Abstract
The incidence of in-hospital cardiovascular adverse events (AEs) in patients with ST-segment elevation myocardial infarction (STEMI) following primary percutaneous coronary intervention (PCI) is relatively high. Identification of metabolic markers could improve our understanding of the underlying pathological changes in these patients. We aimed to identify associations between concentrations of plasma metabolites on admission and development of in-hospital AEs in post-PCI patients with STEMI. We used targeted mass spectrometry to measure plasma concentrations of 26 amino acid metabolites on admission in 96 patients with STEMI who subsequently developed post-PCI AEs and in 96 age- and sex-matched patients without post-PCI cardiovascular AEs. Principal component analysis (PCA) revealed that PCA-derived factors, including branched chain amino acids (BCAAs), were associated with increased risks of all three pre-specified outcomes: cardiovascular mortality/acute heart failure (AHF), cardiovascular mortality, and AHF. Addition of BCAA to the Global Registry of Acute Coronary Events risk score increased the concordance C statistic from 0.702 to 0.814 (p < 0.001), and had a net reclassification index of 0.729 (95% confidence interval, 0.466–0.992, p < 0.001). These findings demonstrate that high circulating BCAA concentrations on admission are associated with subsequent in-hospital AEs after revascularization in patients with STEMI.
Similar content being viewed by others
Introduction
Primary percutaneous coronary intervention (PCI) is currently the recommended treatment for patients presenting with ST-segment elevation myocardial infarction (STEMI)1, salvaged viable myocardium, limited myocardial infarction (MI) size, and preserved ventricular systolic function. However, these patients may develop in-hospital cardiovascular adverse events (AEs) such as cardiac rupture, malignant arrhythmias, and onset of heart failure (HF) after PCI as a result of myocardial ischemia and ischemia–reperfusion injury2. The 30-day mortality rate and incidence of in-hospital acute heart failure (AHF) among patients with STEMI who undergo primary PCI are reportedly 7.9% and 28%, respectively3,4. An early and efficient interventional strategy could reduce the incidence of cardiovascular AEs and improve these patients’ short- and long-term prognoses5.
Current optimal secondary prevention therapies for patients with STEMI who have undergone PCI primarily involve drugs such as antiplatelet agents, statins, β-blockers, angiotensin-converting enzyme inhibitors, and aldosterone antagonists6. However, the limited effectiveness of these strategies in preventing and treating post-MI cardiovascular AEs suggests that interruption of the pathways responsible for the AEs are needed to improve outcomes. Maladaptive molecular processes may be a significant cause of such AEs; thus, a metabolomics approach may help to address these problems.
Following primary PCI, there are metabolic changes in the hearts of patients with STEMI and emerging evidence suggests that metabolic remodelling in the heart is the key to development and progression of heart failure7,8. Amino acids are important metabolites of nutrients that are vital to survival and growth of cells and serve as substrates for intracellular biosynthesis and signalling molecules9. Previous studies have shown that ischemia and ischemia–reperfusion injury lead to disordered amino acid catabolism, especially of the branched-chain amino acids (BCAAs) leucine, isoleucine, valine, and taurine10,11,12. Defective amino acid catabolism sensitizes the heart to ischemia–reperfusion injury, leading to adverse myocardial remodelling and post-MI HF13.
In this study, we aimed to assess abnormalities in circulating amino acid metabolites in admission blood samples of patients with STEMI who went on to undergo PCI and who developed in-hospital cardiovascular AEs and compare them with those in age- and sex-matched controls who did not develop AEs.
Results
Baseline characteristics of patients with STEMI and post-PCI AEs
Of the 96 patients with STEMI who developed the composite primary outcome of cardiovascular death or AHF during hospitalization, 20 had cardiovascular deaths and 76 AHF. The baseline clinical characteristics of patients who did and did not develop cardiovascular AEs are listed in Table 1. The patients were similarly distributed with regard to sex, age, body mass index (BMI), blood pressure, heart rate, Gensini score, interval between symptom onset and reperfusion14, history of hypertension, diabetes mellitus and smoking, and objective laboratory measures, including blood lipids, glucose, and creatine concentrations. Troponin I (TnI) and N-terminal B-type natriuretic peptide (NT-pro BNP) concentrations were significantly higher in the AE than the No-AE group (7.7 ± 19 µg/mL vs. 8.8 ± 6.9 µg/mL, p = 0.001 and 298.5 ± 119.4 ng/L vs. 214.3 ± 80.8 ng/L, p < 0.001, respectively). Furthermore, Global Registry of Acute Coronary Events (GRACE) risk scores were significantly higher in the AE than the No-AE group (131.6 ± 16.9 vs. 145.0 ± 21.8, p < 0.001).
Plasma concentrations of 26 amino acids
Differences in admission plasma concentrations of 26 amino acids between the AE and No-AE groups are shown in Supplementary Table 1. Plasma concentrations of valine (p < 0.001), isoleucine (p < 0.001), leucine (p < 0.001), tyrosine (p < 0.001), phenylalanine (p < 0.001), ornithine (p < 0.001), glutamate (p < 0.001), creatine (p < 0.001), creatinine (p < 0.001), serine (p < 0.001), urea (p = 0.006), kynurenine (p = 0.008), and glycine (p = 0.017) were significantly higher in the AE than No-AE group, whereas those of glutamine (p < 0.001), arginine (p = 0.011), and histidine (p = 0.044) were significantly lower in the AE group. Plasma concentrations of the other tested amino acids were similar between the AE and No-AE groups.
Association between admission amino acid concentrations and in-hospital clinical outcomes
Six meaningful metabolomics factors were identified by principal component analysis (PCA) (Supplementary Table 2): Factor 1 (ornithine, glycine, serine), Factor 2 (BCAAs leucine, isoleucine, valine), Factor 3 (phenylalanine), Factor 4 (urea, creatinine), Factor 5 (taurine), and Factor 6 (threonine).
The associations between these six factors and clinical outcomes were therefore analysed using univariate and multivariate logistic regression models. After adjusting for clinical covariates (including age, BMI, interval between symptom onset and reperfusion, Gensini score, anterior MI, history of hypertension, diabetes mellitus and smoking), Factors 1, 2, and 3 were found to be associated with an increased risk of the composite primary outcome of cardiovascular death or AHF (Factor 1: odds ratio [OR] = 1.73, 95% confidence interval [CI] = 1.12–2.66, p = 0.013; Factor 2: OR = 3.36, 95% CI = 1.98–5.69, P < 0.001; Factor 3: OR = 2.35, 95% CI = 1.52–3.61, p = 0.001) (Table 2). With regard to each of the primary outcomes separately, Factor 2 was found to be associated with greater risks of AHF (OR = 2.07, 95% CI = 1.34–3.19, p = 0.001) and of cardiovascular death (OR = 2.22, 95% CI = 1.23–4.00, p = 0.008). Notably, neither Factor 1 nor Factor 3 was associated with risk of cardiovascular death and Factor 1 was also not associated with risk of AHF.
Random survival forest (RSF) analysis identified the same six metabolomics factors, Factor 2 having the highest value for distinguishing between patients with and without AEs (Fig. 1).
Patients in the highest tertile for Factor 2 were at a 9.45-fold greater risk of developing a primary outcome than those in the lowest tertile (adjusted OR: 9.45; 95% CI = 3.18–28.09, p = 0.001) (Supplementary Fig. 1).
These results suggest that Factor 2 is significantly associated with cardiovascular death and AHF in post-PCI patients with STEMI.
Associations of traditional biomarkers and PCA factor 2 with in-hospital clinical outcomes
TnI15 and NT-pro BNP16 are well-established biomarkers for diagnosing MI and predicting primary outcomes. Univariate and multivariate logistic regression models (adjusting for age, BMI, interval between symptom onset and reperfusion time, Gensini score, anterior MI, history of hypertension, history of diabetes, and history of current smoking) identified both NT-pro BNP and Factor 2 as predictors of primary outcomes (adjusted ORs 2.14, 95% CI = 1.652–3.00, P < 0.001 and 3.36, 95% CI = 1.98–5.69, p < 0.001, respectively). However, TnI was not identified as a predictor in the current study (OR = 1.29, 95% CI = 0.89–1.29, p = 0.089) (Supplementary Table 3).
Abilities of NT-pro BNP and Factor 2 to predict in-hospital clinical outcomes
The abilities of NT-pro BNP and Factor 2 to discriminate between patients with and without AEs was determined by calculating the area under (AUC) the receiver-operating characteristic (ROC) curves. According to this analysis, Factor 2 had a slightly higher predictive value than NT-pro BNP (AUC: 0.69 vs. 0.74 for the composite primary outcome) (Supplementary Fig. 2), whereas the combination of NT-pro BNP plus Factor 2 yielded an higher AUC value (AUC 0.82, p < 0.001) for primary outcome (Supplementary Fig. 2).
Extent of benefit of adding Factor 2 and NT-pro BNP to the GRACE prediction model
GRACE risk score is a recommended in-hospital risk assessment tool17. Whether addition of Factor 2 improved risk prediction compared with that provided by GRACE score alone was therefore investigated. Addition of Factor 2 to the GRACE score significantly improved prediction of cardiovascular AEs, increasing the C statistic from 0.702 to 0.814 (p < 0.001); the net reclassification index (NRI) was 0.729 (95% CI, 0.466–0.992, p < 0.001) and the integrated discrimination improvement (IDI) 0.186 (95% CI, 0.131–0.240, p < 0.001). Adding NT-pro BNP only moderately improved prediction by GRACE score, increasing the C statistic to 0.760 (p = 0.069). Importantly, adding a combination of Factor 2 and NT-pro BNP to the GRACE score further improved risk prediction, increasing the C statistic to 0.869 (p < 0.001). The NRI (NRI: 1.000, 95% CI, 0.756–1.244, p < 0.001) and IDI (IDI: 0.306, 95% CI, 0.241–0.371, p < 0.001) were also significantly higher (Table 3).
Discussion
We examined associations between plasma metabolomics profiles on admission in patients with STEMI and occurrence of in-hospital cardiovascular AEs post-PCI. Metabolite Factor 2, which comprises the BCAAs leucine, isoleucine, and valine, was identified by PCA as independently associated with both cardiovascular death and AHF during hospitalization. Addition of Factor 2 and NT-pro BNP to the GRACE score remarkably improved the predictive value compared with that provided by GRACE score alone.
The association between high admission concentrations of BCAAs and cardiovascular AEs after STEMI may be linked to previously identified correlations between abnormal BCAA catabolism and MI and ischemia–reperfusion injury13. BCAAs, including leucine, isoleucine, and valine, are essential amino acids and are acquired from the diet18. Although increased dietary protein intake can contribute to high circulating concentrations of BCAAs19, recent studies have demonstrated that high plasma BCAA concentrations in individuals with cardiovascular disease are caused by obstructed BCAA catabolism in the myocardium, which results in a build-up of myocardial BCAA that spills over into the circulation20,21,22. The key limiting enzyme of the BCAA catabolic process, branched-chain α-keto acid dehydrogenase (BCKDH), is activated by dephosphorylation with mitochondrial targeted 2C-type serine/threonine protein phosphatase (PP2Cm)23. Transcriptomic and metabolomics studies have revealed that expression of PPM1K (the gene encoding PP2Cm) is suppressed in cardiomyopathy and that proteins involved in the rate-limiting step of BCAA degradation, including BCKDH E1 α/β and E2 subunits, are downregulated during pathological stress, leading to marked accumulation of branched-chain alpha-keto acids (BCKAs) in stressed heart tissue24. Abundant BCAAs directly inhibit mitochondrial respiratory function, leading to superoxide accumulating in the mitochondria of cardiomyocytes24. Furthermore, plasma concentrations of BCAAs and BCKAs are significantly increased in PPM1K gene knockout mice, whereas in PPM1K-null mice cardiac function is compromised at a young age and deteriorates more quickly with mechanical overload25. Accumulation of BCAAs has been shown to suppress glucose metabolism and sensitize the heart to ischemic injury26. Oral BCAA administration activates mammalian target of rapamycin (mTOR) signalling and exacerbates cardiac dysfunction and remodelling in mice with surgically induced MI26. These lines of evidence support the hypothesis that abnormal BCAA catabolism in the ischemic or otherwise pathologically stressed myocardium may cause marked accumulation of BCAAs and BCKAs, which may in turn promote mitochondrial dysfunction, superoxide accumulation, and cardiomyocyte death9, resulting in heart failure or death post-MI.
Several other patient-based studies have shown that disturbances of BCAA metabolism are associated with multiple cardiovascular diseases, including coronary artery disease, MI, and HF. Yang et al.27 have reported that serum concentrations of BCAAs are remarkably higher in patients with coronary artery disease than in healthy individuals, and are independent of diabetes, hypertension, dyslipidaemia, and BMI. Fan et al.28 demonstrated that plasma leucine concentrations are up-regulated in patients with acute MI compared with patients with unstable angina. A case-control study22 showed that plasma leucine and isoleucine concentrations are higher in patients with chronic heart failure than in healthy controls. Several recent studies have investigated the correlation between metabolic disorders of BCAAs and prognosis in patients with cardiovascular disease. Ruiz-Canela et al.29 reported that high plasma baseline BCAA concentrations are associated with higher cardiovascular disease risk during 1-year follow-up. Furthermore, another recent study8 demonstrated that the prognostic value of several metabolites, involving BCAAs, exceeded that of B-type natriuretic peptide in terms of AEs in patients with chronic heart failure.
In the current study, we found that high plasma BCAA concentrations on admission are associated with high risk of in-hospital cardiovascular AEs in patients treated with primary PCI for STEMI. These results suggest that increasing BCAA catabolism to lower plasma concentrations of BCAAs may improve the prognosis of patients with STEMI after PCI. Wang et al.30 reported finding remarkable impairment of BCAA catabolism in the myocardium in response to infarction in a murine MI model; oral administration of BCAA further increased BCAA concentrations, activated mTOR signalling, and exacerbated cardiac dysfunction and remodelling. Importantly, these researchers showed that inhibition of mTOR by rapamycin or pharmacological inhibition of branched chain keto acid dehydrogenase kinase, a down-regulator of myocardial BCAA catabolism, significantly improves cardiac BCAA catabolism, reduces amounts of myocardial BCAA, and ameliorates post-MI cardiac dysfunction and remodelling. Additional data, including data from experimental studies in larger animals, are needed to clarify whether BCAAs are a potential therapeutic target.
The present study had several limitations. First, this was a small single centre study; our results should therefore be validated in larger cohorts. However, in light of the small samples sizes, we used RSF analysis to validate and increase confidence in our results31. Second, the study was also limited by the fact that blood samples were taken in a non-fasting state. Third, our findings are based on blood metabolite profiles, which are not necessarily representative of myocardial metabolism.
In conclusion, we found that high plasma BCAA concentrations on admission are associated with in-hospital cardiovascular AEs in post-PCI patients with STEMI and that addition of plasma BCAA concentrations and NT-pro BNP improves the predictive ability of GRACE risk scores. Of note, BCAAs have been shown to be involved in the pathogenesis of ischemia–reperfusion injury and adverse cardiac remodelling in experimental studies, supporting the hypothesis that disordered BCAA metabolism may represent a novel insight into the pathological mechanisms underlying prognosis after MI. Further studies of BCAA metabolism aimed at improving the prognosis of patients with myocardial infarction and ischemic–reperfusion injury are warranted.
Materials and Methods
Study cohort
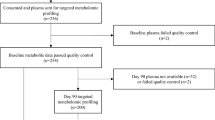
This is a case-control study of patients with STEMI undergoing primary PCI at the First Hospital of Ji Lin University from January 2014 to January 2017. Details of the study design have been published previously32. Briefly, patients >18 years of age referred for primary PCI were enrolled if they met the following criteria: (i) first STEMI; and (ii) onset of chest pain <12 h before presentation. STEMI was defined as continuous chest pain for at least 20 minutes, and new ST-segment elevation ≥0.1 mV in at least two contiguous leads, or new-onset left bundle branch block. Patients with Killip class > I on admission, heart failure, serum creatinine >250 µmol/L, alanine aminotransferase >135 U/L, severe fatty liver or liver cirrhosis, or malignant tumours were excluded, as were patients who had taken amino acid supplements in the preceding 3 months. Aspirin and clopidogrel (both 300 mg) or ticagrelor (180 mg) were administered before catheterization. The postoperative therapeutic strategy was performed in accordance with standard guidelines1. The Medical Ethics Committee of the First Hospital, Jilin University approved the study protocol and the study participants provided written informed consent. The primary clinical outcome was the composite variable of cardiovascular death and AHF after PCI during hospitalization. Secondary clinical outcomes included the individual components of the primary outcome separately were also investigated. Cardiovascular death included death from pump failure, arrhythmia, or mechanical complications including ventricular septal rupture and free wall rupture. AHF was defined as presence of dyspnoea, peripheral oedema and pulmonary rales on physical examination, radiological evidence of pulmonary congestion, need for administration of intravenous diuretic agents, or continuous positive airway pressure. A total of 96 patients with STEMI treated with primary PCI who developed in-hospital cardiovascular death or AHF comprised the AE group. We analysed selected metabolites in these 96 AE patients and 96 No-AE patients matched (1:1 ratio) for age group (±3 years) and sex (Supplementary Fig. 3).
Blood sampling and examination
Blood was drawn from the arterial sheath before coronary angiography and centrifuged at 3000 × g for 10 minutes at room temperature to obtain plasma and then frozen and stored at −80 °C. TnI and NT-pro BNP were measured as described previously32.
Metabolomics
Amino acid profiling by liquid chromatography–tandem mass spectrometry (LC–MS) was conducted using an Ultimate 3000 UHPLC system coupled to a mass spectrometry system (Q-Enactive MS; Thermo Scientific, Logan, UT, USA),which provided quantitative assessment of concentrations of 26 amino acids. Details of methods can be found in the Data Supplement.
Traditional biomarker assays
Blood samples were collected from patients with STEMI on admission.
Calculation of GRACE scores
The GRACE score is widely used to evaluate the risk of death or recurrent MI33. In the current study, GRACE risk scores were calculated using age, heart rate, systolic blood pressure, creatinine, Killip class, presence or absence of cardiac arrest on admission, presence or absence of ST-segment elevation, and cardiac enzyme concentrations on admission.
Statistical methods
Baseline characteristics were compared between the No-AE and AE groups using Mann–Whitney U-tests for continuous variables and χ2 tests for categorical variables.
PCA was used to reduce the large number of correlated metabolites to a smaller number of uncorrelated factors. Varimax rotation34 was used to identify significant factors: only factors with an eigenvalue of ≥1.0 were considered. Individual metabolites with an absolute value for factor load of ≥0.7 are reported as components of a given factor (weighted sum of the standardized metabolites within that factor, weighted on the factor loading for each metabolite). A large cut-off was chosen to avoid false positives. The relationships between PCA-derived factors and clinical outcomes were evaluated using univariate and multivariate logistic regression models, adjusting for traditional STEMI risk factors (age, BMI, symptom onset to reperfusion time, Gensini score, anterior MI, history of hypertension, diabetes, and smoking). ORs and 95% CIs are given.
The PCA results were validated by RSF analysis35, this being a powerful machine-learning statistical algorithm for validating results of studies with small sample sizes. PCA factors were used as covariates and events as the outcome. The results were validated by ranking the PCA factors on the basis of importance of the variable as determined by RSF.
Model performance was assessed by calculating the C statistic, NRI, and IDI. The 95% CIs were calculated for each variable. ROC curves were generated and the AUCs calculated to compare the accuracies of traditional biomarkers and the PCA factors. Statistical analyses were performed using commercial software (SPSS software version 25.0, Chicago, IL USA) and R version 3.3.3 (R Foundation for Statistical Computing, Vienna, Austria). A two-tailed P value of <0.05 was considered to denote statistical significance.
References
Ibanez, B. et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 39, 119–177, https://doi.org/10.1093/eurheartj/ehx393 (2018).
Hausenloy, D. J. & Yellon, D. M. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest 123, 92–100, https://doi.org/10.1172/JCI62874 (2013).
Pedersen, F. et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J Am Coll Cardiol 64, 2101–2108, https://doi.org/10.1016/j.jacc.2014.08.037 (2014).
Desta, L. et al. Incidence, temporal trends, and prognostic impact of heart failure complicating acute myocardial infarction. The SWEDEHEART Registry (Swedish Web-System for Enhancement and Development of Evidence-Based Care in Heart Disease Evaluated According to Recommended Therapies): a study of 199,851 patients admitted with index acute myocardial infarctions, 1996 to 2008. JACC. Heart failure 3, 234–242, https://doi.org/10.1016/j.jchf.2014.10.007 (2015).
Zhou, X. et al. Prognostic Value of Plasma Soluble Corin in Patients With Acute Myocardial Infarction. J Am Coll Cardiol 67, 2008–2014, https://doi.org/10.1016/j.jacc.2016.02.035 (2016).
Minicucci, M. F., Azevedo, P. S., Polegato, B. F., Paiva, S. A. & Zornoff, L. A. Heart failure after myocardial infarction: clinical implications and treatment. Clinical cardiology 34, 410–414, https://doi.org/10.1002/clc.20922 (2011).
Huang, Y., Zhou, M., Sun, H. & Wang, Y. Branched-chain amino acid metabolism in heart disease: an epiphenomenon or a real culprit? Cardiovascular research 90, 220–223, https://doi.org/10.1093/cvr/cvr070 (2011).
Cheng, M. L. et al. Metabolic disturbances identified in plasma are associated with outcomes in patients with heart failure: diagnostic and prognostic value of metabolomics. J Am Coll Cardiol 65, 1509–1520, https://doi.org/10.1016/j.jacc.2015.02.018 (2015).
Sun, H. & Wang, Y. Branched chain amino acid metabolic reprogramming in heart failure. Biochim Biophys Acta 1862, 2270–2275, https://doi.org/10.1016/j.bbadis.2016.09.009 (2016).
Backstrom, T., Goiny, M., Lockowandt, U., Liska, J. & Franco-Cereceda, A. Cardiac outflow of amino acids and purines during myocardial ischemia and reperfusion. J Appl Physiol (1985) 94, 1122–1128, https://doi.org/10.1152/japplphysiol.00138.2002 (2003).
Song, D., O’Regan, M. H. & Phillis, J. W. Mechanisms of amino acid release from the isolated anoxic/reperfused rat heart. European journal of pharmacology 351, 313–322 (1998).
Akdemir, O. et al. Effects of taurine on reperfusion injury. Journal of plastic, reconstructive & aesthetic surgery: JPRAS 64, 921–928, https://doi.org/10.1016/j.bjps.2010.12.007 (2011).
Li, T. et al. Defective Branched-Chain Amino Acid Catabolism Disrupts Glucose Metabolism and Sensitizes the Heart to Ischemia-Reperfusion Injury. Cell metabolism 25, 374–385, https://doi.org/10.1016/j.cmet.2016.11.005 (2017).
Avci, A. et al. Association between the Gensini Score and Carotid Artery Stenosis. Korean circulation journal 46, 639–645, https://doi.org/10.4070/kcj.2016.46.5.639 (2016).
Antman, E. M. et al. Cardiac-specific troponin I levels to predict the risk of mortality in patients with acute coronary syndromes. The New England journal of medicine 335, 1342–1349, https://doi.org/10.1056/nejm199610313351802 (1996).
Heeschen, C., Hamm, C. W., Mitrovic, V., Lantelme, N. H. & White, H. D. N-terminal pro-B-type natriuretic peptide levels for dynamic risk stratification of patients with acute coronary syndromes. Circulation 110, 3206–3212, https://doi.org/10.1161/01.cir.0000147611.92021.2b (2004).
Ibanez, B. et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J, https://doi.org/10.1093/eurheartj/ehx393 (2017).
Harper, A. E., Miller, R. H. & Block, K. P. Branched-chain amino acid metabolism. Annual review of nutrition 4, 409–454, https://doi.org/10.1146/annurev.nu.04.070184.002205 (1984).
Layman, D. K. The role of leucine in weight loss diets and glucose homeostasis. The Journal of nutrition 133, 261s–267s, https://doi.org/10.1093/jn/133.1.261S (2003).
McCormack, S. E. et al. Circulating branched-chain amino acid concentrations are associated with obesity and future insulin resistance in children and adolescents. Pediatric obesity 8, 52–61, https://doi.org/10.1111/j.2047-6310.2012.00087.x (2013).
Wang, T. J. et al. Metabolite profiles and the risk of developing diabetes. Nature medicine 17, 448–453, https://doi.org/10.1038/nm.2307 (2011).
Wang, J. et al. Metabolomic identification of diagnostic plasma biomarkers in humans with chronic heart failure. Molecular bioSystems 9, 2618–2626, https://doi.org/10.1039/c3mb70227h (2013).
Lu, G. et al. Protein phosphatase 2Cm is a critical regulator of branched-chain amino acid catabolism in mice and cultured cells. The Journal of clinical investigation 119, 1678–1687, https://doi.org/10.1172/jci38151 (2009).
Sun, H. et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 133, 2038–2049, https://doi.org/10.1161/circulationaha.115.020226 (2016).
Sun, H., Lu, G., Ren, S., Chen, J. & Wang, Y. Catabolism of branched-chain amino acids in heart failure: insights from genetic models. Pediatric cardiology 32, 305–310, https://doi.org/10.1007/s00246-010-9856-9 (2011).
Cediel, G. et al. Prognostic Value of New-Generation Troponins in ST-Segment-Elevation Myocardial Infarction in the Modern Era: The RUTI-STEMI Study. Journal of the American Heart Association 6, https://doi.org/10.1161/jaha.117.007252 (2017).
Yang, R. Y. et al. Association of branched-chain amino acids with coronary artery disease: A matched-pair case-control study. Nutrition, metabolism, and cardiovascular diseases: NMCD 25, 937–942, https://doi.org/10.1016/j.numecd.2015.06.003 (2015).
Fan, Y. et al. Comprehensive Metabolomic Characterization of Coronary Artery Diseases. J Am Coll Cardiol 68, 1281–1293, https://doi.org/10.1016/j.jacc.2016.06.044 (2016).
Ruiz-Canela, M. et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clinical chemistry 62, 582–592, https://doi.org/10.1373/clinchem.2015.251710 (2016).
Wang, W. et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. American journal of physiology. Heart and circulatory physiology 311, H1160–h1169, https://doi.org/10.1152/ajpheart.00114.2016 (2016).
Kacar, K. et al. Overcoming the clinical-MR imaging paradox of multiple sclerosis: MR imaging data assessed with a random forest approach. AJNR. American journal of neuroradiology 32, 2098–2102, https://doi.org/10.3174/ajnr.A2864 (2011).
Du, X. et al. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life sciences 209, 167–172, https://doi.org/10.1016/j.lfs.2018.08.011 (2018).
Tang, E. W., Wong, C. K. & Herbison, P. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. American heart journal 153, 29–35, https://doi.org/10.1016/j.ahj.2006.10.004 (2007).
Kaiser, H. F. The varimax criterion for analytic rotation in factor analysis. Psychometrika 23, 187–200, https://doi.org/10.1007/bf02289233 (1958).
Ishwaran, H. et al. Random survival forests for competing risks. Biostatistics (Oxford, England) 15, 757–773, https://doi.org/10.1093/biostatistics/kxu010 (2014).
Acknowledgements
The authors thank Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac) for editing the English in a draft of this manuscript. The present study was supported by grants from the Beijing Municipal Science and Technology Commission (Z171100000417002) and the Ministry of Science and Technology of China (2016YFC0900903).
Author information
Authors and Affiliations
Contributions
X.D. collected the samples, analysed the data, and wrote the manuscript. H.Y. analysed the data. Y.L. designed the experiments and revised the paper. Y.W. detected the amino acid metabolites by mass spectrometry. P.H. and W.Z. contributed to sample collection. S.Z. contributed to data acquisition. B.Q. and J.L. helped to analyse the data. Y.Z. contributed to the design of the clinical study cohort. J.D. approved the final version and acquired the funding.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, X., You, H., Li, Y. et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Sci Rep 8, 15809 (2018). https://doi.org/10.1038/s41598-018-34245-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-34245-6
Keywords
This article is cited by
-
A terminal metabolite of niacin promotes vascular inflammation and contributes to cardiovascular disease risk
Nature Medicine (2024)
-
The Role of Branched-chain Amino Acids and Their Metabolism in Cardiovascular Diseases
Journal of Cardiovascular Translational Research (2024)
-
Branched-chain amino acids in cardiovascular disease
Nature Reviews Cardiology (2023)
-
Serum branched amino acids and the risk of all-cause mortality: a meta-analysis and systematic review
Amino Acids (2023)
-
Excessive branched-chain amino acid accumulation restricts mesenchymal stem cell-based therapy efficacy in myocardial infarction
Signal Transduction and Targeted Therapy (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.