory activities mediated by GalXM, we first evaluated the production of IL-6 in a GalXM-stimulated bronchoalveolar lavage. The results confirmed that GalXM induced the production of high concentrations of IL-6 from the second day through the last day of observation (Fig. 7).
The capsular GalXM constituent induces IL-6 production in bronchoalveolar lavage cells from C57BL/6 mice. Total bronchoalveolar lavage cells (1 × 105/well) were incubated for 24 h before adding 50 μg/mL of capsular GalXM. The culture supernatant was collected at Days 2, 4 and 7 after adding the GalXM, and IL-6 production was determined with ELISA assays. The results represent one of two independent experiments.
We then analyzed the role of IL-6 during C. neoformans infection. Our results suggest IL-6 is an essential cytokine for the protective immune response against murine cryptococcosis. In the absence of IL-6 (IL-6−/− mice), the number of CFUs increased approximately 50-fold over the control (Fig. 8). These observations were confirmed by an analysis of lung histological sections (Fig. 9A–F), where a high number of fungal cells were seen in the lung alveoli of the IL-6−/− mice compared to the control animals, suggesting an exacerbation of the infection in the IL-6−/− mice. We also observed important morphological changes in the lungs of the IL-6−/− mice infected with C. neoformans relative to the wild-type controls. To evaluate the involvement of GalXM in the IL-17 cytokine-mediated response, we performed the GalXM treatment 24 h before infection and evaluated the number of CFUs in the lungs of animals 30 days after infection. We found that in the absence of IL-6 or IL-17, the capsular constituent GalXM no longer had a protective effect (Fig. 10), suggesting the immunoprotective mechanism of capsular GalXM during a C. neoformans infection is Th17 dependent.
GalXM loses the ability to induce the removal of fungal cells in the lung of C. neoformans-infected IL-6−/− mice. Mice were pretreated with PBS or capsular GalXM (250 μg/mL intratracheally) 24 h prior to an intratracheal injection with 106 C. neoformans cells. After 30 days, the mice were euthanized, and the lungs were recovered. Viable fungal cells were quantified in petri dishes containing agar from the homogenized organ. The results are expressed in CFUs (dilutions: 1:10 for wild type; 1:1000 for IL-6−/−). The results represent one of four independent experiments (n = 5 mice/group). The asterisk indicates *P < 0.05 relative to the control. The hash symbol (#) indicates P < 0.001 compared to the wild-type mice.
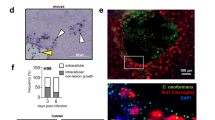
IL-6−/− mice infected with C. neoformans produce increased numbers of fungal cells. Interleukin-6 deficient mice (D–F) were intratracheally pretreated with PBS (B,E) or 250 μg/mL capsular GalXM (C,F) 24 h before an intratracheal injection with 106 C. neoformans cells. After 30 days, the mice were euthanized, and the lungs were recovered. Fungal cells were identified in histological sections positive for mucicarmine. The black arrows in Panels B and C indicate C. neoformans. Panels E and F show an abundance of C. neoformans. The results represent one of three independent experiments (n = 5 mice/group). The photographs were taken under an optical microscope at 100× (A–C) and 200 × (D–F) magnification.
IL-17−/− mice treated with GalXM present a defect similar to that seen in IL6−/− mice in the control of pulmonary infection. Mice (Wt, IL6−/− and IL17−/−) were intratracheally pretreated with 250 μg/mL capsular GalXM 24 h before an intratracheal injection with 106 C. neoformans cells. After 30 days, the mice were euthanized, and the lungs were recovered. Viable fungal cells were quantified in petri dishes containing agar from the homogenized organ. The results are expressed in CFUs (dilutions: 1:10 for wild type; 1:1000 for IL-6−/− and IL17−/−). The results represent one of four independent experiments (n = 5 mice/group). The asterisk indicates *P < 0.05 relative to the control.
Discussion
In this work, we evaluated the immunomodulatory role of the capsular polysaccharides GXM and GalXM from C. neoformans in dendritic cells that are, together with alveolar macrophages, important in regulating the innate immune response against lung infection by C. neoformans61,62,63,64. Initial results using dendritic cells as a model showed that GXM and GalXM induced the increased expression of MHC II and CD86, which are molecules characteristic of mature dendritic cells. In addition, we observed a positive release of cytokine IL-12p40 after the maturation of dendritic cells. The release of IL-12p40 in the presence of different concentrations of the capsule constituents indicated that small concentrations of the two polysaccharides stimulate the dendritic cells to produce IL-12p40, suggesting they contribute to dendritic cell maturation and may induce efficient T-cell responses. These hypotheses are supported by results obtained by Wozniak and colleagues65, who showed that dendritic cells isolated from the lungs of mice infected with encapsulated C. neoformans had an increase in the expression of the CD80, CD86, and MHC II maturation markers. These results agree with the observation that CD4+ T cells in bronchoalveolar infiltrate coincide with the increased expression of two subunits (p40 and p35) of the IL-12 cytokine and with the presence of IFN-γ in the pulmonary homogenate of mice 14 days after infection with C. neoformans66,67.
It has been shown that acapsular C. neoformans is easily phagocytosed by immature dendritic cells, inducing increases in MHC I and II and an increase in the expression of CD40 and CD8345. The addition of monoclonal antibodies against GXM facilitated the maturation of dendritic cells, promoting the ingestion of the fungal cells. This maturation allowed for the efficient activation of T cells, their differentiation, and an increase in the production of IFN-γ45,68. These authors also investigated the effect of the polysaccharide capsule on genetic expression in dendritic cells by examining the interaction with an acapsular mutant (CAP59) of C. neoformans. The results showed that a large number of genes involved in dendritic cell maturation were positively regulated after treatment with the acapsular yeast that was not seen with the capsular cells. However, the author observed a completely different pattern of gene expression in the cells treated with an encapsulated strain69. Together, these results suggest that GXM interferes with the activation and maturation of dendritic cells, indicating the fungus may impair the capability for an efficient T-cell response. However, it is important to note that the acapsular yeasts of the CAP59 strain produce GalXM70,71 and that these molecules are involved in the maturation of dendritic cells and T-cell activation.
To confirm our hypothesis, we verified the ability of GXM- or GalXM-stimulated dendritic cells to activate T lymphocytes. Our results showed that both the GalXM- and GXM-stimulated dendritic cells were able to induce the proliferation of MLN cells in the presence or absence of ConA. However, unlike the GalXM polysaccharide, GXM was unable to induce the proliferation of MLN cells directly, that is, without the participation of dendritic cells. These data are consistent with the work of Yauch and colleagues46, who observed that at high concentrations (300–1000 μg/mL), the GXM capsular component inhibited the proliferation of activated T cells in the presence or absence of dendritic cells. It has also been shown that the inhibition of T cell proliferation was not due to apoptosis or necrosis, but rather due to the inhibition of the IL-2 produced by these cells. This suggests that GXM may have a direct effect on T-cell proliferation in late cryptococcal infections when GXM is present at higher concentrations due to its accumulation in tissues72.
The Th1 and Th2 cytokines are involved in infection caused by C. neoformans. Although cytokines associated with the Th1 profile are essential for natural immunity, cytokines associated with the Th2 profile do not confer protection in mice31,73. It is well described in the literature that the increased expression of Th1 cytokines is related to protection against cryptococcosis through increased phagocytic capacity and macrophage fungicidal activity, resulting in the control of the infection74,75,76. Experiments using IFN-γ−/− mice show an increased fungal load77. In addition, an IL-4−/–dependent Th2 response exacerbates pulmonary infection78,79, and IL-13 (another Th2 cytokine) contributes to fatal allergic inflammation during murine infection with C. neoformans80.
In our studies, GalXM-stimulated dendritic cells induced the production of IFN-γ, but not IL-4, in CD4+ T cells. As already mentioned, GalXM also induces the release of TNF-α in PBMCs, monocytes, and murine macrophages29,35,81. These data suggest that GalXM may induce the production of Th1 cytokines in different cell types. However, GXM-stimulated dendritic cells were unable to induce IFN-γ or IL-4 production in CD4+ T cells. The GXM capsular polysaccharide has been described as being associated with several immunoregulatory effects and could be considered an immunosuppressive molecule29,32,82,83, as its immunosuppressive activity has been well characterized in the literature in recent years25,29,32,33,83,84.
Kleinschek and colleagues85 showed that the Th17-mediated response is dependent on IL-23 and contributes to protection against infection by C. neoformans. In the murine model for C. neoformans infection, IL-12 p40- and p35-deficient mice were described as developing a Th2 response associated with high levels of susceptibility86. These data strongly suggest that IL-12 plays an essential role in immunity against C. neoformans87. In addition, IL-17 secretion by splenocytes from C. neoformans-infected mice was observed in resistant (IL-13−/−) mice, but not in susceptible mice expressing IL-1380.
The proliferation of yeast inside macrophages has been characterized as significantly reduced after treatment with the Th1 cytokines IFN-γ, TNF-α, and IL-1773. Surprisingly, our results showed that co-cultures of GalXM-stimulated dendritic cells and CD4+ T lymphocytes efficiently induced the release of IL-17 and IFN-γ, indicating that this capsular constituent may lead to a Th17 response. These data are supported by Annunziato and colleagues88, who found Th17 cells producing IFN-γ in the gut of individuals with Crohn’s disease, which is characterized by a dysregulated Th1/Th17 immune response. Another important study demonstrated that murine C. neoformans infection results in the induction of the Th1 response with the release of the IL-17 cytokine, classical macrophage activation, and infection resolution89. In 2009, Lin and colleagues90 reported that mice vaccinated with Als3p (Candida albicans adhesin) for 14 days who then underwent the removal of their splenocytes/lymph nodes and were subsequently re-stimulated with Als3p for 5 days showed an increased production of Th1 (CD4+IFN-γ+), Th17 (CD4+IL-17+), and Th1/Th17 (CD4+IFN-γ+IL-17+) cytokines. Moreover, our results showed an increase in the production of cytokines that are essential for the Th17 profile such as the production of TGF-β required for the onset of the phenotype and IL-6, a cytokine characterized as a critical cofactor for the differentiation of Th1760,91. We also demonstrated that the production of IL-23 by GalXM-stimulated dendritic cells amplifies and/or stabilizes the Th17 phenotype52,92.
To date, we know that the capsular constituent of C. neoformans GalXM can induce the maturation of dendritic cells and that these cells can activate T lymphocytes and drive the response to the Th17 phenotype with IFN-γ production, which could be called the Th1/Th17 profile. Some studies have suggested that the Th17 subpopulation, that could be involved in immunoprotection in the fungal-infection model93. Recently, Meya and colleagues94 have demonstrated that stimulation in patients with ex vivo IFN-γ induced a high production of monocytes producing TNF-α and IL-6. Thus, the presence of IFN-γ would favor a differentiation for a Th17 profile. However, a better approach to IFN-γ production is needed in our study model. In addition to the studies of C. neoformans infection, the importance of IL-17 has also been described in other fungal-infection models58,95,96,97. Based on this, we decided to examine whether the response to the Th17 subpopulation induced by capsular GalXM-stimulated dendritic cells is related to protection in the murine model during experimental cryptococcosis, despite Zelante and colleagues98 having demonstrated that in fungal infections with C. albicans and Aspergillus fumigatus, an IL-23- and IL-17-mediated response subverted the inflammatory neutrophil program, resulting in severe inflammatory tissue pathology associated with the infections. Surprisingly, our data showed that if treated with GalXM prior to infection, the fungal load was removed, and the effect lasted for the 30-days post infection period that we observed.
The Th1 and Th17 responses resulted in the removal of yeast from the lungs, but they did not prevent the systemic spread of the highly virulent C. neoformans strain H99. This suggests that the Th2 response induced by the C. neoformans H99 strain is a virulence mechanism in this strain and that the use of Th2 profile-deficient mice (IL-13−/− and IL-4−/−) leads to a strong response involving the Th1 and Th17 profiles76. However, other mechanisms of virulence could be responsible for the strong central nervous system tropism76,99. The mechanisms observed by the authors were not enough to prevent the death of mice that presented with severe pathology in the brain. In our work, we suggested that the capsular polysaccharide GalXM could lead to a protective response mediated by Th1 and Th17 subpopulations, but there are probably other mechanisms involved in preventing the death of mice infected with C. neoformans that deserve to be studied. Our results suggest that the capsular polysaccharide GalXM is involved in the protection of mice infected with C. neoformans. The data showed that the IL-6 cytokine was essential for the protective immune response against murine cryptococcosis, as demonstrated by the large increase in CFUs in IL-6−/− mice, the high number of fungal cells found within the lung alveoli and the morphological changes in the lung relative to the control. These data suggest that the protective mechanism used by GalXM during C. neoformans infection is IL-6 dependent and may involve the Th17 phenotype. The importance of IL-6 during C. neoformans infection has also been confirmed in previous work. For example, C. neoformans infection was exacerbated in IL-6−/− and IL-12−/− mice, confirming the hypothesis that Th17 and Th1 responses are involved in natural resistance to infection and the induction of a protective response31,100.
Administering IL-6 exogenously 24 and 3 hours before intracerebral infection with viable C. neoformans cells resulted in a significant reduction in the number of CFUs in the brain and blood, and it increased the survival of infected mice101. In addition, PBMCs pre-stimulated in vitro for 12 days with heat-killed C. neoformans before the addition of viable encapsulated yeasts produced high concentrations of IL-6 compared to PBMCs that were pre-stimulated with feasible fungi102. We also showed that the capsular constituent of GalXM induced an increase in IL-6 production by alveolar macrophages and co-cultures of dendritic cells and CD4+ T cells.
Finally, we observed the deleterious effect of a lack of the cytokine IL-17103,104, which, like IL-6, is important for the resolution and control of C. neoformans infection.
It is known that the variations in the extent and position of O-acetylation on the backbone of GXM induces antigenic mutability, which gives rise to the classification of C. neoformans strains into five serotypes (A, B, C, D and AD)105. Variations related to the capsular polysaccharide GalXM are not yet well characterized. But, it is possible to occur in the constitutions of capsular polysaccharides, and this phenomenon happens mainly by the variation of the culture conditions used and depending on the culture condition used, is a phenomenon often observed in fungi106,107. De Jesus et al.108 demonstrated that differences in sugar composition, physicochemical properties and serological reactivity may occur among GalXM preparations of C. neoformans grown under varied culture conditions.
An interesting example mentioned above, the O-acetyl groups are substitutes for polyssacharide capsular GXM and play an important role in the modulation of host immune protectors. For example, the higher virulence observed in C. gatti as compared to C. neoformans depends on different types of O-acetylation of GXM capsule109.
Given the bulky nature of O-acetyl groups and their positioning on the GalXM side chains, we suspect that the extent of O-acetylation may affect immunoregulatory properties of GalXM.
Taken together, our data demonstrated that the capsular polysaccharide GalXM induced maturation of dendritic cells. The presence of this capsular polysaccharide also increased expression of the CD86 and MHC II molecules on the cell surface, followed by IL-23 cytokine production. In addition, GalXM-treated dendritic cells induced T cell proliferation and production of IL-6, IFN-γ and IL-17, suggesting a bias to Th17 response. The properties of GalXM observed in dendritic cells and T cells allowed evaluating their involvement during cryptococcosis. Mice that received GalXM capsular 24 h prior to C. neoformans infection were protected as demonstrated by reduced lung, brain and splenic fungal load, suggesting significant involvement of the Th17 subtype in the infection.
Methods
Cryptococcus strains
Cryptococcus neoformans wild-type (B3501 serotype D) and GXM negative (CAP67 serotype D) strains were kindly provided by Professor Tarnara Doering (Department of Molecular Microbiology, Washington University School of Medicine, St Louis, MO, USA) and Professor Robert Cherniak (Georgia State University, Atlanta, GA, USA), respectively. The cells were cultured in a liquid defined medium (LDM)110 at 28 °C −30 °C with continuous shaking (100 rpm) for 5 days for polysaccharides purification and 2 days for infection experiments before removal by centrifugation.
Ethics statement
This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health (USA). The protocol was approved by the Committee on the Ethics of Animal Experiments of the Health Science Center of the Federal University of Rio de Janeiro (CEUA-CCS, Permit Number: A17/17-061-14) and all efforts were made to minimize suffering.
Mice
Six to eight week-old Balb/c, C57BL/6, C57BL/6 IL-6−/− (IL-6 deficient), and C57BL/6 IL-17−/− (IL-17 deficient) mice were purchased from the University of São Paulo. The mice were kept in polypropylene boxes in a temperature-controlled environment between 23 °C and 25 °C and had free access to water and standard feed during the experiments.
Isolation and purification of C. neoformans capsular polysaccharides (GalXM and GXM)
Isolation and purification procedures were adapted from protocols mentioned before19,29. The GalXM was purified from culture supernatant from C. neoformans CAP67 mutant strain grown as described above. Supernatants were separated from the cells by centrifugation at 1200 × g for 30 min at 4 °C, concentrated by ultrafiltration using a 10 kDa-cutoff spiral cartridge (Amicon/Millipore, USA), and submitted to overnight precipitation at 4 °C after addition of three volumes of ethanol. The precipitate containing GalXM and mannoprotein was dissolved in distilled water, filtered through a 0.45 μm filter and lyophilized. The freeze-dried mixture of GalXM and mannoprotein was dissolved in 10 mL of 5 mM sodium acetate buffer pH 5.2, containing 1 mM calcium chloride, 1 mM magnesium chloride, 1 mM manganese chloride and 0.15 M sodium chloride (Con-A buffer), and applied to a XK-26/40 column packed with 70 mL ConA-Sepharose 4B (GE Healthcare, Sweeden), equilibrated in the same buffer and connected to an HPLC system (AKTApurifier GE Healthcare, Sweeden) at a flow rate of 0.25 mL/min. The column was washed with 5 column volumes (CV) of Con-A buffer to elute the GalXM. The flow through and washes were collected as fractions of 10 mL and assayed with phenol-sulfuric acid reaction111. Fractions containing GalXM were pooled, concentrated, dialyzed against distilled water and lyophilized.
The GXM capsular polysaccharide was isolated from C. neoformans B3501 wild type strain and purified by differential precipitation with CTAB12. Briefly, capsular polysaccharides isolated from the culture supernatant by precipitation with ethanol were dissolved in 0.2 M NaCl, CTAB (3 mg/mg of polysaccharides) was added slowly. Then, a solution of 0.05% CTAB was added and the GXM was selectively precipitated. The precipitate was collected by centrifugation and washed with 2% acetic acid in ethanol then in 90% ethanol. The precipitate was dissolved in 1 M NaCl and three volumes of ethanol were added to precipitate the GXM. The precipitate was centrifuged, washed with 2% acetic acid in ethanol and then with ethanol, dissolved in water and lyophilized.
To eliminate potential LPS contamination, 10 mg of GalXM or GXM preparations were dissolved in LPS-free water and further purified through chromatography on a column of Polymyxin B-Agarose (Sigma) equilibrated with LPS free-water. LPS-free GalXM or GXM were eluted with 12 mL of water, recovered and lyophilized.
Intratracheal infection
The animals were injected intratracheally with 250 μg/mL GalXM or PBS. After 24 h, the animals were intratracheally infected with the encapsulated yeast strain C. neoformans (strain B3501 serotype D). Through the hemocytometer count, an inoculum containing 106 viable cells (or 5 × 106 for the survival assay) was made and observed for 14 (C57BL/6 and Balb/c) or 30 (C57BL/6, IL-17−/−, and IL-6−/−) days.
Dendritic cells
Dendritic cells were obtained as described by Lutz and colleagues112. The tibia and femur of C57BL/6 mice were dissected and the marrow removed using a syringe containing RPMI medium. The cells were homogenized, counted, and adjusted to 2 × 106 cells/plate in RPMI medium supplemented with 2 mM L-glutamine, 5 × 10−5 M β-mercaptoethanol, 1 mM sodium pyruvate, 1 mM non-essential amino acids, 10% SFB, and 20 ng/mL rmGM-CSF (recombinant granulocyte macrophage mouse growth factor; R&D Systems). A 10-mL suspension was transferred to a petri dish and incubated at 37 °C in a 5% CO2 atmosphere for 10 days. Fresh rmGM-CSF (20 ng/mL) was added every third day of culture.
Syngeneic lymphocyte reaction
Differentiated C57BL/6 dendritic cells (5 × 105/well) were incubated with 50 μg/mL GXM or GalXM (isolated from the wild-type B3501 strain) in supplemented RPMI medium containing 10% SFB for 24 h at 37 °C in a 5% CO2 atmosphere. The cells were then removed, washed, and re-incubated (3 × 104/well) in microplates with purified CD4+ T cells (3 × 105/well) for another 48 h.
Lymphoproliferative response
Differentiated dendritic cells (5 × 105/well) from C57BL/6 mice were incubated with 50 μg/mL GXM or GalXM for 24 h. The cells were then removed, washed, and re-incubated (3 × 104 cells/well) with mesenteric lymph node (MLN) cells (3 × 105/well) with or without ConA (5 μg/mL) for 72 h. For the direct lymphoproliferative response assay, we used MLN cells (3 × 105/well) incubated with 50-μg/mL GXM or GalXM29 in the presence of anti-CD3 (5 μg/mL) for 72 h. After this period, 10 μL of a 1:10 dilution of a 5 mCi (185 MBq) 3H-thymidine stock solution was added to the culture. The incorporation of 3H-thymidine was determined by liquid scintillation (LS6500 Beckman Coulter scintillation counter) after further incubation for 18 h.
Cytokine assay
Dendritic cells from C57BL/6 mice (2 × 105/well) were incubated for 24 h with 50 μg/mL of GXM derived from C. neoformans. The supernatants were then collected, and enzyme-linked immunosorbant assays (ELISAs) were used to determine the production of the cytokines IL-4, IFN-γ, IL-12p40, IL-10, IL-23, IL-12p70, IL-17, IL-6, TNF-α, and TGF-β. We also used an ELISA assay (BD Biosciences) to quantify the production of IL-6 in total bronchoalveolar lavage cells.
Histopathological studies
The mice were euthanized after 30 days of infection, and the lungs, brain, and spleen were removed and fixed in 10% buffered formalin for 24 h. The organs were embedded in paraffin, sliced (5–6 μm thick) with a Leica microtome, and stained with the mucicarmine technique. The stained sections were analyzed under a Nikon Eclipse E400 microscope.
Colony forming units (CFUs)
The lungs, brains, and spleens were macerated in 1-mL sterile PBS and 10-fold serial dilutions were plated in duplicate on Sabouraud dextrose-agar plates. Neat and 1:10 dilutions of freshly extracted blood were similarly plated. The plates were allowed to grow for 72 h at 30 °C before counting the visible colonies to determine the total CFUs per organ.
Statistical analysis
All calculations were performed using GraphPad Prism v5.0. Results in the figures are expressed as the mean and standard deviation (SD). The number (N) of animals per group is indicated in the figure legends. Data were compared by analyses of variance (ANOVA) followed by a Dunnett or Tukey post-test. Significant differences are indicated for *P < 0.05 **P < 0.01, and ***P < 0.001.
References
Kidd, S. E. et al. A rare genotype of Cryptococcus gattii caused the cryptococcosis outbreak on Vancouver Island (British Columbia, Canada). Proceedings of the National Academy of Sciences of the United States of America 101, 17258–17263, https://doi.org/10.1073/pnas.0402981101 (2004).
Li, S. S. & Mody, C. H. Cryptococcus. Proceedings of the American Thoracic Society 7, 186–196, https://doi.org/10.1513/pats.200907-063AL (2010).
Kwon-Chung, K. J. et al. Cryptococcus neoformans and Cryptococcus gattii, the etiologic agents of cryptococcosis. Cold Spring Harbor perspectives in medicine 4, a019760, https://doi.org/10.1101/cshperspect.a019760 (2014).
Amaral, D. M., Rocha, R. C., Carneiro, L. E., Vasconcelos, D. M. & Abreu, M. A. Disseminated cryptococcosis manifested as a single tumor in an immunocompetent patient, similar to the cutaneous primary forms. Anais brasileiros de dermatologia 91, 29–31, https://doi.org/10.1590/abd1806-4841.20164582 (2016).
Alzahrani, Y. A., Aziz, H. A., Shrestha, N. K., Biscotti, C. V. & Singh, A. D. Cryptococcal iridociliary granuloma. Survey of ophthalmology 61, 498–501, https://doi.org/10.1016/j.survophthal.2015.12.005 (2016).
Chang, C. M. et al. Donor-derived Cryptococcus infection in liver transplant: case report and literature review. Experimental and clinical transplantation: official journal of the Middle East Society for Organ Transplantation 12, 74–77, https://doi.org/10.6002/ect.2012.0288 (2014).
Merkler, A. E. et al. Direct Invasion of the Optic Nerves, Chiasm, and Tracts by Cryptococcus neoformans in an Immunocompetent Host. The Neurohospitalist 5, 217–222, https://doi.org/10.1177/1941874415569072 (2015).
Casadevall, A. Cryptococci at the brain gate: break and enter or use a Trojan horse? The Journal of clinical investigation 120, 1389–1392, https://doi.org/10.1172/JCI42949 (2010).
Sorrell, T. C. et al. Cryptococcal transmigration across a model brain blood-barrier: evidence of the Trojan horse mechanism and differences between Cryptococcus neoformans var. grubii strain H99 and Cryptococcus gattii strain R265. Microbes and infection 18, 57–67, https://doi.org/10.1016/j.micinf.2015.08.017 (2016).
Feretzaki, M., Hardison, S. E., Wormley, F. L. Jr. & Heitman, J. Cryptococcus neoformans hyperfilamentous strain is hypervirulent in a murine model of cryptococcal meningoencephalitis. PloS one 9, e104432, https://doi.org/10.1371/journal.pone.0104432 (2014).
Todaro-Luck, F., Reiss, E., Cherniak, R. & Kaufman, L. Characterization of Cryptococcus neoformans capsular glucuronoxylomannan polysaccharide with monoclonal antibodies. Infection and immunity 57, 3882–3887 (1989).
Cherniak, R., Morris, L. C., Anderson, B. C. & Meyer, S. A. Facilitated isolation, purification, and analysis of glucuronoxylomannan of Cryptococcus neoformans. Infection and immunity 59, 59–64 (1991).
Ellerbroek, P. M. et al. O-acetylation of cryptococcal capsular glucuronoxylomannan is essential for interference with neutrophil migration. Journal of immunology 173, 7513–7520 (2004).
Gates, M. A., Thorkildson, P. & Kozel, T. R. Molecular architecture of the Cryptococcus neoformans capsule. Molecular microbiology 52, 13–24, https://doi.org/10.1111/j.1365-2958.2003.03957.x (2004).
Nimrichter, L. et al. Self-aggregation of Cryptococcus neoformans capsular glucuronoxylomannan is dependent on divalent cations. Eukaryotic cell 6, 1400–1410, https://doi.org/10.1128/EC.00122-07 (2007).
James, P. G. & Cherniak, R. Galactoxylomannans of Cryptococcus neoformans. Infection and immunity 60, 1084–1088 (1992).
Vaishnav, V. V., Bacon, B. E., O’Neill, M. & Cherniak, R. Structural characterization of the galactoxylomannan of Cryptococcus neoformans Cap67. Carbohydrate research 306, 315–330 (1998).
Heiss, C., Klutts, J. S., Wang, Z., Doering, T. L. & Azadi, P. The structure of Cryptococcus neoformans galactoxylomannan contains beta-D-glucuronic acid. Carbohydrate research 344, 915–920, https://doi.org/10.1016/j.carres.2009.03.003 (2009).
Previato, J. O. et al. Distribution of the O-acetyl groups and beta-galactofuranose units in galactoxylomannans of the opportunistic fungus Cryptococcus neoformans. Glycobiology 27, 582–592, https://doi.org/10.1093/glycob/cww127 (2017).
Alvarez, M., Burn, T., Luo, Y., Pirofski, L. A. & Casadevall, A. The outcome of Cryptococcus neoformans intracellular pathogenesis in human monocytes. BMC microbiology 9, 51, https://doi.org/10.1186/1471-2180-9-51 (2009).
Redlich, S., Ribes, S., Schutze, S., Eiffert, H. & Nau, R. Toll-like receptor stimulation increases phagocytosis of Cryptococcus neoformans by microglial cells. Journal of neuroinflammation 10, 71, https://doi.org/10.1186/1742-2094-10-71 (2013).
Retini, C., Vecchiarelli, A., Monari, C., Bistoni, F. & Kozel, T. R. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infection and immunity 66, 664–669 (1998).
Levitz, S. M. Receptor-mediated recognition of Cryptococcus neoformans. Nihon Ishinkin Gakkai zasshi = Japanese journal of medical mycology 43, 133–136 (2002).
Chiapello, L. S., Aoki, M. P., Rubinstein, H. R. & Masih, D. T. Apoptosis induction by glucuronoxylomannan of Cryptococcus neoformans. Medical mycology 41, 347–353 (2003).
Chiapello, L. S. et al. Immunosuppression, interleukin-10 synthesis and apoptosis are induced in rats inoculated with Cryptococcus neoformans glucuronoxylomannan. Immunology 113, 392–400, https://doi.org/10.1111/j.1365-2567.2004.01970.x (2004).
Monari, C., Bistoni, F. & Vecchiarelli, A. Glucuronoxylomannan exhibits potent immunosuppressive properties. FEMS yeast research 6, 537–542, https://doi.org/10.1111/j.1567-1364.2006.00072.x (2006).
Piccioni, M. et al. A critical role for FcgammaRIIB in up-regulation of Fas ligand induced by a microbial polysaccharide. Clinical and experimental immunology 165, 190–201, https://doi.org/10.1111/j.1365-2249.2011.04415.x (2011).
Retini, C. et al. Interdependency of interleukin-10 and interleukin-12 in regulation of T-cell differentiation and effector function of monocytes in response to stimulation with Cryptococcus neoformans. Infection and immunity 69, 6064–6073, https://doi.org/10.1128/IAI.69.10.6064-6073.2001 (2001).
Villena, S. N. et al. Capsular polysaccharides galactoxylomannan and glucuronoxylomannan from Cryptococcus neoformans induce macrophage apoptosis mediated by Fas ligand. Cellular microbiology 10, 1274–1285, https://doi.org/10.1111/j.1462-5822.2008.01125.x (2008).
Blackstock, R., McElwee, N., Neller, E. & Shaddix-White, J. Regulation of cytokine expression in mice immunized with cryptococcal polysaccharide, a glucuronoxylomannan (GXM), associated with peritoneal antigen-presenting cells (APC): requirements for GXM, APC activation, and interleukin-12. Infection and immunity 68, 5146–5153 (2000).
Beenhouwer, D. O., Shapiro, S., Feldmesser, M. & Casadevall, A. & Scharff, M. D. Both Th1 and Th2 cytokines affect the ability of monoclonal antibodies to protect mice against Cryptococcus neoformans. Infection and immunity 69, 6445–6455, https://doi.org/10.1128/IAI.69.10.6445-6455.2001 (2001).
Rocha, J. D. et al. Capsular polysaccharides from Cryptococcus neoformans modulate production of neutrophil extracellular traps (NETs) by human neutrophils. Scientific reports 5, 8008, https://doi.org/10.1038/srep08008 (2015).
Monari, C. et al. Cryptococcus neoformans capsular glucuronoxylomannan induces expression of fas ligand in macrophages. Journal of immunology 174, 3461–3468 (2005).
Monari, C., Paganelli, F., Bistoni, F., Kozel, T. R. & Vecchiarelli, A. Capsular polysaccharide induction of apoptosis by intrinsic and extrinsic mechanisms. Cellular microbiology 10, 2129–2137, https://doi.org/10.1111/j.1462-5822.2008.01196.x (2008).
Chaka, W. et al. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infection and immunity 65, 272–278 (1997).
Pericolini, E. et al. The microbial capsular polysaccharide galactoxylomannan inhibits IL-17A production in circulating T cells from rheumatoid arthritis patients. PloS one 8, e53336, https://doi.org/10.1371/journal.pone.0053336 (2013).
Monari, C. et al. Microbial immune suppression mediated by direct engagement of inhibitory Fc receptor. Journal of immunology 177, 6842–6851 (2006).
De Jesus, M. et al. Galactoxylomannan-mediated immunological paralysis results from specific B cell depletion in the context of widespread immune system damage. Journal of immunology 183, 3885–3894, https://doi.org/10.4049/jimmunol.0900449 (2009).
Vecchiarelli, A. et al. Cryptococcus neoformans galactoxylomannan is a potent negative immunomodulator, inspiring new approaches in anti-inflammatory immunotherapy. Immunotherapy 3, 997–1005, https://doi.org/10.2217/imt.11.86 (2011).
Moyrand, F., Fontaine, T. & Janbon, G. Systematic capsule gene disruption reveals the central role of galactose metabolism on Cryptococcus neoformans virulence. Molecular microbiology 64, 771–781, https://doi.org/10.1111/j.1365-2958.2007.05695.x (2007).
Hart, D. N. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood 90, 3245–3287 (1997).
Moser, M. & Murphy, K. M. Dendritic cell regulation of TH1-TH2 development. Nature immunology 1, 199–205, https://doi.org/10.1038/79734 (2000).
Watowich, S. S. & Liu, Y. J. Mechanisms regulating dendritic cell specification and development. Immunological reviews 238, 76–92, https://doi.org/10.1111/j.1600-065X.2010.00949.x (2010).
De Smedt, T. et al. Regulation of dendritic cell numbers and maturation by lipopolysaccharide in vivo. The Journal of experimental medicine 184, 1413–1424 (1996).
Vecchiarelli, A. et al. The polysaccharide capsule of Cryptococcus neoformans interferes with human dendritic cell maturation and activation. Journal of leukocyte biology 74, 370–378 (2003).
Yauch, L. E., Lam, J. S. & Levitz, S. M. Direct inhibition of T-cell responses by the Cryptococcus capsular polysaccharide glucuronoxylomannan. PLoS pathogens 2, e120, https://doi.org/10.1371/journal.ppat.0020120 (2006).
Hill, J. O. & Harmsen, A. G. Intrapulmonary growth and dissemination of an avirulent strain of Cryptococcus neoformans in mice depleted of CD4+ or CD8+ T cells. The Journal of experimental medicine 173, 755–758 (1991).
Huffnagle, G. B., Yates, J. L. & Lipscomb, M. F. Immunity to a pulmonary Cryptococcus neoformans infection requires both CD4+ and CD8+ T cells. The Journal of experimental medicine 173, 793–800 (1991).
Huffnagle, G. B., Yates, J. L. & Lipscomb, M. F. T cell-mediated immunity in the lung: a Cryptococcus neoformans pulmonary infection model using SCID and athymic nude mice. Infection and immunity 59, 1423–1433 (1991).
Huffnagle, G. B., Lipscomb, M. F., Lovchik, J. A., Hoag, K. A. & Street, N. E. The role of CD4+ and CD8+ T cells in the protective inflammatory response to a pulmonary cryptococcal infection. Journal of leukocyte biology 55, 35–42 (1994).
Qin, H. J., Feng, Q. M., Fang, Y. & Shen, L. Type-I interferon secretion in the acute phase promotes Cryptococcus neoformans infection-induced Th17 cell polarization in vitro. Experimental and therapeutic medicine 7, 869–872, https://doi.org/10.3892/etm.2014.1517 (2014).
Ivanov, I. I., Zhou, L. & Littman, D. R. Transcriptional regulation of Th17 cell differentiation. Seminars in immunology 19, 409–417, https://doi.org/10.1016/j.smim.2007.10.011 (2007).
Li, Q. et al. IL-17 and IFN-gamma production in peripheral blood following BCG vaccination and Mycobacterium tuberculosis infection in human. European review for medical and pharmacological sciences 16, 2029–2036 (2012).
Mudigonda, P. et al. Interleukin-23 and interleukin-17: importance in pathogenesis and therapy of psoriasis. Dermatology online journal 18, 1 (2012).
Chen, X., Li, N., Bi, S., Wang, X. & Wang, B. Co-Activation of Th17 and Antibody Responses Provides Efficient Protection against Mucosal Infection by Group A Streptococcus. PloS one 11, e0168861, https://doi.org/10.1371/journal.pone.0168861 (2016).
Gao, S. et al. Attenuated Streptococcus pneumoniae vaccine candidate SPY1 promotes dendritic cell activation and drives a Th1/Th17 response. Immunology letters 179, 47–55, https://doi.org/10.1016/j.imlet.2016.08.008 (2016).
Mengesha, B. G. & Conti, H. R. The Role of IL-17 in Protection against Mucosal Candida Infections. Journal of fungi 3, https://doi.org/10.3390/jof3040052 (2017).
Deepe, G. S. Jr. & Gibbons, R. S. Interleukins 17 and 23 influence the host response to Histoplasma capsulatum. The Journal of infectious diseases 200, 142–151, https://doi.org/10.1086/599333 (2009).
Basu, S., Quilici, C., Zhang, H. H., Grail, D. & Dunn, A. R. Mice lacking both G-CSF and IL-6 are more susceptible to Candida albicans infection: critical role of neutrophils in defense against Candida albicans. Growth factors 26, 23–34, https://doi.org/10.1080/08977190801987513 (2008).
Romagnani, S. Human Th17 cells. Arthritis research & therapy 10, 206, https://doi.org/10.1186/ar2392 (2008).
Osterholzer, J. J. et al. Role of dendritic cells and alveolar macrophages in regulating early host defense against pulmonary infection with Cryptococcus neoformans. Infection and immunity 77, 3749–3758, https://doi.org/10.1128/IAI.00454-09 (2009).
Gibson, J. F. & Johnston, S. A. Immunity to Cryptococcus neoformans and C. gattii during cryptococcosis. Fungal genetics and biology: FG & B 78, 76–86, https://doi.org/10.1016/j.fgb.2014.11.006 (2015).
Voelz, K. & May, R. C. Cryptococcal interactions with the host immune system. Eukaryotic cell 9, 835–846, https://doi.org/10.1128/EC.00039-10 (2010).
Chen, G. H. et al. Local GM-CSF-Dependent Differentiation and Activation of Pulmonary Dendritic Cells and Macrophages Protect against Progressive Cryptococcal Lung Infection in Mice. Journal of immunology 196, 1810–1821, https://doi.org/10.4049/jimmunol.1501512 (2016).
Wozniak, K. L., Vyas, J. M. & Levitz, S. M. In vivo role of dendritic cells in a murine model of pulmonary cryptococcosis. Infection and immunity 74, 3817–3824, https://doi.org/10.1128/IAI.00317-06 (2006).
Osterholzer, J. J. et al. CCR2 mediates conventional dendritic cell recruitment and the formation of bronchovascular mononuclear cell infiltrates in the lungs of mice infected with Cryptococcus neoformans. Journal of immunology 181, 610–620 (2008).
Wozniak, K. L. et al. Insights into the mechanisms of protective immunity against Cryptococcus neoformans infection using a mouse model of pulmonary cryptococcosis. PloS one 4, e6854, https://doi.org/10.1371/journal.pone.0006854 (2009).
Pietrella, D., Corbucci, C., Perito, S., Bistoni, G. & Vecchiarelli, A. Mannoproteins from Cryptococcus neoformans promote dendritic cell maturation and activation. Infection and immunity 73, 820–827, https://doi.org/10.1128/IAI.73.2.820-827.2005 (2005).
Lupo, P. et al. The presence of capsule in Cryptococcus neoformans influences the gene expression profile in dendritic cells during interaction with the fungus. Infection and immunity 76, 1581–1589, https://doi.org/10.1128/IAI.01184-07 (2008).
Grijpstra, J., Gerwig, G. J., Wosten, H., Kamerling, J. P. & de Cock, H. Production of extracellular polysaccharides by CAP mutants of Cryptococcus neoformans. Eukaryotic cell 8, 1165–1173, https://doi.org/10.1128/EC.00013-09 (2009).
Grijpstra, J., Tefsen, B., van Die, I. & de Cock, H. The Cryptococcus neoformans cap10 and cap59 mutant strains, affected in glucuronoxylomannan synthesis, differentially activate human dendritic cells. FEMS immunology and medical microbiology 57, 142–150, https://doi.org/10.1111/j.1574-695X.2009.00587.x (2009).
Lu, H., Zhou, Y., Yin, Y., Pan, X. & Weng, X. Cryptococcal antigen test revisited: significance for cryptococcal meningitis therapy monitoring in a tertiary chinese hospital. Journal of clinical microbiology 43, 2989–2990, https://doi.org/10.1128/JCM.43.6.2989-2990.2005 (2005).
Voelz, K., Lammas, D. A. & May, R. C. Erratum for Voelz et al., Cytokine Signaling Regulates the Outcome of Intracellular Macrophage Parasitism by Cryptococcus neoformans. Infection and immunity 84, 3656, https://doi.org/10.1128/IAI.00820-16 (2016).
Kawakami, K. Regulation by innate immune T lymphocytes in the host defense against pulmonary infection with Cryptococcus neoformans. Japanese journal of infectious diseases 57, 137–145 (2004).
Uicker, W. C., McCracken, J. P. & Buchanan, K. L. Role of CD4+ T cells in a protective immune response against Cryptococcus neoformans in the central nervous system. Medical mycology 44, 1–11 (2006).
Zhang, Y. et al. Robust Th1 and Th17 immunity supports pulmonary clearance but cannot prevent systemic dissemination of highly virulent Cryptococcus neoformans H99. The American journal of pathology 175, 2489–2500, https://doi.org/10.2353/ajpath.2009.090530 (2009).
Arora, S. et al. Role of IFN-gamma in regulating T2 immunity and the development of alternatively activated macrophages during allergic bronchopulmonary mycosis. Journal of immunology 174, 6346–6356 (2005).
Blackstock, R. & Murphy, J. W. Role of interleukin-4 in resistance to Cryptococcus neoformans infection. American journal of respiratory cell and molecular biology 30, 109–117, https://doi.org/10.1165/rcmb.2003-0156OC (2004).
Kawakami, K. et al. Interleukin-4 weakens host resistance to pulmonary and disseminated cryptococcal infection caused by combined treatment with interferon-gamma-inducing cytokines. Cellular immunology 197, 55–61, https://doi.org/10.1006/cimm.1999.1557 (1999).
Muller, U. et al. IL-13 induces disease-promoting type 2 cytokines, alternatively activated macrophages and allergic inflammation during pulmonary infection of mice with Cryptococcus neoformans. Journal of immunology 179, 5367–5377 (2007).
Delfino, D. et al. Tumor necrosis factor-inducing activities of Cryptococcus neoformans components. Infection and immunity 64, 5199–5204 (1996).
Oliveira, D. L. et al. Extracellular vesicles from Cryptococcus neoformans modulate macrophage functions. Infection and immunity 78, 1601–1609, https://doi.org/10.1128/IAI.01171-09 (2010).
Scriven, J. E. et al. A Glucuronoxylomannan-Associated Immune Signature, Characterized by Monocyte Deactivation and an Increased Interleukin 10 Level, Is a Predictor of Death in Cryptococcal Meningitis. The Journal of infectious diseases 213, 1725–1734, https://doi.org/10.1093/infdis/jiw007 (2016).
Vecchiarelli, A. et al. Downregulation by cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1 beta secretion from human monocytes. Infection and immunity 63, 2919–2923 (1995).
Kleinschek, M. A. et al. IL-23 enhances the inflammatory cell response in Cryptococcus neoformans infection and induces a cytokine pattern distinct from IL-12. Journal of immunology 176, 1098–1106 (2006).
Decken, K. et al. Interleukin-12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infection and immunity 66, 4994–5000 (1998).
Firacative, C. et al. Identification of T helper (Th)1- and Th2-associated antigens of Cryptococcus neoformans in a murine model of pulmonary infection. Scientific reports 8, 2681, https://doi.org/10.1038/s41598-018-21039-z (2018).
Annunziato, F. et al. Phenotypic and functional features of human Th17 cells. The Journal of experimental medicine 204, 1849–1861, https://doi.org/10.1084/jem.20070663 (2007).
Hardison, S. E. et al. Pulmonary infection with an interferon-gamma-producing Cryptococcus neoformans strain results in classical macrophage activation and protection. The American journal of pathology 176, 774–785, https://doi.org/10.2353/ajpath.2010.090634 (2010).
Lin, L. et al. Immunological surrogate marker of rAls3p-N vaccine-induced protection against Staphylococcus aureus. FEMS immunology and medical microbiology 55, 293–295, https://doi.org/10.1111/j.1574-695X.2008.00531.x (2009).
Wozniak, K. L., Kolls, J. K. & Wormley, F. L. Jr. Depletion of neutrophils in a protective model of pulmonary cryptococcosis results in increased IL-17A production by gammadelta T cells. BMC immunology 13, 65, https://doi.org/10.1186/1471-2172-13-65 (2012).
Antachopoulos, C. & Walsh, T. J. Immunotherapy of Cryptococcus infections. Clinical microbiology and infection: the official publication of the European Society of Clinical Microbiology and Infectious Diseases 18, 126–133, https://doi.org/10.1111/j.1469-0691.2011.03741.x (2012).
Isailovic, N., Daigo, K., Mantovani, A. & Selmi, C. Interleukin-17 and innate immunity in infections and chronic inflammation. Journal of autoimmunity 60, 1–11, https://doi.org/10.1016/j.jaut.2015.04.006 (2015).
Meya, D. B. et al. Monocyte Phenotype and IFN-gamma-Inducible Cytokine Responses Are Associated with Cryptococcal Immune Reconstitution Inflammatory Syndrome. Journal of fungi 3, https://doi.org/10.3390/jof3020028jof3020028 [pii] (2017).
Conti, H. R. et al. Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine 206, 299–311, https://doi.org/10.1084/jem.20081463 (2009).
Zelante, T., De Luca, A., D’Angelo, C., Moretti, S. & Romani, L. IL-17/Th17 in anti-fungal immunity: what’s new? European journal of immunology 39, 645–648, https://doi.org/10.1002/eji.200839102 (2009).
Werner, J. L. et al. Neutrophils produce interleukin 17A (IL-17A) in a dectin-1- and IL-23-dependent manner during invasive fungal infection. Infection and immunity 79, 3966–3977, https://doi.org/10.1128/IAI.05493-11 (2011).
Zelante, T. et al. IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. European journal of immunology 37, 2695–2706, https://doi.org/10.1002/eji.200737409 (2007).
Esher, S. K., Zaragoza, O. & Alspaugh, J. A. Cryptococcal pathogenic mechanisms: a dangerous trip from the environment to the brain. Memorias do Instituto Oswaldo Cruz 113, e180057, https://doi.org/10.1590/0074-02760180057 (2018).
Shen, L. et al. Increased activity of the complement system in cerebrospinal fluid of the patients with Non-HIV Cryptococcal meningitis. BMC infectious diseases 17, 7, https://doi.org/10.1186/s12879-016-2107-9 (2017).
Blasi, E. et al. Biomolecular events involved in anticryptococcal resistance in the brain. Infection and immunity 63, 1218–1222 (1995).
Siddiqui, A. A., Shattock, R. J. & Harrison, T. S. Role of capsule and interleukin-6 in long-term immune control of Cryptococcus neoformans infection by specifically activated human peripheral blood mononuclear cells. Infection and immunity 74, 5302–5310, https://doi.org/10.1128/IAI.00661-06 (2006).
Mukaremera, L. & Nielsen, K. Adaptive Immunity to Cryptococcus neoformans Infections. Journal of fungi 3, https://doi.org/10.3390/jof3040064 (2017).
Shourian, M., Ralph, B., Angers, I., Sheppard, D. C. & Qureshi, S. T. Contribution of IL-1RI Signaling to Protection against Cryptococcus neoformans 52D in a Mouse Model of Infection. Frontiers in immunology 8, 1987, https://doi.org/10.3389/fimmu.2017.01987 (2017).
Belay, T. & Cherniak, R. Determination of antigen binding specificities of Cryptococcus neoformans factor sera by enzyme-linked immunosorbent assay. Infection and immunity 63, 1810–1819 (1995).
Kudoh, A., Okawa, Y. & Shibata, N. Significant structural change in both O- and N-linked carbohydrate moieties of the antigenic galactomannan from Aspergillus fumigatus grown under different culture conditions. Glycobiology 25, 74–87, https://doi.org/10.1093/glycob/cwu091cwu091 (2015).
Gates-Hollingsworth, M. A. & Kozel, T. R. Phenotypic heterogeneity in expression of epitopes in the Cryptococcus neoformans capsule. Molecular microbiology 74, 126–138, https://doi.org/10.1111/j.1365-2958.2009.06855.x (2009).
De Jesus, M., Chow, S. K., Cordero, R. J., Frases, S. & Casadevall, A. Galactoxylomannans from Cryptococcus neoformans varieties neoformans and grubii are structurally and antigenically variable. Eukaryotic cell 9, 1018–1028, https://doi.org/10.1128/EC.00268-09EC.00268-09 (2010).
Urai, M. et al. Evasion of Innate Immune Responses by the Highly Virulent Cryptococcus gattii by Altering Capsule Glucuronoxylomannan Structure. Front Cell Infect Microbiol 5, 101, https://doi.org/10.3389/fcimb.2015.00101 (2015).
Cherniak, R., Reiss, E., Slodki, M. E., Plattner, R. D. & Blumer, S. O. Structure and antigenic activity of the capsular polysaccharide of Cryptococcus neoformans serotype A. Molecular immunology 17, 1025–1032 (1980).
Dubois, M., Gilles, K., Hamilton, J. K., Rebers, P. A. & Smith, F. A colorimetric method for the determination of sugars. Nature 168, 167 (1951).
Lutz, M. B. et al. An advanced culture method for generating large quantities of highly pure dendritic cells from mouse bone marrow. Journal of immunological methods 223, 77–92 (1999).
Acknowledgements
This work was supported by Brazilian National Research Council (CNPq), Rio de Janeiro State Science Foundation (FAPERJ), and Programa Institutos Nacionais de Ciência e Tecnologia (INCT), CNPq, Brazil. We thank Lindomar Miranda for helpful technical assistance. CGF-de-L, AM, JOP and LMP are senior investigators from CNPq.
Author information
Authors and Affiliations
Contributions
C.G.F.-de-L., L.M.-P. and J.O.P. wrote the main manuscript text and I.F.L.R.-de-F. and D.O.N. prepared all figures; J.D.B.R., M.P.N., A.M., P.A.V.O., L.F.L., C.M.T. and D.D.R. conducted the experiments; C.G.F.L., G.A.D.R., L.M.P. and D.D.R. analyzed the data and revised the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
LaRocque-de-Freitas, I.F., Rocha, J.D.B., Nunes, M.P. et al. Involvement of the capsular GalXM-induced IL-17 cytokine in the control of Cryptococcus neoformans infection. Sci Rep 8, 16378 (2018). https://doi.org/10.1038/s41598-018-34649-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34649-4
Keywords
This article is cited by
-
Non-self glycan structures as possible modulators of cancer progression: would polysaccharides from Cryptococcus spp. impact this phenomenon?
Brazilian Journal of Microbiology (2023)
-
Role of IL-17 family cytokines in the progression of IPF from inflammation to fibrosis
Military Medical Research (2022)
-
X-linked immunodeficient (XID) mice exhibit high susceptibility to Cryptococcus gattii infection
Scientific Reports (2021)
-
The role of Toll-like receptor 9 in a murine model of Cryptococcus gattii infection
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.