Abstract
Mitogen-activated protein kinase (MAPK) cascades are fundamental signal transduction modules in all eukaryotic organisms, controlling cell division, growth, development, and hormone signaling. Additionally, they can be activated in response to a variety of biotic and abiotic stressors. Although the evolution and expression patterns of MAPK cascade families have been systematically investigated in several model plants (e.g., Arabidopsis, rice, and poplar), we still know very little about MAPK, MAPKK, and MAPKKK families in Jatropha curcas, an economically important species. Therefore, this study performed genome-wide identification and transcriptional expression analysis of these three families in J. curcas. We identified 12 J. curcas MAPK (JcMAPKs), 5 JcMAPKKs, and 65 JcMAPKKKs. Phylogenetic analysis classified all JcMAPKs and JcMAPKKs into four subgroups, whereas JcMAPKKKs were grouped into three subfamilies (MEKK, RAF, and ZIK). Similarities in exon/intron structures supported the evolutionary relationships within subgroups and subfamilies. Conserved motif analysis indicated that all J. curcas MAPK cascades possessed typical, 200–300 amino-acid protein kinase domains. MAPK cascade genes were presented throughout all 11 chromosomes. Gene duplication analysis suggested that after JcMAPK and JcMAPKKK diverged, 3 and 19 tandem duplicates occurred under strong purifying selection. Furthermore, RNA-seq and qRT-PCR analyses revealed that some MAPK cascade genes are predominantly expressed in specific tissues. Moreover, their expression levels significantly increased under cold treatment. Our results should provide insight into the roles of MAPK cascade genes in regulating J. curcas stress responses and in hormonal signal transduction. Furthermore, these data have important applications in the genetic improvement of J. curcas.
Similar content being viewed by others
Introduction
Plants often experience biotic and abiotic stressors, including pathogen infection, cold, drought, heat, and high salinity. In response, plants have evolved mechanisms to sense and transmit environmental stimuli, including the universal regulatory mechanism of phosphorylation/dephosphorylation mediated by protein kinases and phosphatases1. Mitogen-activated protein kinase (MAPK) cascades are evolutionarily conserved signaling modules in eukaryotes, comprising three consecutive serine/threonine protein kinases: MAPKK kinase (MAPKKK), MAPK kinase (MAPKK), and MAPK2,3. The cascade plays a crucial role in diverse cellular processes, including growth, proliferation, development, differentiation, programmed cell death, stress response, and signal transduction4.
With more members than the other two families, MAPKKKs (MAPK3Ks) are further classified into three subfamilies according to kinase motifs: MEKK-like, RAF-like, and ZIK-like5,6. MEKK-like MAPKKKs have a conserved motif of -G(T/S)Px(W/Y/F)MAPEV-, and parts of them participate in the classical MAPK cascade (e.g., 10 members of 21 Arabidopsis thaliana MEKK-like MAPKKK), other MEKK-like MAPKKKs, RAF-like MAPKKKs (conserved motif -GTxx(W/Y)MAPE-) and ZIK-like MAPKKKs (-GTPEEMAPE(L/V/M)(Y/F/L)-) have no biological function in MAPK signal transduction (e.g., MAP3Kε1 and MAP3Kε2 in A. thaliana)7. The functions of some RAF-like members (e.g., CTR1, Constitutive-triple response 1; EDR1, Enhanced-disease resistance (1) have been comprehensively investigated in A. thaliana. CTR1 inhibits MKK9-MPK3/MPK6 pathway during ethylene signaling, whereas EDR1 encodes a CTR1-like kinase that negatively regulates ethylene-induced senescence and participates in salicylic acid-inducible pathogen resistance8,9,10. To date, MAPKKK gene family members have been systematically identified in many plant species, 80 putative MAPKKK genes are known from A. thaliana, including 21 MEKK-like, 48 RAF-like, and 11 ZIK-like6,11, 75 in Oryza sativa12,13, 74 in Zea mays14, and 62 in Vitis vinifera15,16.
At the top of MAPK cascades, MAPKKKs activate MAPKKs (MAP2k or MEKs or MKKs) through phosphorylating two amino acids in the -S/T-x3–5-S/T- motif (x: random amino acid) of the MAPKK activation loop. MAPKKs also contain several conserved motifs that facilitate MAPKK and MAPK interactions, including catalytic sites (-VGTxxYMSPER-) and active sites (-D(L/I/V)K-)17. Phylogenetic analyses have revealed four subcategories (groups A–D) of MAPKKs11. The functional elucidations of MAPKK-MAPK cascades in A. thaliana have been well studied. Group A MKK1/MKK2-MPK4/MPK6 cascades play important roles in plant responses to cold, salt, and pathogens18,19. Additionally, group A MKK6-MPK4/MPK11 cascades have essential regulatory functions in plant cell division20,21. Group B MKK3-MPK6 cascades are involved in pathogen resistance and the jasmonate signal transduction pathway22. Group C MKK4/MKK5-MPK3/MPK6 cascades mediate biotic stress and function in plant stomatal development23,24. Finally, group D MKK9-MPK3/MPK6 cascades play a roles in ethylene signal transduction and antitoxin biosynthesis25,26. Genome-wide identification revealed that the estimated numbers of MAPKK are 10 in A. thaliana6,11, 8 in O. sativa12,13, 9 in Z. mays14, 5 in V. vinifera15,16, 11 in Populus trichocarpa27, and 9 in Malus pumila28.
Sequence alignment analysis found 11 kinase subdomains (I–XI) in MAPK. MAPKs are activated through double phosphorylation by activated MAPKKs of highly conserved threonine and tyrosine residues (-TxY- motif) in their activation loop (T-loop) between subdomains VII and VIII29. In plants, MAPKs (MPK or MMK) are divided into groups A–D based on -TxY- motifs (MAPKK phosphorylation site, -TxYVxTRWYRAPE(L/V)-, x: random amino acid). Groups A, B, and C possess a -TEY- motif in their activation loop, while group D activation loops contain a -TDY- motif. Numerous studies have confirmed that MAPK genes are involved in various biological functions. Group A members (e.g., MPK3 and MPK6 in A. thaliana30) influence stress response, specifically with relation to hormonal signaling pathways involving abscisic acid, salicylic acid, jasmonic acid, and ethylene31,32,33. Group B members mainly participate in the regulation of abiotic stress, pathogen defense, and cell division, some examples are MPK4 in A. thaliana, MMK3 in Medicago sativa, and MPK13 in Nicotiana tabacum19,34,35,36. Less data are available on group C, but one study demonstrated up-regulation of members MPK7 in A. thaliana and their ortholog GhMAPK in Gossypium hirsutum under cold, salt, salicylic acid, H2O2, and pathogen infection37. Group D MAPKs have attracted considerable attention. For example, fungal infection induces BWMK1 expression in O. sativa38, while wounding induces TDY1 in M. sativa39. Overall, the availability of complete, fully annotated genome databases have allowed for genome-wide surveys of MAPK genes in plants, identifying 20 in A. thaliana6,11, 15 in O. sativa12,13, 21 in P. trichocarpa27, 16 in Solanum lycopersicum40, and 14 in V. vinfera15,16,41.
Jatropha curcas (Physic nut), which belongs to the family of Euphorbiaceae, is a small perennial tree with high oil content and extensive adaptability. However, MAPK cascade gene families have thus far not been systematically characterized for this species. In this study, we aimed to better understanding the function of J. curcas MAPK cascades. We identified 12 MAPK, 5 MAPKK, and 65 MAPKKK genes through searching the published J. curcas genome database. We then conducted analyses to clarify genome structure, chromosomal location, conserved consensus motifs, and phylogeny. Subsequently, we used DGE (Digital Gene Expression) and qRT-PCR (Quantitative real-time polymerase chain reaction) to investigate the transcript profile of identified genes in different tissues and under cold stress. Our results will provide a useful basis for further studies on the roles of MAPK cascades in J. curcas growth and stress response.
Results
Identification of MAPK, MAPKK, and MAPKKK genes in J. curcas
Our genome-wide analysis resulted in the identification of 12 JcMAPKs (JcMAPK1–12), 5 JcMAPKKs (JcMAPKK1–5), and 65 JcMAPKKKs (JcMAPKKK1–65, including 16 MEKKs, 40 RAFs, and 9 ZIKs) (Table S1).
The 12 JcMAPKs ranged in gene length from 3050 (JcMAPK4) to 10080 (JcMAPK9) bp. Predicted proteins were 370 (JcMAPK1) to 639 (JcMAPK5) amino acids, with putative molecular weights (Mw) of 42.75–72.40 kDa and theoretical isoelectric points (pI) ranging from 5.07 (JcMAPK4) to 9.23 (JcMAPK8). Predicted localization was in the cytoplasm and nucleus. The 5 predicted JcMAPKKs possessed 324 (JcMAPKK4) to 560 (JcMAPKK3) amino acids, with Mw of 36.60–62.41 kDa and pI ranging from 5.70 (JcMAPKK3) to 9.45 (JcMAPKK5). Subcellular localization of JcMAPKKs were generally in the nucleus, with the exception of JcMAPKK3 in the plasma membrane and JcMAPKK4 in mitochondria. The 65 predicted JcMAPKKKs ranged from 256 (JcMAPKKK41) to 2057 (JcMAPKKK54) amino acids, with Mw of 28.94–228.10 kDa and pI ranging from 4.58 (JcMAPKKK1) to 9.45 (JcMAPKKK13). Most (59/65) JcMAPKKKs were located in the cytoplasm or nucleus, the remainder were presented in the plasma membrane (Table S1).
Analysis of MAPK, MAPKK, and MAPKKK gene structure and conserved motifs
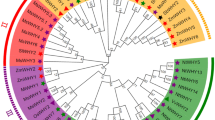
Phylogenetic analysis classified the 12 JcMAPKs into four different group (A, B, C, and D), in accordance with previous phylogeny in A. thaliana30. Groups A and B contained one gene, while groups C and D each had five genes. Gene structure analysis showed that all JcMAPKs possessed 5′-UTR and 3′-UTR regions. Group A (JcMAPK10) had 18 exons, while group B (JcMAPK12) had three. Group C JcMAPKs had six to seven exons, while Group D members had 10–12, these numbers were similar to other plants, including A. thaliana11 and P. trichocarpa27 (Fig. 1A). The MEME program was used to identify the conserved motifs of JcMAPKs to explore structural diversity. As shown in Fig. 1B, 10 conserved motifs were found. Together with the analyzed results of GenBank CDD and Pfam, all of the identified JcMAPKs contained the protein kinase domain with approximate length of 280 aa (Fig. 1B; Table S1).
Phylogenetic relationship, intron-exon structure (A) and conserved motifs (B) of MAPK family genes in J. curcas. The amino acid sequences of all J. curcas MAPK proteins were aligned using the ClustalW program and subjected to phylogenetic analysis by the distance with neighbor joining method using MEGA5.0 program. The gene structures were drawn using GSDS. Introns and exons are represented by lines and boxes, respectively. All motifs were identified by MEME database.
Phylogenetic analysis also classified the five JcMAPKKs into four groups (A, B, C, and D) together with their orthologs. Groups A, B, and C each had one gene with 8, 10, and 12 exons, respectively. Group D contained two genes of one exon each (Fig. 2A). In addition, all JcMAPKKs possessed a 260 aa protein kinase domain, located in the middle of polypeptides (Fig. 2B; Table S1). The classification and gene structure results suggest that JcMAPKKs in different groups have distinct functions.
Phylogenetic relationship, intron-exon structure (A) and conserved motifs (B) of MAPKK family genes in J. curcas. The amino acid sequences of all J. curcas MAPKK proteins were aligned using the ClustalW program and subjected to phylogenetic analysis by the distance with neighbor joining method using MEGA5.0 program. The gene structures were drawn using GSDS. Introns and exons are represented by lines and boxes, respectively. All motifs were identified by MEME database.
Phylogenetic analysis classified JcMAPKKKs into MEKK, RAF, and ZIK subfamilies. Most J. curcas MEKK genes had 1 (JcMAPKKK4, JcMAPKKK9, JcMAPKKK11, and JcMAPKKK14)–17 (JcMAPKKK5 and JcMAPKKK12) exons (Fig. 3), which were different with the exon count in Arabidopsis6 and rice12. Nearly all RAF members possessed 3 (JcMAPKKK27, JcMAPKKK31, JcMAPKKK32, and JcMAPKKK49)–17 (JcMAPKKK24) and ZIK members owned 3 (JcMAPKKK58) –9 (JcMAPKKK60) exons. The one exception was JcMAPKKK54 of the RAF subfamily, with 51 exons, and the observation that exons 1–24 and exons 27–50 shared the same structure and length suggests a gene-duplication origin for JcMAPKKK54 (Fig. 3). Our results indicate that even within the same subfamily, JcMAPKKK gene structure was highly divergent. However, contrastive results of gene structure suggested that those genes clustering together on the phylogenetic tree often had similar exon-intron patterns. For example, JcMAPKKK4, JcMAPKKK9, JcMAPKKK11, and JcMAPKKK14 of MEKK subfamily clustered closely, and all contained only one exon (Fig. 3). Nevertheless, conserved motif analysis showed that motif distribution had remarkable subfamily specificity. MEKK and ZIK members shared analogous polypeptide length and distribution patterns, with Motif1 (serine/threonine protein kinase) located in the front or middle of the protein sequence. In contrast, Motif1 in RAF members were located at the end of the sequence. Moreover, half of the identified RAF members had shorter polypeptide length than MEKK and ZIK proteins, while the other half had longer polypeptide length (Fig. 4).
Phylogenetic relationship and intron-exon structure of MAPKKK family genes in J. curcas. The amino acid sequences of all J. curcas MAPKKK proteins were aligned using the ClustalW program and subjected to phylogenetic analysis by the distance with neighbor joining method using MEGA5.0 program. The gene structures were drawn using GSDS. Introns and exons are represented by lines and boxes, respectively.
Phylogenetic relationship and conserved motifs of MAPKKK family in J. curcas. The amino acid sequences of all J. curcas MAPKKK proteins were aligned using the ClustalW program and subjected to phylogenetic analysis by the distance with neighbor joining method using MEGA5.0 program. All motifs were identified by MEME database.
Multiple alignment of MAPK, MAPKK, and MAPKKK genes in J. curcas
The activation-loops of all JcMAPKs in groups A, B, and C contained the -TEY- motif (-TEYVxTRWYRAPE(L/V)-), whereas group D members possessed a -TDY- motif. Residues E (Glutamate) and D (Aspartate) are MAPKK phosphorylation targets, thus interacting with the MAPKK active site (-K/R-K/R-K/RxxxxxL/IxL/I-). We also identified P-loop and C-loop motifs in all JcMAPKs, these regions have substrate binding characteristics and are also present in A. thaliana42,43. Moreover, the C-terminal region of five group C JcMAPK genes (JcMAPK1, JcMAPK2, JcMAPK3, JcMAPK4, and JcMAPK11) possessed CD domains (-LHDxxE/DEPxC-), an anchoring site of upstream MAPKKs42,43,44,45 (Fig. 5).
Sequence alignment and motif analysis of MAPK family in J. curcas. The activation-loop region is marked by a black line. Key motifs of -TEY- in Group A, B, and C and -TDY- in Group D within activation-loop are marked by triangle; P-loop, C-loop, and CD-domain are marked by dotted lines. The 11 kinase subdomains are in roman numerals (I to XI) above the sequence.
The Arabidopsis genome possesses 10 MAPKK members11, whereas the J. curcas genome contains five. Sequence alignments of the 12 JcMAPKs and 5 JcMAPKKs in J. curcas revealed that they all contain 11 subdomains (I–XI) that are conserved regions in the serine/threonine protein kinase of other plant species (Figs 5,6). The conserved motif of activation-loop (-S/TxxxxxS/T- and -VGTxxYMSPER-) located in subdomains of VII and VIII was the phosphorylated object of MAPKKKs, and the active site (-D(L/I/V)L- or -K/R-K/R-K/RxxxxxL/IxL/I-) located in subdomains of VI and VII conduct the phosphorylating process of MAPKs (Fig. 6).
The MEKK, RAF, and ZIK subfamilies in J. curcas have the following conserved signatures of -G(T/S)Px(W/Y/F)MAPEV-, -GTxx(W/Y)MAPE-, and -GTPEEMAPE(L/V/M)(Y/F/L)-, respectively, similar to the MAPKKKs of Arabidopsis and other plant species. Multiple sequence alignments of J. curcas MEKK, RAF, and ZIK members confirmed that most JcMAPKKKs have the corresponding conserved motifs (Fig. 7).
Chromosomal distribution and gene duplication analysis
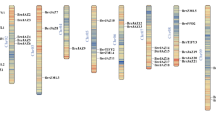
According to a previously published high-density genetic linkage map46, the 12 JcMAPKs, 5 JcMAPKKs, and 65 JcMAPKKKs were distributed non-randomly on the 11 J. curcas chromosomes, with a relatively high density all of three families across chromosomes. Chromosomes LG1, LG 2, LG 8, and LG9 each contained two JcMAPKs, whereas chromosomes LG3, LG4, LG7, and LG11 each contained one (Fig. 8). Five JcMAPKKs were presented across chromosomes LG2, LG5, and LG11, with three on LG11. With the exception of JcMAPKKK2 and JcMAPKKK19 (No anchored data available), the 63 JcMAPKKKs were mapped on all 11 chromosomes. The number of JcMAPKKKs on each chromosome ranged from 1 (Chromosome LG2) to 9 (Chromosome LG7).
Gene duplication events were critical to MAPK, MAPKK, and MAPKKK expansion in J. curcas. Among JcMAPKs, we found three paralogous gene pairs: JcMAPK1/2, JcMAPK2/3, and JcMAPK5/7. Gene duplication events were far more frequent in JcMAPKKKs than in JcMAPKs and JcMAPKKs. Three paralogous gene pairs (JcMAPKKK50/53, JcMAPKKK44/45, and JcMAPKKK31/32) were located on chromosomes LG3, LG5, and LG11, respectively. We also found 16 other paralogs spread across different chromosomes (Fig. 9). All duplicated orthologous gene pairs had Ka/Ks ratios <1, indicating that the MAPK cascade genes in J. curcas mainly experienced purifying selection after their duplication.
Cis-element analysis of J. curcas MAPK cascade genes
We identified stress-related (e.g., heat, cold, wounding, and disease) and hormone-related (e.g., abscisic acid, ethylene, auxin, gibberellin, and salicylic acid) cis-elements in the promoter regions of J. curcas MAPK cascade genes (Table S2). Of the 82 MAPK cascade genes, 66 had a heat shock element in their promoter. We also found anaerobic response elements in 8 of 12 JcMAPKs, 4 of 5 JcMAPKKs, and 54 of 65 JcMAPKKKs. Furthermore, 5 of 12 JcMAPKs, 2 of 5 JcMAPKKs, and 22 of 65 JcMAPKKKs contained the low temperature response element. JcMAPKK3 contained the most cis-elements (27), including abscisic acid responsive element, jasmonic acid methyl ester responsive element (CGTCA-motif/TGACG-motif), ethylene responsive element, salicylic acid responsive element (TCA element), and pathogen responsive element (W-box). The results strongly suggested that MAPK cascade genes function in stress resistance and hormone signaling pathways.
Tissue-specific expression patterns of J. curcas MAPK cascade genes
Using published RNA-seq data and associated FPKM values from three different tissues46, we analyzed tissue-specific transcriptional expression profiles of MAPK cascade genes. Figure 10 shows heatmaps of expression profiles. We found that ESTs (Expressed Sequence Tag) representing 74 of the 82 (90.2%) MAPK cascade genes in J. curcas were detected in all three tissues, with considerable variation in expression levels. Notably, JcMAPK1, JcMAPK7, JcMAPK12, JcMAPKK2, JcMAPKK5, JcMAPKKK3, JcMAPKKK22, JcMAPKKK27, JcMAPKKK31, JcMAPKKK32, JcMAPKKK36, JcMAPKKK51, and JcMAPKKK59 all exhibited relatively high transcript abundance in all tested tissues. Five JcMAPKKKs (JcMAPKKK14, JcMAPKKK15, JcMAPKKK55, JcMAPKKK56, and JcMAPKKK33) were not expressed in some tissues, while three (JcMAPKKK4, JcMAPKKK29, and JcMAPKKK41) were not expressed in any tissue (Fig. 10C–E).
The expression profiles of MAPK (A), MAPKK (B), MEKK (C), RAF (D), and ZIK (E) genes in J. curcas different tissues. The tissue-specific expression levels of J. curcas MAPK cascade genes were obtained from the RNA-Seq data (accession number SRR1639659, SRR1639660, and SRR1639661) and resulting FPKM values. Blue indicates high expression and red indicates low expression.
The majority of JcMAPK members were expressed constitutively in leaves, roots, and seeds (Fig. 10A). In leaves, JcMAPK7 transcript level was highest, followed by JcMAPK12. In contrast, JcMAPK12 transcript level was highest in roots and seeds, followed by JcMAPK3. Among the five JcMAPKK genes, JcMAPKK4 transcript was most abundant in root, whereas JcMAPKK5 transcripts were most abundant in leaves and seeds (Fig. 10B). Of JcMAPKKKs, the MEKK member JcMAPKKK3 had the highest expression in all three tissues, hinting at its core function of connecting MAPK to MAPKKK in MAPK signaling transduction pathways (Fig. 10C). The expression of 35 (87.5%) RAF JcMAPKKKs was detected in all three tissues, with the remainder exhibiting tissue-specific expression. JcMAPKKK51 was highly expressed in leaf, whereas JcMAPKKK27 and JcMAPKKK32 were highly expressed in root and seed, respectively. Additionally, JcMAPKKK56 was specifically expressed in leaf (Fig. 10D). None of ZIK subfamily members differed in expression between tissues, suggesting that they may have house-keeping roles in organ development46. Compared to other ZIK members, JcMAPKKK59 and JcMAPKKK65 had higher expression levels in leaves (Fig. 10E), suggesting a role in leaf-specific development.
Expression patterns of J. curcas MAPK cascade genes under cold stress
Our DGE database indicated that 33 (40.2%) of the 82 MAPK cascade genes are fully expressed throughout the cold stress treatment. The expression of JcMAPK4, JcMAPKK5, and eight JcMAPKKKs (JcMAPKKK41, JcMAPKKK16, JcMAPKKK33, JcMAPKKK50, JcMAPKKK51, JcMAPKKK9, JcMAPKKK29, and JcMAPKKK37) were significantly up-regulated after 12, 24, and 48 h of cold stress (Fig. 11)47,48. Notably, JcMAPKKK41 and JcMAPKKK16 were dramatically up-regulated after 12 h of cold stress, indicating a potentially important function in J. curcas cold response. In contrast, we observed significant down-regulation of JcMAPK7, JcMAPKK1, and nine JcMAPKKKs (JcMAPKKK1, JcMAPKKK15, JcMAPKKK18, JcMAPKKK23, JcMAPKKK34, JcMAPKKK54, JcMAPKKK55, JcMAPKKK56, and JcMAPKKK64) (Fig. 11).
Gene expression patterns usually provide the important clue for its function. We thus used qRT-PCR to verify the expression levels of eight genes that are differentially expressed across tissues (Fig. 12) and 12 cold-responsive genes (Fig. 13). We found that JcMAPKKK9, JcMAPKKK35, JcMAPKKK55, JcMAPKKK58, and JcMAPKKK59 were more highly expressed in all tested tissues, with JcMAPKKK9 exhibiting higher expression in root and JcMAPK7 in leaf (Fig. 12). After 0.5–48 h of cold stress in leaves, the expression of all tested MAPK cascade genes were induced potentially (Fig. 13A–L). The cold-induced expression of MAPKKK16 was particularly notable, reaching the highest expression level (49.13-fold increase) after 12 h in leaves (Fig. 13E). In addition, the response of cold-induced gene in some case is very rapid and examines distinct expression changes at earlier time points. For instance, JcMAPK4, JcMAPKKK9, JcMAPKKK29, JcMAPKKK37, and JcMAPKKK50 were significantly up-regulated after 0.5 h cold stress (Fig. 13A,D,F,H,J). In roots, cold stress significantly up-regulated JcMAPK4, JcMAPKKK9, JcMAPKKK41, and JcMAPKKK50 (Fig. 13a,d,i,j), while significantly down-regulated JcMAPKKK58 (Fig. 13l). These qRT-PCR results were consistent with RNA-seq and DGE data, suggesting that our conclusions regarding gene expression were reasonable.
qRT-PCR differential expression analysis of MAPK cascade genes in different tissues. The 24h-imbibed seeds, leaves and roots from 2-week-old seedlings were harvested for total RNA extraction and expression analysis. The relative expression levels were compared to expressions in the sample of leaves. Data represent a mean value of three repeats from three independent qRT-PCR assays. ** and * indicate significant differences in comparison with the leaves at p < 0.01 and p < 0.05, respectively.
qRT-PCR relative expression levels of MAPK cascade genes in leaves and roots under cold stress. 2-week-old seedlings stressed in 12 °C cold for 0.5, 3, 12, 24, and 48 h. Control seedlings were continuously cultivated under normal growth conditions of 26 °C. Relative expression levels of JcMAPK4 (A,a), JcMAPK10 (B,b), JcMAPKK5 (C,c), JcMAPKKK9 (D,d), JcMAPKKK16 (E,e), JcMAPKKK29 (F,f), JcMAPKKK33 (G,g), JcMAPKKK37 (H,h), JcMAPKKK41 (I,i), JcMAPKKK50 (J,j), JcMAPKKK51 (K,k), and JcMAPKKK58 (L,l) in leaves and roots were presented. Data are presented as the mean fold changes between treated and control samples at each time point ± standard deviations (SDs). ** and * indicate significant differences in comparison with the control at p < 0.01 and p < 0.05, respectively.
Interaction network analysis
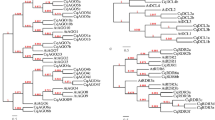
In recent years, interaction networks of gene families have become a very useful method to investigate the gene interactions and regulatory relationship. In order to identify potential biological function of J. curcas MAPK cascade members, interaction networks of JcMAPK4, JcMAPKK3, JcMAPKKK16, JcMAPKKK41, JcMAPKKK51, and JcMAPKKK55 were created based on experimentally validated interactions in Arabidopsis using STRING software. The results showed that JcMAPK4-mediated network may be involved in lateral root formation, sugar signaling, polar auxin transport, and in a signaling pathway that modulates the expression of genes responding to biotic and abiotic stresses (Fig. 14A). The interactive proteins involved in the JcMAPKK3 network, including PTP1, MPK2, MPK4, MPK6, and MPK7. It has been reported that these interactions might function in the regulation of jasmonate signal transduction, oxidative stress-mediated signaling cascade (such as ozone), and plant cytokinesis during meiosis and mitosis (Fig. 14B). Notable, there were 10 high confidence interactive proteins involved in the JcMAPKKK16 network, including MAPK cascade proteins (MEK1 and MKK2) that are involved in biotic or abiotic stress and pathogen defense, MKK9 involved in ethylene signaling and in salt stress response, and MKK4 involved in cell proliferation (Fig. 14C). Furthermore, JcMAPKKK41 associates with ABI2 (ABA insensitive 2), and plays an essential role in the regulation of the stomatal closure, high light stress, response to glucose, seed germination, and inhibition of vegetative growth by repressing the abscisic acid signaling pathway (Fig. 14D). In addition, JcMAPKKK51 and JcMAPKKK55 showed interaction with ACC1 (acetyl-CoA carboxylase 1) and ACC3, indicating its possible roles in fatty acid synthesis and elongation (Fig. 14E,F). These results indicated that a single MAPK cascade may participate in various stresses or signal response, suggesting possible roles of MAPK cascade in multiple signaling pathways in J. curcas.
Interaction network analysis of J. curcas MAPK cascade proteins and related genes in Arabidopsis. The line thickness relates to the combined score. The homologous genes of J. curcas are presented in red font in parentheses. (A) JcMAPK4; (B) JcMAPKK3; (C) JcMAPKKK16; (D) JcMAPKKK41; (E) JcMAPKKK51; (F) JcMAPKKK55.
Discussion
Genome-wide exploration of the MAPK, MAPKK, and MAPKKK families have been performed in several plants, laying an important foundation for further functional characterization. In this study, we identified 12 MAPKs, 5 MAPKKs, and 65 MAPKKKs in J. curcas genome. Identified JcMAPK and JcMAPKK gene families were each classified into four subgroups that exhibited similar intron-exon organizations, suggesting that MAPK and MAPKK evolutionary origins were conserved across species. J. curcas possesses far fewer JcMAPKs and JcMAPKKs than Arabidopsis (20/10)6,11, rice (15/8)12,13, maize (20/9)14, and poplar (21/11)27. Moreover, only a fraction of the JcMAPK and JcMAPKK genes in J. curcas have orthologs in Arabidopsis, hinting at an ancestor that experience gene duplication prior to the eudicot-monocot divergence49. Our findings confirm previous MAPKKK reports in other species11,12,14,15,16 and classified JcMAPKKKs into three subfamilies (16 MEKKs, 40 RAFs, and 9 ZIKs) (Figs 3, 4). We also found that kinase domains were located at different sites across JcMAPKKK proteins. In the RAF subfamily, most proteins had a C-terminal kinase domain and a long N-terminal regulatory domain. In contrast, most ZIK members had an N-terminal kinase domain. Protein structure in MEKK members were less conserved, with kinase domains variously at the N-terminal, C-terminal, or central part of the protein, consistent with their orthologs in rice12 and cucumber44. This diversity may allow MAPKKKs to regulate multiple specific metabolic activities in plants. For instance, two of the best-studied RAF MAPKKKs in Arabidopsis are CTR1 (AtRAF1) and EDR1 (AtRAF2), respectively negative regulators in ethylene-induced signaling9,10,50 and in response to powdery mildew attack8. However, neither protein participates in a classic MAPK cascade14. The relatively limited numbers of MAPKKs and MAPKs in J. curcas also support the idea that MAPKKKs may not involved in typical MAPK cascade signaling. Our BLAST analysis revealed that JcMAPKKK25/48 and JcMAPKKK46 are CTR1 and EDR1 orthologs, opening the door for future research on their potentially analogous functions.
In MAPK, the conserved CD domain -LHDxxE/DEPxC- is a docking site for MAPKK, and the domain’s two adjacent D and E residues play crucial role in interacting with alkaline residues K (Lysine) and R (Arginine) in MAPKK. We found a CD domain in all JcMAPK group C members, but not in groups A, B, and D (Fig. 5). This result was consistent with finding in B. distachyon45. Analysis of conserved motifs in MEKK (-G(T/S)Px(W/Y/F)MAPEV-), RAF (-GTxx(W/Y)MAPE-), and ZIK (-GTPEEMAPE(L/V/M)(Y/F/L)-) indicated that -MAPE- are the core amino acid residues within the MAPKKK kinase domain. Furthermore, MEME did not detect additional protein domains in J. curcas MEKK and ZIK subfamilies, whereas additional domains were presented in most RAF members, such as PAS domain (At3g06620.1, At3g06640.1, At4g23050.2, and At5g49470.2) in Arabidopsis11 and PB1 domain in Arabidopsis (At1g04700.1) and grapevine16, which were accordant with the orthologs in J. curcas of JcMAPKKK39/43 and JcMAPKKK55, respectively.
Abundance varies considerably across members of MAPK cascade gene families, given their participation in a wide range of physiological processes51. Within the 22 duplicated gene pairs identified in J. curcas, only five (JcMAPKKK25/JcMAPKKK48, JcMAPKKK28/JcMAPKKK46, JcMAPKKK31/JcMAPKKK32, JcMAPKKK23/JcMAPKKK36, and JcMAPKKK44/JcMAPKKK45) shared similar expression patterns in nearly all tested tissues. Our observations are in accord with previous findings of preferential tissue expression among MAPK cascade gene pairs14,46,51. In general, duplicated gene pairs may differ considerably in expression profiles and functions across tissues. In support of this, expression profile clustering (Fig. 10) did not reflect phylogenetic similarities (Figs 1A, 2A, and 3). For instance, JcMAPKKK50 expression was higher in leaf, root, and seed, but this was not the case in its closely duplicated sister gene JcMAPKKK53. Likewise, JcMAPKKK57 expression was higher in leaf, but its duplicate JcMAPKKK64 was predominantly expressed in root. Orthologous genes also displayed different expression patterns across species. For instance, JcMAPKKK9 in J. curcas had higher expression in root than in seed or leaf (Fig. 10C), whereas its ortholog OsMAPKKK2 in O. sativa was constitutively and highly expressed in nearly all tissues12. Thus, although duplicated genes have similar amino acid and nucleotide sequences, they may not share functions or involvement in the same metabolic pathways44,47. Some may lose or gain functions after duplication in J. crucas evolution.
Multiple studies have examined MAPK cascade involvement in cold stress responses of different plants. In the BJ and FJ banana varieties, cold treatment up-regulated 50% and 60% of MAPKKs, respectively, as well as 43.4% and 65.8% of MAPKKK genes52. Likewise, cold stress activates A. thaliana MAPK cascades of AtMEKK1-AtMKK1/2-AtMPK453 and AtMPK6-p44MAPK54, furthermore, cold temperature also induces the expression of CRLK1 (Ca2+ dependent receptor-like kinase 1) located upstream of AtMEKK155. Moreover, the up-regulation of MAPK cascade genes under cold stress has been demonstrated in numerous plants, including ZmMPK3 and ZmMPK5 in maize56,57, CsMPK3, CsMPK7, and CsMPK13 in cucumber44, SlMAPKK in tomato58, TaRAF36 and TaRAF49 in Triticum aestivum59, and FvMAPK7 in Fragaria vesca43. Here, we identified 10 MAPK cascade genes that were up-regulated across 12, 24, and 48 h of cold stress (Fig. 11). Promoter analysis revealed that most of these genes possess LTR and TC-rich repeats, cis-acting elements involved in cold stress response (Table S2). Overall, the presence of such cis-elements in promoter of the MAPK cascade genes suggests their involvement in J. curcas stress response pathways.
Methods
Identification of MAPK, MAPKK, MAPKKK genes in J. curcas
Completed genome sequences and predicted peptide sequences of J. curcas were downloaded from Kazusa DNA Research Institute (http://www.kazusa.or.jp/jatropha/) and GenBank (http://www.ncbi.nlm.nih.gov/genome/915/, accession number: AFEW00000000.1) to construct a local genome and protein database using NCBI BLAST (Windows v2.2.27). Arabidopsis and rice MAPK, MAPKK, and MAPKKK protein sequences were download from the TAIR database (https://www.arabidopsis.org/) and the rice genome annotation database (http://rice.plantbiology.msu.edu/). The Ricinus communis and P. trichocarpa protein sequences were downloaded from Phytozome (https://phytozome.jgi.doe.gov/pz/portal.html). All the amino-acid sequences were aligned for constructing HMM profiles with the HMMER v3.0 hmm build tool. Next, HMM profiles were searched against the local J. curcas genome and protein database with threshold e-value and identity of 1e-10 and 50%, respectively. Obtained sequences were self-BLASTed to remove redundancy. Only MAPK genes with the -TxY- motif, MAPKK genes with -D(L/I/V)K- and -S/TxxxxxS/T- sequences, as well as MAPKKK genes with one of three consensus sequences (-G(T/S)Px(W/Y/F)MAPEV-, -GTxx(W/Y)MAPE-, and -GTPEEMAPE(L/V/M)(Y/F/L)-) were included. Potential proteins were further verified using the GenBank CDD tool (http://www.ncbi.nlm.nih.gov/sturcture/cdd/wrpsb.cgi) and Pfam (http://pfam.xfam.org/search/) to confirm MAPK domain presence (PF00069).
Molecular weights (Mw) and isoelectric points (pI) were calculated on the ExPASy server (http://web.expasy.org/protparam/). Predicted subcellular localization of MAPK cascade members was conducted on the CELLO v2.5 server (http://cello.life.nctu.edu.tw/). Specific interaction networks of selected MAPK cascade proteins were constructed using STRING (http://string-db.org/).
Multiple sequence alignment and phylogenetic analysis
Multiple sequence alignments of putative MAPK cascade proteins were performed in ClustalW v2.0. Domains and motifs were detected using MEME (http://meme-suite.org/tools/meme). A neighbor-joining (NJ) phylogenetic tree (bootstrapping: 1000 replicates) was constructed in MEGA v5.0.
Gene structure and cis-element analysis of promoter regions
Exon, intron, and UTR (untranslated region) distribution patterns of each gene was generated in the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/), based on a comparison of the J. curcas genome with CDS. To perform cis-element analysis, 1.5 kb of genomic DNA sequences upstream of each gene’s initiation codon (ATG) were downloaded, and then were detected by PlantCARE database.
Chromosomal location, gene duplication, and synteny analysis
Specific positions of MAPK cascade genes on the corresponding scaffold were obtained from the J. crucas genome, and a previously constructed linkage map46 was used to complete chromosomal location in MapChart v2.1. Gene duplication was investigated based on the following criteria: (1) alignment length >70% of longer genes; (2) alignment length with >70% identity; (3) only one duplication event for tightly linked genes. Duplicated gene pairs, along with their corresponding Ka (Number of nonsynonymous substitutions per nonsynonymous site) and Ks (Number of synonymous substitutions per synonymous site), were also characterized using the PAL2NAL web server (http://www.bork.embl.de/pal2nal/)60. Duplicated regions in the J. curcas genome were visualized using Circos v0.67 (http://circos.ca).
Expression analysis of MAPK cascade genes using RNA-seq
Illumina RNA sequencing data were obtained from J. curcas seed (NCBI SRA accession number SRR1639661), as well as the leaf (SRR1639660) and root (SRR1639659) of closely related J. integerrima46. Clean reads were mapped to CDS using Bowtie 2, and transcript levels were determined as FPKM (Fragments per kilobase of exon per million fragments mapped). Transcriptome and DGE data from leaves sequenced in our previous studies47,48 were used to determine expression profiling under cold stress. Relative gene expression was determined through normalizing the number of unambiguous clean tags per gene to transcripts per million clean tags (TPM)61,62. Expression data were analyzed, clustered, and displayed using gplots and pheatmap in R version 3.4.1.
Plant material treatment, RNA isolation, and qRT-PCR analysis
Seeds of J. curcas were surface-sterilized in 1.5% CuSO4 for 20 min, rinsed thoroughly with sterile distilled water63, and then soaked in distilled water for 24 h. Imbibed seeds were sown on trays containing six layers of wetted filter paper and germinated in a dark, 26 °C climate chamber for 5 d. Geminated seeds were transferred to pots containing a sterilized perlite, peat, and sand mixture (1:2:1), then grown for 14 d in a climate chamber set to 26/20 °C (day/night), 75% relative humidity, and 16/8 h photoperiod. Cold treatments followed our previously published methods64,65, 2-week-old seedlings were subjected to 12 °C for 0.5, 3, 12, 24, and 48 h. Control seedlings were continuously cultivated under normal growth conditions. 24 h-imbibed seeds, leaves and roots from each treatment (including control) were harvested, frozen in liquid nitrogen, and stored at −80 °C.
Total RNA were extracted from experimental tissue with Trizol reagent (Invitrogen, USA). First strand cDNAs were synthesized using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, USA). Expression profiles in different tissues and under cold stress were detected through qRT-PCR, performed with Power SYBR Green PCR Master Mix (Thermo, USA) on a CFX Connect (Bio-Rad, USA) (for primers, see Table S3). The internal control for expression analysis was GAPDH, and relative expression was calculated using the 2−ΔΔCt method. Each sample contained three replicates.
Statistical analysis
At minimum, all experiments were performed in triplicate. Data were analyzed using a paired Student’s t-test in SPSS version 24.0 (Chicago, USA). Figures were drawn in Sigma Plot 13.0 (Systat Software Inc., UK). Each data are presented as means ± SE of at least three replicates.
References
Bohnert, H. J., Nelson, D. E. & Jensen, R. G. Adaptation to environmental stresses. Plant Cell 7, 1099–1111 (1995).
Bogre, L., Meskiene, I., Heberle-Bors, E. & Hirt, H. Stressing the role of the MAP kinases in mitogenic stimulation. Plant Mol Biol 43, 705–718 (2000).
Uimari, Q. et al. Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Hum Reprod Update 32, 780–793 (2017).
Pedley, K. F. & Martin, G. B. Role of mitogen-activated protein kinase in plant immunity. Curr Opin Plant Biol 8, 541–547 (2005).
Favata, M. F. et al. Identification of a novel inhibitor of mitogen-activated protein kinase. J Biol Chem 273, 18623–18632 (1998).
Ichimura, K. et al. Mitogen-activated protein kinase cascades in plants: a new nomenclature. Trends Plant Sci 7, 301–308 (2002).
Champion, A., Picaud, A. & Henry, Y. Reassessing the MAP3K and MAP4K relationships. Trends Plant Sci 9, 123–129 (2004).
Frye, C. A., Tang, D. & Innes, R. W. Negative regulation of defense response in plants by a conserved MAPKK kinase. P Natl Acad Sci USA 98, 373–378 (2001).
Huang, Y., Li, H., Hutchison, C. E., Laskey, J. & Kiebe, J. J. Biochemical and functional analysis of CTR1, a protein kinase that negatively regulates ethylene signaling in Arabidopsis. Plant J 33, 221–233 (2003).
Kieber, J. J., Rothenbery, M., Roman, G., Feldmann, K. A. & Ecker, J. R. CTR1, a negative regulator of the ethylene response pathway in Arabidopsis, encodes a member of the raf family of protein kinase. Cell 72, 427–441 (1993).
Hamel, L. P. et al. Ancient signals: comparative genomics of plant MAPK and MAPKK gene families. Trends Plant Sci 11, 192–198 (2006).
Rao, K. P., Richa, T. & Kumar, K. In silico analysis reveals 75 members of mitogen-activated protein kinase kinase kinase gene family in rice. DNA Res 17, 139–153 (2010).
Reyna, N. S. & Yang, Y. Molecular analysis of the rice MAP kinase gene family in relation on Magnaporthe grisea infection. Mol Plant Microbe In 19, 530–540 (2006).
Kong, X. et al. Genome-wide identification and analysis of expression profiles of maize mitogen-activated protein kinase kinase kinase. PLoS One 8, e57714 (2013).
Cakir, B. & Kilickaya, O. Mitogen-activated protein kinase cascades in Vitis vinifera. Front Plant Sci 6, 556 (2015).
Wang, G. et al. Genome-wide identification and analysis of mitogen activated protein kinase kinase kinase gene family in grapevine (Vitis vinifera). BMC Plant Biol 14, 219 (2014).
Bardwell, L. & Thorner, J. A. A conserved motif at the amino termini of MEKs might mediate high-affinity interaction with the cognate MAPKs. Trends Biochem Sci 21, 373–374 (1996).
Meszaros, T. et al. The Arabidopsis MAP kinase kinase MKK1 participates in defence responses to the bacterial elicitor flagellin. Plant J 48, 485–498 (2006).
Qiu, J. L. et al. Arabidopsis MAP kinase 4 regulates gene expression through transcription factor release in the nucleus. EMBO J 27, 2214–2221 (2008).
Beck, M., Komis, G., Ziemann, A., Menzel, D. & Samaj, J. Mitogen-activated protein kinase 4 is involved in the regulation of mitotic and cytokinetic microtubule transitions in Arabidopsis thaliana. New Phytol 189, 1069–1083 (2010).
Takahashi, Y., Soyano, T., Kosetsu, K., Sasabe, M. & Machida, Y. HINKEL kinase, ANP MAPKKKs and MKK6/ANQ MAPKK, which phosphorylates and activates MPK4 MAPK, constitute a pathway that is required for cyotkinesis in Arabidopsis thaliana. Plant Cell Physiol 51, 1766–1776 (2010).
Takahashi, F. et al. The mitogen-activated protein kinase cascade MKK3-MPK6 is an important part of the jasmonate signal transduction pathway in Arabidopsis. Plant Cell 19, 805–818 (2007).
Asai, T., Tena, G., Plotnikova, J., Willmann, M. R. & Chiu, W. L. MAP kinase signalling cascade in Arabidopsis innate immunity. Nature 415, 977–983 (2002).
Wang, H., Ngwenyama, N., Liu, Y., Walker, J. C. & Zhang, S. Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19, 63–73 (2007).
Xu, J. et al. Activation of MAPK kinase 9 induces ethylene and camalexin biosynthesis and enhances sensitivity to salt strss in. Arabidopsis. J Biol Chem 283, 26996–27006 (2008).
Zhou, C., Cai, Z., Guo, Y. & Gan, S. An Arabidopsis mitogen-activated protein kinase cascade, MKK9-MPK6, plays a role in leaf senescence. Plant Physiol 150, 167–177 (2009).
Nicole, M. C., Hamel, L. P., Beaudoin, N. & Ellis, B. E. MAP-ping genomic organization and organ-specific expression profiles of poplar MAP kinase and MAP kinase kinases. BMC Genomics 7, 223 (2006).
Zhang, S., Xu, R. R., Luo, X. C., Jiang, Z. S. & Shu, H. R. Genome-wide identification and expression analysis of MAPK and MAPKK gene family in Malus domestica. Gene 531, 377–387 (2013).
Camps, M., Nichols, A. & Arkinstall, S. Dual specificity phosphatases: a gene family for control of MAP kinase funtion. FASEB J 14, 6–16 (1999).
Rodriguez, M. C., Petersen, M. & Mundy, J. Mitogen-activated protein kinase signaling in plants. Annu Rev Plant Biol 61, 621–649 (2010).
Kiegerl, S. et al. SIMKK, a mitogen-activated protein kinase (MAPK) kinase, is a specific activator of the salt stress-induced MAPK, SIMK. Plant Cell 12, 2247–2258 (2000).
Seo, S., Katou, S., Seto, H., Gomi, K. & Ohashi, Y. The mitogen-activated protein kinase WIPK and SIPK regulate the levels of jasmonic and salicylic acids in wounded tobacco plants. Plant J 49, 899–909 (2007).
Zhang, S. & Klessig, D. F. MAPK cascades in plant defense signaling. Trends Plant Sci 6, 520–527 (2001).
Andreasson, E. et al. The MAP kinase substrate MKS1 is a regulator of plant defense responses. EMBO J 24, 2579–2589 (2005).
Brodersen, P. et al. Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J 47, 532–546 (2006).
Droillard, M. J., Boudsocq, M., Barbier-Brygoo, H. & Lauriere, C. Involvement of MPK4 in osmotic stress response pathways in cell suspensions and plantlets of Arabidopsis thaliana: activation by hypoosmolarity and negative role in hyperosmolarity tolerance. FEBS Lett 574, 42–48 (2004).
Schaffer, R. et al. Microarray analysis of diurnal and circadian-regulated genes in Arabidopsis. Plant Cell 13, 113–123 (2001).
He, C., Fong, S. H., Yang, D. & Wang, G. L. BWMK1, a novel MAP kinase induced by fungal infection and mechanical wounding in rice. Mol Plant Microbe In 12, 1064–1073 (1999).
Schoenbeck, M. A. et al. The alfalfa (Medicago sativa) TDY1 gene encodes a mitogen-activated protein kinase homolog. Mol Plant Microbe In 12, 882–893 (1999).
Kong, F., Wang, J. & Cheng, L. Genome-wide analysis of the mitogen-activated protein kinase gene family in Solanum lycopersicum. Gene 499, 108–120 (2012).
Hyun, T., Kim, J. S. & Kwon, S. Y. Comparative genomic analysis of mitogen activated protein kinase gene family in grapevine. Genes & Genom 32, 275–281 (2010).
Lu, K. et al. Genome-wide survey and expression profile analysis of the mitogen-activated protein kinase (MAPK) gene family in Brassica rapa. PLoS One 10, e0132051 (2015).
Wei, W. et al. Bioinformatics identification and transcript profile analysis of the mitogen-activated protein kinase gene family in the diploid woodland strawberry Fragaria vesca. PLoS One 12, e0178596 (2017).
Wang, J. et al. Genome-wide identification of MAPK, MAPKK, and MAPKKK gene families and transcriptional profiling analysis during development and stress response in cucumber. BMC Genomics 16, 386 (2015).
Chen, L. H. et al. Genome-wide identification and analysis of MAPK and MAPKK gene families in Brachypodium distachyon. PLoS One 7, e46744 (2012).
Wu, P. Z. et al. Integrated genome sequence and linkage map of physic nut (Jatropha curcas L.), a biodiesel plant. Plant J 81, 810–821 (2015).
Wang, H. B., Zou, Z. R., Wang, S. S. & Gong, M. Deep sequencing-based transcriptome analysis of the oil-bearing plant Physic Nut (Jatropha curcas L.) under cold treatments. Plant omics 7, 178–187 (2014).
Wang, H. B., Zou, Z. R., Wang, S. S. & Gong, M. Global analysis of transcriptome responses and gene expression profiles to cold stress of Jatropha curcus L. PLos ONE 8, e82817 (2013).
Kim, S. et al. Genome sequence of the hot pepper provides insights into the evolution of pungency in Capsicum species. Nature Genet 46, 270–278 (2014).
Adams-Phillips, L. et al. Evidence that CTR1-mediated ethylene signal transduction in tomato is encoded by a multigene family whose members display distinct regulatory features. Plant Mol Biol 54, 387–404 (2004).
Liu, Z. N. et al. Identification, expression, and comparative genomic analysis of the IPT and CKX gene families in Chinese cabbage (Brassica rapa ssp pekinensis). BMC Genomics 14, 594 (2013).
Wang, L. et al. The MAPKKK and MAPKK gene families in banana: identification, phylogeny and expression during development, ripening and abiotic stress. Sci Rep 7, 1159 (2017).
Ichimura, K. et al. Isolation of AtMEKK1 (a MAP kinase kinse kinase)-interaction proteins and analysis of a MAP kinase cascade in Arabidopsis. Biochem Bioph Res Co 253, 532–543 (1998).
Ichimura, K., Mizoguchi, T., Yoshida, R., Yuasa, T. & Shinozaki, K. Various abiotic stresses rapidly activate Arabidopsis MAP kinase AtMPK4 and AtMPK6. Plant J 24, 655–665 (2000).
Yang, T., Chaudhuri, S., Yang, L., Du, L. & Poovaiah, B. W. A calcium/calmodulin-regulated member of the receptor-like kinase family confers cold tolerance in plants. J Biol Chem 285, 7119–7126 (2010).
Wang, J. et al. A novel mitogen-activated protein kinase gene in maize (Zea mays), ZmMPK3, is involved in response to diverse environmental cues. J Integr Plant Biol 52, 442–452 (2010).
Berberich, T., Sano, H. & Kusano, T. Involvement of a MAP kinase, ZmMPK5, in senescence and recovery from low-temperature stress in maize. Mol Gen Genet 262, 534–542 (1999).
Wu, J. et al. Genome-wide identification of MAPKK and MAPKKK gene families in tomato and transcriptional profiling analysis during development and stress response. PLoS One 9, e103032 (2014).
Wang, M. et al. Genome-wide identification, phylogeny and expressional profiles of mitogen activated protein kinase kinase kinase (MAPKKK) gene family in bread wheat (Triticum aestivum L.). BMC Genomics 17, 668 (2016).
Suyama, M., Torrents, D. & Bork, P. PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res 34, 609–612 (2006).
Morrissy, A. S. et al. Next-generation tag sequencing for cancer gene expression profiling. Genome Res 19, 1825–1835 (2009).
tHoen, P. A. et al. Deep sequencing-based expression analysis shows major advances in robustness, resolution and inter-lab portability over five microarray platforms. Nucleic Acids Res 36, e141 (2008).
Li, Z. G. & Gong, M. Effects of different chemical disinfectant on seed germination and seedling growth of Jatropha curcas L. Seed 30(4–7), 12 (2011).
Ao, P. X., Li, Z. G., Fan, D. M. & Gong, M. Involvement of antioxidant defense system in chill hardening-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35, 153–160 (2013a).
Ao, P. X., Li, Z. G. & Gong, M. Involvement of compatible solutes in chill hardening-induced chilling tolerance in Jatropha crucas seedlings. Acta Physiol Plant 35, 3457–3464 (2013b).
Acknowledgements
This work was supported by several grants from the National Foundations of Natural Sciences, China (No. 31460179, 31260064, and 31460561).
Author information
Authors and Affiliations
Contributions
H.W. and M.G. conceived and designed the experiments. H.W. wrote the paper; H.W., L.T. and Y.G. analyzed the data. J.G., H.X. and C.L. improved the English. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, H., Gong, M., Guo, J. et al. Genome-wide Identification of Jatropha curcas MAPK, MAPKK, and MAPKKK Gene Families and Their Expression Profile Under Cold Stress. Sci Rep 8, 16163 (2018). https://doi.org/10.1038/s41598-018-34614-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34614-1
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.