Abstract
Human brucellosis, a chronic disease contracted through contact with animals and consuption of unpasteurized dairy products is underreported in limited-resource countries. This cross-sectional study aimed to determine the prevalence and risk factors of brucellosis among febrile patients attending a community hospital in South western Uganda. A questionnaire that captured socio-demographic, occupational and clinical data was administered. Blood samples were tested for Brucella antibodies using Rose Bengal Plate Test (RBPT) and blood culture with standard aerobic BACTEC bottle was done. Of 235 patients enrolled, prevalence of brucellosis (RBPT or culture confirmed) was 14.9% (95% CI 10.6–20.1) with a culture confrmation in 4.3% of the participants. The factors independently associated with brucellosis were consumption of raw milk (aOR 406.15, 95% CI 47.67–3461.69); history of brucellosis in the family (aOR 9.19, 95% CI 1.98–42.54); and selling hides and skins (aOR 162.56, 95% CI 2.86–9256.31). Hepatomegaly (p < 0.001), splenomegaly (p = 0.018) and low body mass index (p = 0.032) were more common in patients with brucellosis compared to others. Our findings reveal a high prevalence of brucellosis among febrile patients and highlight a need for implementing appropiate tests, public awareness activities and vaccination of animals to control and eliminate the disease.
Similar content being viewed by others
Introduction
Brucellosis is a widespread zoonosis caused by Brucella species that can induce considerable human suffering and huge economic losses in livestock1,2,3. The disease is transmitted to humans by contact with fluids from infected animals by several routes such as direct inoculation (cuts and skin abrasions from handling animal carcasses and placent), inhalation of infectious aerosols and ingestion of contaminated milk and meat products4,5,6. The control of brucellosis involves, among other things, implementation of epidemiological surveillance for early detection of cases7. Lack of sufficient knowledge about the disease among the physicians, low index of suspicion, under-diagnosis or misdiagnosis have been attributed to wide spread of the disease8.
Human brucellosis typically, begins as an acute febrile illness with non-specific flu-like signs that can mimic a variety of acute febrile illnesses9. Therefore, laboratory tests are essential for diagnosis but present a particular challenge in settings with limited laboratory capacity and where better-known causes of fever, such as malaria or typhoid, co-occur10,11,12,13,14. Thus, it is generally considered that brucellosis is under-diagnosed in many of the areas in which it is endemic1. In resource limited and malaria endemic settings, such as Uganda, after exclusion of malaria by rapid tests, fever is often managed based solely on clinical symptoms15 using non specific antibiotic regimens that often do not cover Brucella species. If left untreated, the disease can progress to osteoarticular, cutaneous, genitourinary, nervous and other complications16,17.
Although data are scarce, the existing evidence strongly suggests that brucellosis might be a widespread problem in Africa12,18,19. The annual human incidence rate in Uganda was estimated to be 5.8 per 10,000 people20. The objective of this study was to determine prevalence and risk factors of brucellosis among febrile patients attending Rushere community Hospital, a cattle keeping area of western Uganda and to compare clinical characteristics between brucellosis and non-brucellosis patients.
Materials and Methods
Study setting and population
The cross-sectional study was carried out at Rushere community hospital, Kiruhura district, Western Uganda. Kiruhura District is a farming district where livestock forms the backbone of economic activity. Cow milk and meat are important products produced in the district. The western region is the leading producer of milk in the country per farm, accounting for 33.7% of the total national daily milk production that is estimated at 6.8 million litres21,22,23. Kiruhura district produces more than 100,000 liters of milk daily22.
The study participants were patients aged 5 years and above with clinical suspicion of brucellosis defined by a reported or recorded history of fever for a minimum of 7 days and at least one or more of the following criteria: night sweats, headache, weight loss, fatigue, myalgia or arthralgia, anorexia24. Participants with other confirmed diagnosis such as smear positive tuberculosis and those who did not consent were excluded from the study. A semi-structured, standardized questionnaire was used to capture socio-demographic characteristics, information on animal exposure and clinical signs.
Laboratory procedures
The blood tests performed on the sample were Rose Bengal plate test (RBPT), bacterial blood culture, malaria smear microscopy and HIV serology. Additionally, sputum smear microscopy was done in participants with clinical suspicion of tuberculosis. Tests for malaria, HIV and RBPT were done at Rushere community hospital laboratory while tests for bacterial blood cultures were done at Epicentre research laboratory in Mbarara. A minimum of 10 mL of blood sample was collected from each participant using a vacutainer needle: 5 to 7 ml were first collected into blood culture bottle followed by 4 ml in a plain tube for HIV serology and Rose bengal plate test (RBPT). For malaria microscopy, we collected capillary blood from the side of the finger for each participant.
Blood cultures were processed with BACTEC 9240 (Becton Dickinson, Franklin Lakes, USA) in a Biosafety Level 3 (BSL3) laboratory that is maintained at negative pressure. All sample manipulations were performed in a regularly calibrated and certified biosafety cabinet. Between 5 to 7 ml of blood from patients were inoculated in one standard aerobic BACTEC bottle and incubated for seven days and subcultured on Brucella base blood agar whenever positive signal occurred by instrument. Suspected colonies were identified by colonial morphology, Gram-staining, standard biochemical procedures (oxidase, catalase, production of H2S and urease) and agglutination test using specific antisera. At the end of the first week, bottles not detected positive by the instrument were kept for additional three weeks and subcultures were performed weekly. Cultures were considered negative for Brucella in the face of no growth after four weeks of incubation.
Microscopy for malaria parasites was done using Field’s staining technique25 for thick blood smears for all participants. HIV testing was done using two rapid tests (Alere Determine™ HIV-1/2, Abbott, Illinois, USA and HIV 1/2 STAT-PAK® Assay,Chembio Diagnostic system, New York, USA) and a third one (Uni-Gold HIV, Trinity Biotech, Co Wicklow, Ireland) in case the first two tests were discordant following the standard national algorithm26.
For RBPT, we used two protocols: the standard one and the modified RBPT for testing serum dilutions27,28. For the former, 30 µL of plain serum was dispensed on a white glossy ceramic tile and mixed with an equal volume of RBPT antigen (Veterinary Laboratory Agency; England, United Kingdom) using a toothpick. The antigen was previously equilibrated at room temperature and shaken to re-suspend any bacterial sediment. The tile was then rocked at room temperature for 8 minutes (instead of the 4 minutes recommended for animal brucellosis)29 and any visible agglutination and/or the appearance of a typical rim was taken as a positive result. For the modified RBPT, positive sera were tested further as follows. Eight 30 µL drops of saline were dispensed on the tile and the first one mixed with an equal volume of the positive plain serum (1/2 serum dilution). Then, 30 µL of this first dilution was transferred to the second drop with the help of a micropipette and mixed to obtain the 1/4 dilution. From this, the 1/8 to 1/128 dilutions were obtained by successive transfers and mixings taking care of rinsing the pipette tip between transfers. Finally, each drop was tested with an equal volume (30 µL) of the RBPT reagent, so that the final dilutions range from 1/2 to 1/256.
Patients diagnosed with brucellosis based on RBPT or blood culture were treated with intravenous gentamycin for two weeks and oral doxycycline for six weeks free of charge. Children below 8 years received cotrimoxazole instead of doxycycline as per Uganda clinical guidelines30.
Sample size and statistical analysis
The sample size of 236 participants was calculated using an estimated prevalence of brucellosis of 15%31 with a 5% precision at a 95% confidence interval after a 20% inflation for non-response rate using Epi Info(version 7.1.4.0, CDC, Atlanta US).
Data was entered in EpiData3.1software (EpiData, Odense, Denmark), then exported to STATA version 13 (StataCorp, College Station, Texas, USA) for analysis.
Descriptive analysis of independent variables namely (participant’s age, gender, education level, marital status, religion, income status, occupation, family history of brucellosis, consumption of raw milk and other products) was done. Prevalence of brucellosis was the ratio of the number of patients with fitting definition of probable or confirmed brucellosis by the total number of included patients tested for brucellosis. A probable case was a clinically suspected case with a positive RBPT not confirmed by culture and a confirmed case was as a clinically suspected case confirmed by culture and identification of Brucella spp.
Chi-square was used to compare categorical variables while continuous variables were compared using student t-test. Wilcoxon rank sum was used to compare nonparametric continuous data. Bivariate analysis was done to evaluate associations between patients’ characteristics and diagnosis of brucellosis (confirmed or probable brucellosis). Covariates associated with p value ≤ 0.2) in the bivariate analysis were entered into multivariate logistic regression model through backward stepwise elimination method to obtain the final predictive model of covariates that were independently associated (p < 0.05) with brucellosis.
Ethical approval to conduct the study was obtained from Mbarara University of Science and Technology Research and Ethics Committee (MUST-REC) and the Faculty Research Committee (FRC). The study was given study number 02/03-17 by the REC. We respected the guidelines of Helsinki and CIOMS-2002 (Council for International Organizations of Medical Sciences) regarding research with humans, avoiding any type of physical or moral damage. Written informed consent was obtained by research assistants from all adult participants (18 years and above) and in case of minors (below 18 years) from their parents or guradians. Assent was obtained from children who were older than 7 years.
The datasets generated and analysed during the study are available from the corresponding author on request.
Results
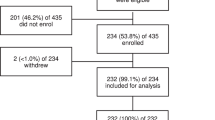
Over a two consecutive months period, out of 480 patients attending the hospital with fever, 239 were recruited and four were secondarily excluded because they subsequently refused blood drawing (Fig. 1). We thus present results from 235 participants enrolled into the study between May and August 2017.
Patients’ baseline and clinical characteristics
Characteristics of those with and without probable brucellosis are presented in Table 1. The median age of the study participants was 30 years (IQR 22, 45). Participants with probable brucellosis were significantly younger than those without (p = 0.003). Majority of the participants were cattle keepers (56.2%), had acquired formal education (71.5%) and were male (54.0%). The proportion of males with probable brucellosis was 11.5%. The median duration of fever was 9 days (IQR 8,14 days). Fourty-six (19.6%) participants reported history of consumption of raw milk, 3 (1.3%) had history of consumption of raw blood from animals, 54 (23.0%) reported positive history of brucellosis in the family.
The most common symptoms overall were headache, joint or back pains and chills. Hepatomegaly (p < 0.001), splenomegaly (p = 0.018) and low body mass index (<18.5 Kg/m2) (p = 0.032) were significantly more common among patients with probable brucellosis compared to the others. Other clinical signs and symptoms were similar among patients with and without probable brucellosis (Table 2).
Prevalence of brucellosis and other co-infections
Thirty five out of the 235 participants had a positive RBPT result and 10 had a blood culture positive for Brucella spp giving a prevalence of probable and confirmed brucellosis of 14.9% (95% CI 10.6–20.1) and 4.3% (2.1–7.7), respectively. All confirmed cases were also positive with RBPT and 10/35 (28.6%) of cases with positive RBPT were culture positive (Table 3). None of the cases (21/35) with a RBPT titre of <1:8 had positive results on culture.
Malaria was diagnosed in 37/235 (15.7%) of the participants. Three out of 35 (8.6%) patients with probable brucellosis also had malaria. HIV prevalence among the participants was 19/235 (8.1%) and 5.7% (2/35) among brucellosis probable or confirmed cases. Salmonella Typhi was isolated in one participant. Cryptococcus neoformans was also isolated in one participant who was HIV positive.
Risk factors for probable brucellosis
Consumption of raw milk (adjusted OR – aOR 406.15; 95% CI 47.67–3461.69), history of family member with brucellosis (aOR 9.19; 95% CI 1.98–42.54) and selling of skins and hides (aOR 162.56; 95% CI 2.86–9256.31) were identified as independent risk factors for probable brucellosis. There was no association with the HIV status (Table 4).
Discussion
Our results show a high prevalence of probable brucellosis among patients consulting for fever in this hospital based survey (14.9%). This is close to the prevalence of 13.3% among febrile patients in Kampala31 and is consistent with the high prevalence reported in general population in rural area in Uganda (11.7% and 13.4%)32,33 or among butchers in Mbarara (7%) and Kampala (12%) districts34.
Outside Uganda, a similar hospital based study in a predominantly pastoral community in nearby Kenya indicated a comparable high sero-prevalence (13.7%)35 among febrile patients, and 32.5% in Nigeria36, highlighting brucellosis as an important cause of fever. The prevalence of probable cases in our study is however higher than that reported among febrile patients in other countries: 7% in Egypt (p < 0.001)37; 7.7% in Mali (p = 0.03)38. This is probably because of the high prevalence of brucellosis in livestock in Western Uganda (55.6%)39. Indeed, a strong association has been reported between prevalence of the disease in animals and in humans40. This variation from our findings can also perhaps be attributed to variations in different serodiagnostic approaches to the disease. In this particular study, we used the RBPT whose sensitivity and specificity vary from 87% to >99%27,28,41,42.The fact that vaccination of animals against brucellosis in Uganda using Brucella abortus S19 vaccine is a voluntary exercise43 and is not routinely done, most likely because of economic and logistic reasons18,may further explain the high prevalence of the disease.
The prevalence of culture confirmed brucellosis reported in our study of 4.3% is similar to that reported among hospitalized febrile patients in Northern Tanzania of 3.5%14. In our study, the culture positivity was 28.6% among the brucellosis probable cases. This is comparable to the one reported in the study in Jordan of 23.4%44 but lower than the culture positivity of 74.1% reported in Kuwait45. The lower culture positivity in our study might be attributed to high proportion of study participants who received antibiotics prior to hospital visit (63.4%). All patients with positive cultures in our study had positive serology results with titres ≥1:8 on RBPT, contrary to other studies that have reported positive cultures with negative serological test results46. Although the study was not powered to look at the association between the titer level of the serology and the culture results, this supports the proposed cut-off of 1:8 by Diaz et al.28,47.
Our findings also show that consumption of raw cow milk, history of brucellosis in a family member and selling cattle’s hides and skins are independent risk factors of brucellosis. This is in agreement with findings from other studies7,48,49,50. Therefore, in high brucellosis burden countries, individuals selling hides and skins are at risk of occupational exposure7. Consumption of raw milk is also associated with higher rates of recurrent disease7,51. Consistent with our findings, the existence of another infected family member is a well known major risk factor for brucellosis7,48. This is because families are likely to share a common infected food source7.
Most clinical signs and symptoms were similar among patients with brucellosis and those without brucellosis except hepatomegaly and splenomegaly that were more common in probable or confirmed brucellosis cases(p < 0.05), in agreement with findings from other studies35,46,52,53,54. This is due to persistent bacterial colonization of reticuloendothelial system54,55. As previously reported, we did not find an association between HIV status and the risk of brucellosis56,57.
This study had some limitations: first, it was done in one hospital in one district and so the findings may not be generalizable to the entire region; second, the study was based on relatively small numbers which impacted on some results of the multivariate analysis that had very wide confidence intervals; third, the study may also be prone to recall bias since some of the data came from self-reporting and participants were asked for information about exposure to animal products that could have taken place longer period preceding the interview date (more than one month).
Conclusions
Brucellosis is frequent among patients with prolonged fever attending Rushere Community Hospital mostly due to high exposure within this community from cultural practice regarding consumption of raw milk in the Western region of Uganda. Based on our findings, we recommend that patients with fever for at least one week, living at high risk of exposure should be routinely screened for brucellosis for early diagnosis and prompt treatment. This may require the development of rapid, affordable and easy to use point of care tests in areas where people are more exposed. Our findings also highlight the need for vaccination of animals, carrying out public education activities to sensitize the community on boiling and/or pasteurization of milk before consumption, and use of personal protective gear while handling animal as well as screening of family members of brucellosis cases for early disease detection in order to control and eliminate the disease.
References
Pappas, G., Papadimitriou, P., Akritidis, N., Christou, L. & Tsianos, E. V. The new global map of human brucellosis. The Lancet infectious diseases 6, 91–99 (2006).
Roth, F. et al. Human health benefits from livestock vaccination for brucellosis: case study. Bulletin of the World health Organization 81, 867–876 (2003).
Young, E. J. Brucella spp. Principles and Practice of Clinical Bacteriology, 2nd ed. 265–272(John Wiley & Sons Ltd, West Sussex, England, 2006).
Young, E. J. An overview of human brucellosis. Clinical infectious diseases 21, 283–290 (1995).
John, K. et al. Quantifying risk factors for human brucellosis in rural northern Tanzania. PloS one 5, e9968 (2010).
Godfroid, J. et al. A “One Health” surveillance and control of brucellosis in developing countries: moving away from improvisation. Comparative immunology, microbiology and infectious diseases 36, 241–248 (2013).
Corbel, M. J. Brucellosis in humans and animals. (World Health Organization, 2006).
Thakur, S., Kumar, R. & Thapliyal, D. Human brucellosis: review of an under-diagnosed animal transmitted disease. The Journal of communicable diseases 34, 287–301 (2002).
Mantur, B., Amarnath, S. & Shinde, R. Review of clinical and laboratory features of human brucellosis. Indian journal of medical microbiology 25, 188 (2007).
Crump, J. A. et al. Estimating the incidence of typhoid fever and other febrile illnesses in developing countries. Emerging infectious diseases 9, 539 (2003).
Marcotty, T. et al. Zoonotic tuberculosis and brucellosis in Africa: neglected zoonoses or minor public-health issues? The outcomes of a multi-disciplinary workshop. Annals of Tropical Medicine & Parasitology 103, 401–411 (2009).
McDermott, J. J. & Arimi, S. Brucellosis in sub-Saharan Africa: epidemiology, control and impact. Veterinary microbiology 90, 111–134 (2002).
Kunda, J. et al. Health-seeking behaviour of human brucellosis cases in rural Tanzania. BMC Public Health 7, 315 (2007).
Bouley, A. J. et al. Brucellosis among hospitalized febrile patients in northern Tanzania. The American journal of tropical medicine and hygiene 87, 1105–1111 (2012).
Amexo, M., Tolhurst, R., Barnish, G. & Bates, I. Malaria misdiagnosis: effects on the poor and vulnerable. The Lancet 364, 1896–1898 (2004).
Gur, A. et al. Complications of brucellosis in different age groups: a study of 283 cases in southeastern Anatolia of Turkey. Yonsei Medical Journal 44, 33–44 (2003).
Franco, M. P., Mulder, M., Gilman, R. H. & Smits, H. L. Human brucellosis. The Lancet infectious diseases 7, 775–786 (2007).
Ducrotoy, M. et al. Brucellosis in Sub-Saharan Africa: Current challenges for management, diagnosis and control. Acta tropica 165, 179–193 (2017).
McDermott, J., Grace, D. & Zinsstag, J. Economics of brucellosis impact and control in low-income countries. Scientific and Technical Review of the Office International des Epizooties (Paris) 32, 249–261 (2013).
Makita, K., Waiswa, F. E., Eisler, C., Welburn, M. C. & How, S. C. human brucellosis incidence in urban Kampala can be reduced most efficiently? A stochastic risk assessment of informally-marketed milk. PLoS ONE. 12, e14188 (2010).
Balikowa, D. Dairy development in Uganda. A review of Uganda’s dairy industry. Dairy Dev. Authority Uganda 3202, 1–215 (2011).
Tijjani, I. & Yetişemiyen, A. A Dairy Cattle and Dairy Industry in Uganda: Trends and Challenges. Res. J. Agriculture and Forestry Sci 3, 14–18 (2015).
Wikipedia. Dairy industry in Uganda, https://en.wikipedia.org/wiki/Dairy_industry_in_Uganda (May 4, 2018).
Ministry of Heath. (ed. Ministry of Heath (Uganda)) 48 (2012).
Warhurst, D. & Williams, J. ACP Broadsheet no 148. July 1996. Laboratory diagnosis of malaria. Journal of clinical pathology 49, 533 (1996).
MoH, U. Uganda National Policy Guidelines for HIV counselling and testing. Kampala, Uganda (2003).
Mantur, B. G., Amarnath, S. K., Patil, G. A. & Desai, A. S. Clinical utility of a quantitative Rose Bengal slide agglutination test in the diagnosis of human brucellosis in an endemic region. Clinical laboratory 60, 533–541 (2014).
Díaz, R., Casanova, A., Ariza, J. & Moriyon, I. The Rose Bengal Test in human brucellosis: a neglected test for the diagnosis of a neglected disease. PLOS Neglected tropical diseases 5, e950 (2011).
Alton, G. G., Jones, L. M., Pietz, D. E. & Organization, W. H. Laboratory techniques in brucellosis. (1975).
Ministry of Heath. (ed. Uganda Ministry of Health) 136–138 Ministry of Health, Uganda, Kampala (2016).
Mutanda, L. Selected laboratory tests in febrile patients in Kampala, Uganda. East African medical journal 75, 68–72 (1998).
Miller, R. et al. The prevalence of brucellosis in cattle, goats and humans in rural Uganda: a comparative study. Transboundary and emerging diseases 63 (2016).
Ezama, A., Gonzalez, J.-P., Majalija, S. & Bajunirwe, F. Assessing short evolution brucellosis in a highly brucella endemic cattle keeping population of Western Uganda: a complementary use of Rose Bengal test and IgM rapid diagnostic test. BMC public health 18, 315 (2018).
Nabukenya, I., Kaddu-Mulindwa, D. & Nasinyama, G. W. Survey of Brucella infection and malaria among Abattoir workers in Kampala and Mbarara Districts, Uganda. BMC public health 13, 901 (2013).
Njeru, J. et al. Human brucellosis in febrile patients seeking treatment at remote hospitals, northeastern Kenya, 2014–2015. Emerging infectious diseases 22, 2160 (2016).
Kudi, A. et al. Human Brucellosis: Seroprevalence amongst Patients attending the General Out-patient Department (GOPD), Federal Teaching Hospital Gombe, Nigeria. Nigerian Biomedical Science Journal 11, 31 (2015).
Jennings, G. J. et al. Brucellosis as a cause of acute febrile illness in Egypt. Transactions of the Royal Society of Tropical Medicine and Hygiene 101, 707–713 (2007).
Steinmann, P. et al. Brucellosis seroprevalence and Risk factors for seroconversion among febrile attendants of Urban Health care facilities in Mali. Revue Africaine de Saute et de productions Animates 4, 3-4-117 (2006).
Bernard, F., Vincent, C., Matthieu, L., David, R. & James, D. Tuberculosis and brucellosis prevalence survey on dairy cattle in Mbarara milk basin (Uganda). Preventive Veterinary Medicine 67, 267–281 (2005).
Osoro, E. M. et al. Strong association between human and animal Brucella seropositivity in a linked study in Kenya, 2012–2013. The American journal of tropical medicine and hygiene 93, 224–231 (2015).
Ruiz-Mesa, J. et al. Rose Bengal test: diagnostic yield and use for the rapid diagnosis of human brucellosis in emergency departments in endemic areas. Clinical microbiology and infection 11, 221–225 (2005).
Christopher, S., Umapathy, B. & Ravikumar, K. Brucellosis: review on the recent trends in pathogenicity and laboratory diagnosis. Journal of laboratory physicians 2, 55 (2010).
Kansiime, C. et al. Community perceptions on integrating animal vaccination and health education by veterinary and public health workers in the prevention of brucellosis among pastoral communities of south western Uganda. PloS one 10, e0132206 (2015).
Issa, H. & Jamal, M. Brucellosis in children in south Jordan. (1999).
Dimitrov, T., Panigrahi, D., Emara, M., Awni, F. & Passadilla, R. Seroepidemiological and microbiological study of brucellosis in Kuwait. Medical Principles and Practice 13, 215–219 (2004).
Abdi-Liae, Z., Soudbakhsh, A., Jafari, S. & Tomaj, H. E. K. Haematological manifestations of brucellosis. Acta Medica Iranica 45, 145–148 (2007).
Mantur, B., Amarnath, S. & Shinde, R. Review of clinical and laboratory features of human Brucellosis.
Sofian, M. et al. Risk factors for human brucellosis in Iran: a case–control study. International Journal of Infectious Diseases 12, 157–161 (2008).
Bosilkovski, M. et al. Human brucellosis in Macedonia–10 years of clinical experience in endemic region. Croatian medical journal 51, 327–336 (2010).
Njeru, J. et al. Systematic review of brucellosis in Kenya: disease frequency in humans and animals and risk factors for human infection. BMC public health 16, 853 (2016).
Nematollahi, S. et al. Epidemiological Characteristics of Human Brucellosis in Hamadan province during 2009–2015: Results from National Notifiable Diseases Surveillance System. International Journal of Infectious Diseases (2017).
Aygen, B., Doğanay, M., Sümerkan, B., Yildiz, O. & Kayabaş, Ü. Clinical manifestations, complications and treatment of brucellosis: a retrospective evaluation of 480 patients. Medecine et maladies infectieuses 32, 485–493 (2002).
Andriopoulos, P., Tsironi, M., Deftereos, S., Aessopos, A. & Assimakopoulos, G. Acute brucellosis: presentation, diagnosis, and treatment of 144 cases. International journal of infectious diseases 11, 52–57 (2007).
Ariza, J. Brucellosis: an update. The perspective from the Mediterranean basin. Reviews in medical microbiology 10, 125–135 (1999).
Atluri, V. L., Xavier, M. N., De Jong, M. F., Den Hartigh, A. B. & Tsolis, R. M. Interactions of the human pathogenic Brucella species with their hosts. Annual review of microbiology 65, 523–541 (2011).
Keramat, F., Majzobi, M. M., Poorolajal, J., Ghane, Z. Z. & Adabi, M. Seroprevalence of Brucellosis in Human Immunodeficiency Virus Infected Patients in Hamadan, Iran. Osong public health and research perspectives 8, 282 (2017).
Moreno, S. et al. Brucellosis in patients infected with the human immunodeficiency virus. European Journal of Clinical Microbiology and Infectious Diseases 17, 319–326 (1998).
Acknowledgements
We thank the patients who participated in the study and the staff of Rushere community hospital who contributed in various ways towards the success of the study. We are very grateful to Epicentre Mbarara for funding the study. The contribution of funders of the Institute for Tropical Health (Obra Social la CAIXA, Fundaciones Caja Navarra and Roviralta, PROFAND, Ubesol, ACUNSA and Artai) is also gratefully acknowledged. We also thank Moriyon Ignacio from Institute for Tropical Health, University of Navarra (ISTUN), Pamplona, Spain and Francis Bajunirwe of Mbarara University of Sciences and Technology, Mbarara, Uganda for their technical input into the study.
Author information
Authors and Affiliations
Contributions
R.M., Y.B., F.B., D.N., A.P. and M.B. contributed to the design and implementation of the research; R.M. and M.B analysed the data; A.Z. and R.C. provided support in writing the manuscript. F.B. and M.B. supervised the research. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Migisha, R., Dan Nyehangane, Boum, Y. et al. Prevalence and risk factors of brucellosis among febrile patients attending a community hospital in south western Uganda. Sci Rep 8, 15465 (2018). https://doi.org/10.1038/s41598-018-33915-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33915-9
Keywords
This article is cited by
-
Investigating the etiology of acute febrile illness: a prospective clinic-based study in Uganda
BMC Infectious Diseases (2023)
-
Prevalence and risk factors of human brucellosis and malaria among patients with fever in malaria-endemic areas, attending health institutes in Awra and Gulina district, Afar Region, Ethiopia
BMC Infectious Diseases (2021)
-
Molecular epidemiology of Brucella species in mixed livestock-human ecosystems in Kenya
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.