Abstract
Two members of 6-cysteine (6-cys) protein family, P48/45 and P230, are important for gamete fertility in rodent and human malaria parasites and are leading transmission blocking vaccine antigens. Rodent and human parasites encode a paralog of P230, called P230p. While P230 is expressed in male and female parasites, P230p is expressed only in male gametocytes and gametes. In rodent malaria parasites this protein is dispensable throughout the complete life-cycle; however, its function in P. falciparum is unknown. Using CRISPR/Cas9 methodology we disrupted the gene encoding Pfp230p resulting in P. falciparum mutants (PfΔp230p) lacking P230p expression. The PfΔp230p mutants produced normal numbers of male and female gametocytes, which retained expression of P48/45 and P230. Upon activation male PfΔp230p gametocytes undergo exflagellation and form male gametes. However, male gametes are unable to attach to red blood cells resulting in the absence of characteristic exflagellation centres in vitro. In the absence of P230p, zygote formation as well as oocyst and sporozoite development were strongly reduced (>98%) in mosquitoes. These observations demonstrate that P230p, like P230 and P48/45, has a vital role in P. falciparum male fertility and zygote formation and warrants further investigation as a potential transmission blocking vaccine candidate.
Similar content being viewed by others
Introduction
The s48/45 domain 6-cysteine (6-cys) family of Plasmodium falciparum proteins is a small family with 14 members that show stage-specific expression throughout the parasite life cycle and most members localize at the parasite surface1. Most members have critical roles in parasite development, either in the vertebrate host or in the mosquito vector, and several members are leading targets for malaria vaccines. Four members, P48/45, P47, P230 and P230p are specifically expressed in the sexual stages of the parasite and are encoded by 2 paralogous pairs of genes. Immune responses directed against the proteins P48/45 and P230 can prevent parasite transmission through the mosquito and these antigens are being actively pursued as so called transmission blocking vaccines2,3,4. Using specific antibodies and rodent and human parasite mutants lacking P48/45 and P230 it has been shown that both proteins are crucial for efficient transmission through mosquitoes5,6,7. In the rodent parasite P. berghei these proteins are expressed at the surface of male gametes and are critical for attachment of male gametes to female gametes5. In P. falciparum these proteins are expressed in both male and female gametocytes/gamete8,9,10. P. falciparum P230 has been shown to play a critical role in interactions of male gametes with red blood cells (RBC). In mutants lacking P230 expression the characteristic clusters of uninfected red blood cells that form around male gametes, so-called exflagellation centres, are absent7. This ‘loss of exflagellation centres’ phenotype was not observed for equivalent P. berghei mutants lacking P230 expression5. In P. berghei and P. falciparum the paralog of P48/45, the female specific P47 protein is located on the surface of female gametes, zygotes and ookinetes11. P47 is important in protecting ookinetes from the mosquito’s complement-like immune response in both rodent and human malaria species12,13,14. In addition, P. berghei P47 plays an essential role in the attachment and recognition of the female gamete by the male gamete5,12. In contrast, P. falciparum P47 does not play such a crucial role in gamete fertilization11. These observations indicate that differences exist in the precise function of the sex-specific 6-Cys members between human and rodent malaria species.
In two rodent Plasmodium species the paralog of P230, the male-specific P230p protein, appears to be dispensable throughout the parasite’s complete life cycle5,15,16. P. berghei and P. yoelii mutants lacking expression of P230p can develop in the vertebrate host and in the mosquito vector without a discernible phenotype and p230p knock-out parasites manifest a wild type parasite phenotype. Consequently, as P230p is non-essential, the p230p gene is the most frequently locus used to introduce additional transgenes into rodent malaria parasite genomes15.
The function of P230p of human malaria parasites is unknown but, like in rodent parasites, P. falciparum P230p is male specific5,8,9,10,17,18,19. Recently we generated transgenic P. falciparum parasites where we disrupted the p230p gene by introducing transgenes into this locus using adapted CRISPR/Cas9 methodology20. These PfΔp230p parasites show normal blood stage growth and are able produce gametocytes. In this study, we analysed the phenotype of the sexual stages and subsequent developmental mosquito-stages of these PfΔp230p parasites. We show that P. falciparum P230p has a vital role during mosquito transmission, which is in strong contrast to P230p of rodent malaria parasites. PfΔp230p male and female gametes retain P48/45 and P230 expression on male gametocytes. However, like P. falciparum mutants lacking P230, the capacity of PfΔp230p male gametes to bind to RBC is strongly reduced. In the absence of P230p expression, ookinete and oocyst development in Anopheles stephensi mosquitoes is almost absent. These observations identify P230p as one of a limited number of gamete-specific proteins critical for P. falciparum transmission.
Results
P. falciparum mutants lacking expression of P230p (PfΔp230p-1 and PfΔp230p-2)
We recently generated two transgenic P. falciparum mutants, where different GFP-expression cassettes had been introduced into the p230p gene locus using CRISPR/Cas9 technology20. In these mutants, GFP@cam and GFP@hsp70, GFP expression is driven by promoters of two different genes, calmodulin and hsp70. The introduction of GFP-expression cassette resulted in the disruption of the p230p gene including removal of 259 bp of the p230p coding sequence (Fig. 1a). We name here these two mutants PfΔp230p-1 (GFP@cam) and PfΔp230p-2 (GFP@hsp70). Correct integration of the GFP-expression cassettes in the p230p locus has been demonstrated by diagnostic PCR and Southern analysis20. The p230p gene is a paralogue of the p230 gene, which is located directly down-stream of p230p. To show that the integration of the GFP-expression cassette disrupted only the p230p gene and did not alter the p230 locus, we performed additional Southern analysis of SphI/SpeI restricted DNA of WT and PfΔp230p-1, using a probe targeting the p230p gene-homology region (867 bp) and a probe specific for 745 bp of the p230 open reading frame. The first probe hybridized to expected DNA fragments which differ in size in WT and PfΔp230p-1 (4141 bp and 3755 bp, respectively), and the second probe hybridized to a fragment of the same size (5747 bp) in WT and PfΔp230p-1 (Fig. 1a,b; see Supplementary Table S1 for primer sequences). These hybridisation results indicate the specific targeting of the p230p locus by the CRISPR/Cas9 constructs.
Generation and genotyping of PfΔ230p parasite lines and absence of p230p expression in PfΔp230p parasites. (a) Two PfΔp230p parasite lines were generated using CRISPR/Cas9 methodology as described previously20. The p230p gene was disrupted by insertion of a GFP-expression cassettes using plasmids pLf0026 (cam promoter driving GFP) or pLf0035 (hsp70 promoter driving GFP). A schematic representation of the locus containing the paralogous genes p230p and p230, before and after insertion of the construct showing the location of the restriction sites (SpeI, SphI), sizes of restriction fragments (in red), location of primers (p) and the PCR amplicons and sizes of transcripts (in black) used to analyse correct disruption and transcription of the paralogous genes. (b,c) HR1, HR2: p230p homology regions. The figure is not shown to scale. Primer sequences can be found in Supplementary Table S1. (b) Southern analysis of SphI/SpeI restricted DNA of WT and PfΔp230p-1 parasites confirms the specific and expected disruption of the p230p gene locus. DNA was hybridized with a probe targeting the homology region 2 (HR2; primers p3/p4) of p230p (left panel) and a specific probe of 745 bp (primers p1/p2) of the 5′ p230 open reading frame (right panel). The hybridization pattern observed with first probe identified the expected different-sized DNA fragments in WT and PfΔp230p-1 parasites (4141 bp and 3755 bp); the second probe hybridized to a single expected fragment (5747 bp) in both WT and PfΔp230p-1, indicating an unaltered p230 locus. Uncropped images of the Southerns are shown in Supplementary Fig. S3. (c) Transcription analysis of the 6-Cys family members p230p, p230 and p48/45 in WT and PfΔ230p parasites by RT-PCR and Northern blot. Left panel: RT-PCR amplified transcripts of p230p (primers p5/p6; expected size: 259 bp), p230 (primers p1/p2; expected size: 745 bp), p48/45 (primers p7/p8; expected size: 1219 bp) and 18sRNA (primers p9/p10; expected size: 165 bp). + and − denote the presence or absence of reverse transcriptase. Uncropped images of gels are shown in Supplementary Fig. S4. Right panel: Northern blot analysis of p230p and p230 transcripts confirming the absence of p230p and presence of p230 transcripts in PfΔ230p parasites. Upper panel: hybridization with an internal probe (259 bp) from p230p (primers p5/p6, WT expected size: ~8 kb); middle panel hybridization with a probe against the 5′ p230 open reading frame (primers p1/p2, expected size: ~10 kb); lower panel: ethidium bromide (EtBr) stained RNA as loading control. Uncropped images of the Northern blot analyses are shown in Supplementary Fig. S5. The size of expected RT-PCR products and transcripts are shown in black in (a). Primer sequences are shown in Supplementary Table S1. (d) Immunofluorescence analyses of mature, stage V, WT and PfΔp230p gametocytes. Fixed cells were labelled with mouse anti-P230p polyclonal serum (anti-rMBP.PfB0400w) and with secondary conjugated antibodies anti-IgG Alexa Fluor® 594 (red). Nuclei stained with the DNA-specific dye Hoechst-33342. All pictures were recorded with standardized exposure/gain times; anti-IgG Alexa Fluor® 594 (red). 0.6 s; Hoechst (blue) 0.136 s; bright field 0.62 s (1x gain). Scale bar, 7 µm.
P230p is expressed exclusively in male gametocytes as has been demonstrated by immunofluorescence and PCR analyses as well as RNAseq and proteome analyses of separated P. falciparum male and female gametocytes8,9,10,17,18,19. In contrast, the paralog P230 and P48/45 have comparable expression levels in both males and females parasites8,9,10 (Supplementary Table S2). To demonstrate that gametocytes of PfΔp230p-1 and PfΔp230p-2 were deficient in p230p expression, we performed RT-PCR and Northern blot analysis using mRNA isolated from gametocyte cultures (Fig. 1c). No p230p transcripts were detected by RT-PCR in gametocytes from either PfΔp230p-1 or PfΔp230p-2, whereas a p230p transcript (259 bp) was amplified from WT gametocytes. We were able to amplify p230 and p48/45 transcripts (745 and 1219 bp respectively) from gametocytes of WT and the PfΔp230p mutant lines. The lack of p230p transcripts and presence of p230 transcripts in gametocytes of the mutant lines was confirmed by Northern blot analysis using the probes specific for the p230p and p230 gene loci (Fig. 1c).
In addition, we analysed P230p expression by immunofluorescence microscopy using polyclonal antiserum against P230p17. This antiserum reacted only to WT gametocytes and no signal was detected in PfΔp230p gametocytes (Fig. 1d). Combined our analyses show correct disruption of p230p in PfΔp230p parasites resulting in absence of expression of p230p in gametocytes whereas the paralogous gene p230 is transcribed. The staining pattern in WT gametocytes suggests P230p is located at the surface in the mature stage V gametocyte, and is in agreement with the localisation in P. falciparum gametocytes expressing a GFP-tagged version of P230p (P230p-GFP9). We also analysed P230p expression in activated male gametocytes, both using the polyclonal antiserum against P230p and in gametocytes of the transgenic P230p-GFP line. We were unable to clearly detect P230p in male gametes using the polyclonal serum or in live gametes of the P230P-GFP line but, staining with anti-GFP antibodies demonstrated that the tagged-protein was present either in or on male gametes (Supplementary Fig. S1a–c). While P230p is expressed in/on male gametes it is likely to be weakly expressed given the low GFP fluorescence and that there was no detectable signal with polyclonal serum.
Mosquito transmission of PfΔp230p-1 and PfΔp230p-2 parasites is strongly reduced
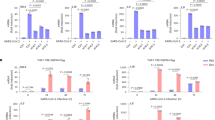
Since multiple members of the 6-cys family play a role in mosquito transmission (i.e. P48/45, P47, P2305,6,7,11,12,13,14) we analysed the ability of mosquitoes to transmit the PfΔp230p lines. A. stephensi mosquitoes were fed with WT and PfΔp230p gametocytes using the standard membrane feeding assay and the number of oocysts and salivary gland sporozoites were determined at day 6 and day 14 respectively. We fed WT and PfΔp230p gametocytes in independent experiments and used different clones of the mutant parasites (WT, 7 experiments (exp.); PfΔp230p-1 clone 0022cl1, 6 exp.; PfΔp230p-1 clone 0022cl5, 5 exp.; PfΔp230p-2 clone 0035cl4, 6 exp). In all experiments we observed a >98% reduction in oocyst development in mosquitoes that had been fed with PfΔp230p parasites; mean oocyst numbers ranging from 4 to 32 for WT-infected mosquitoes compared to 0.1 to 0.7 in PfΔp230p-infected mosquitoes (100 mosquitoes analysed per experiment; Fig. 2a, Supplementary Table S3). In PfΔp230p-infected mosquitoes we observed a maximum of 5 oocysts per mosquito compared to 40 after WT feeding. No sporozoites were observed in salivary glands of PfΔp230p-infected mosquitoes. These results indicate the P. falciparum P230p plays an important role in mosquito transmission.
Mosquito development (ookinete and oocyst formation) and in vitro formation of exflagellation centres of PfΔp230p parasites. (a) Mean oocyst numbers in A. stephensi mosquitoes at day 8 after feeding in different experiments (exp.) with 10–20 mosquitoes/exp.: WT (7 exp.); PfΔp230p-1 (6 exp.); PfΔp230p-2 (6 exp.). ***p = 0.002 (unpaired T-test). (b) Mean ookinete numbers (retort and mature forms) in A. stephensi mosquitoes 24 hours after feeding. Left panel: retort (immature) ookinetes with 10–20 mosquitoes/exp.: WT (7 exp.); PfΔp230p-1 (4 exp.); PfΔp230p-2 (6 exp.). **p = 0.005 and ***p = 0.0006 (unpaired T-test). Right panel: mature ookinetes with 10–20 mosquitoes/exp.: WT (7 exp.); PfΔp230p-1 (6 exp.); PfΔp230p-2 (6 exp.). ***p = 0.0004 (unpaired T-test). (c) Exflagellation centres (EC, circles) as observed by light microscopy analysis of live preparations of male gametocytes between 10 and 20 min after activation examined in a Bürker cell chamber. Only WT activated male gametocytes attach to red blood cells and form characteristic exflagellation centres (left panel; white circles). See also Supplementary Videos S1–6 for the absence/presence of exflagellation centres. Arrows indicate exflagellating male gametocytes (MG) of PfΔp230p. Scale bar, 7 µm.
The formation of ookinetes in PfΔp230p-1 and PfΔp230p-2 parasites is severely compromised
To better define the role of P230p in P. falciparum mosquito transmission we analysed gametocyte, gamete and ookinete formation of the two mutant lines. The in vitro production of male and female gametocytes of both PfΔp230p-1 and PfΔp230p-2 were in the same range as WT parasites (Table 1). However, the number of ‘retort-form’ and mature ookinetes was reduced by >97% in midguts of A. stephensi mosquitoes at 22 h. after feeding (Fig. 2a, Table 1). In PfΔp230p infected mosquitoes we observed no or very low numbers of retorts and mature ookinetes, with mean numbers ranging from only 0 to 1.5 (retorts) and 0 to 0.5 (mature ookinetes). In WT infected mosquitoes the numbers of retorts and mature ookinetes ranged between 6–21 and 7.5–27, respectively. This strong reduction in ookinetes numbers, indicate that either fertilisation or the development of fertilised female gametes is interrupted. We cannot discriminate between these two possibilities, since P. falciparum fertilised female gametes cannot easily be distinguished from unfertilized female gametes, thereby hampering quantification of fertilisation. However, since P230p is specifically expressed in male gametocytes the most likely explanation for the reduced ookinete formation is the inability of PfΔp230p male gametes to fertilize females. We therefore next analysed the formation of male gametes in more detail.
PfΔp230p-1 and PfΔp230p-2 male gametes are unable to generate exflagellation centres
Both gametocyte production and sex ratio of mature gametocytes at day 14 were comparable between WT and PfΔp230p parasites (Table 1) and morphologically, at the light microscopy level, there is no difference between WT and PfΔp230p gametocytes (data not shown). After activation in FCS mature gametocytes from both WT and PfΔp230p cultures readily formed high numbers of exflagellating male gametocytes as observed by light-microscopy. We estimate that >90% of stage V male PfΔp230p gametocytes showed exflagellation (from 3 experiments) (Table 1). These observations indicate that the formation of male gametes is not affected by the absence of P230p. However, a striking difference was the absence of PfΔp230p male gamete attachment to uninfected RBC and the formation of exflagellation centres observed 15–30 min post activation (Fig. 2b and Supplementary Videos S1–S6). Such exflagellation centres generally consist of one or more exflagellating male gametes attaching to a number of RBC21. While WT stage V gametocytes formed such centres by more than 90% of the activated male gametocytes, this was observed in less than 5% of activated PfΔp230p males (Table 1). These results indicate that in the absence of P230p, male gametes were incapable of effectively attaching to RBC. This phenotype is very similar to the phenotype described for P. falciparum mutants lacking P230 and indicates that both P230 and P230p play a role in interactions of male gametes with RBC. Whether the inability of male gametes to interact with RBC is solely responsible for the reduced ookinete formation, or whether P230p has an additional role in fertilisation, is unknown. It has been shown that the 6-cys family members, P230 and P48/45, form complexes with other proteins on the surface of gametes22,23. We therefore examined if the expression of P230 and P48/45 was altered in activated PfΔp230p-1 and PfΔp230p-2 gametocytes by immunofluorescence analysis. Using anti-P230 and P48/45 antibodies we demonstrated that P230 and P48/45 were present in both activated PfΔp230p female and male gametocytes/gametes where staining patterns were comparable to what was observed in activated WT gametocytes (Fig. 3a,b and Supplementary S1d). The combined results indicate that the formation of exflagellation centres and subsequent reduced ookinete formation is directly P230p dependent and not a consequence of the loss of P230 or P48/45 on gametes in the PfΔp230p gametes.
Expression of P230 an P48/45 in activated female and male gametes of PfΔp230p-1. (a) Immunofluorescence analyses of female gametes 30 minutes after gametocyte activation in fetal calf serum. Unfixed parasites were labelled with mouse anti-P230 (MAb 63F2A2) or rat anti-P48/45 (MAb 85RF45.1) antibodies followed by secondary conjugated antibodies (i.e. anti-rat, anti-mouse IgG Alexa Fluor® 488 (green) or anti- mouse IgG Alexa Fluor ® 594 (red)). Nuclei stained with the DNA-specific dye Hoechst-33342. All pictures were recorded with standardized exposure/gain times; Alexa Fluor® 488 (green) 0.7 s; anti-IgG Alexa Fluor ® 594 (red) 0.6 s; Hoechst (blue) 0.136 s; bright field 0.62 s (1x gain). Scale bar, 7 µm. (b) Immunofluorescence analyses of male gametes 15 minutes after gametocyte activation in fetal calf serum. Cells were fixed with methanol and labelled with mouse anti-P230 (MAb 63F2A2) or rat anti-P48/45 (MAb 85RF45.1) antibodies followed by secondary conjugated antibodies (i.e. anti-mouse IgG Alexa Fluor® 488 (green) or anti-rat IgG Alexa Fluor® 594 (red)). Nuclei stained with the DNA-specific dye Hoechst-33342. All pictures were recorded with standardized exposure/gain times; Alexa Fluor ® 488 (green) 0.7 s; anti-IgG Alexa Fluor ® 594 (red). 0.6 s; Hoechst (blue) 0.136 s; bright field 0.62 s (1x gain). Scale bar, 7 µm.
The role of P230 and P230p in P. falciparum gamete binding to RBC is different to the role of these proteins in the rodent parasite P. berghei. Single gene-deletion mutants lacking expression of either P230 or P230p in P. berghei exhibit formation of exflagellation centres like WT, indicating that male gametes of these mutants bind normally to RBC5. To examine a possible compensatory role in RBC binding of the two P. berghei paralogs we generated a p230 and p230p double gene deletion mutant (Supplementary Fig. S2a,b). Activated male gametocytes of this mutant, PbΔp230Δp230p, formed WT-like levels of exflagellation centres (Supplementary Fig. S2c, Supplementary Videos S7–12, Supplementary Table S4) demonstrating an absence of a role of these proteins in RBC binding of P. berghei male gametes.
Finally, we examined fertility of female gametes of the PfΔp230p lines, by crossing the GFP-expressing PfΔp230p gametocytes with WT gametocytes and examining parasite development in mosquitoes. Mosquitoes with both GFP-positive and GFP-negative oocysts were obtained in multiple experiments (Fig. 4; Table 2). GFP-positive oocysts can only result from cross-fertilisation of WT gametes and PfΔp230p gametes. In view of the male-specific expression of P230p and the male phenotype of PfΔp230p parasites, the presence of the GFP-positive oocysts most likely result from cross-fertilisation between WT male gametes and PfΔp230p female gametes. These observations are in support of normal fertility of PfΔp230p female gametes and reduced fertility of PfΔp230p male gametes.
Crossing of GFP-expressing PfΔp230p gametocytes with WT gametocytes results in the formation of GFP-positive oocysts. (a) GFP-positive oocyst in midguts of A. stephensi mosquitoes fed on a mixture of PfΔp230p-2 and WT gametocytes (day 10 after feeding). Arrows indicate GFP-positive oocysts in the WT and PfΔp230p-2 cross and GFP-negative oocysts in WT fed mosquitoes. (b) GFP-positive and GFP-negative oocysts in mosquitoes fed on a mixture of PfΔp230p-2 and WT gametocytes or only WT gametocytes (day 10 after feeding). See Table 25 for the ratio of GFP-positive and GFP-negative oocysts in mosquitoes fed on a mixture of PfΔp230p-2 and WT gametocytes. All pictures were recorded with standardized exposure/gain times to visualize differences in fluorescence intensity (GFP 0.7 s; bright field 0.62 s (1x gain)).
Discussion
We demonstrate that P. falciparum P230p plays a vital role in parasite transmission through mosquitoes. Mutants lacking expression of P230p (PfΔp230p) have a strong reduction (>98%) in ookinete formation, which in turn results in a strong reduction in oocyst formation and absence of sporozoites in salivary glands. We show that the PfΔp230p male gametes have lost the capacity to bind to RBC and could not form the characteristic exflagellation centres. A function of P230p in male gamete fertility is in agreement with male specific expression of PfP230p and concomitant absence in female gametocytes/gametes8,9,10,17,18,19. Indeed the results of crossing experiments in mosquitoes, performed using a mixture of WT and PfΔp230p gametocytes, indicate that PfΔp230p females retain their fertility. The important role that P. falciparum P230p plays in mosquito transmission does not match the redundant function of P230p in the rodent parasites P. berghei and P. yoelii. Rodent parasites also express P230p specifically in male gametocytes24, but mutants lacking P230p have no discernible defect and exhibit normal gametocyte/gamete formation, are fully able to form exflagellation centres and mosquito transmission is similar to WT parasites5,15. We also demonstrate that the P. berghei P230 is not compensating for the loss of its paralogue P230p, since activated male gametocytes of P. berghei mutants lacking expression of both P230 and P230p can still bind to RBCs and form exflagellation centres. These observations demonstrate a critical difference in the function P230p performs in rodent and human malaria parasites. For a few other 6-Cys proteins there has been evidence for functional differences between the orthologs of rodent and human parasites. For example, the female-specific P47 protein is vital for the fertility of P. berghei female gametes, while this protein appears not to be crucial for P. falciparum female gamete fertility5,11. Analysis of P. berghei mutants lacking P45/48 and P230 demonstrates that these proteins are male-specific fertility factors5,6. In contrast, P. falciparum P48/45 and P230 are expressed in both males and female gametes8,9,10, which may suggest a role for these proteins in both male and female gamete fertility. Also other proteins expressed in gametocytes/gametes functional differences have been demonstrated between the equivalent proteins in rodent and human malaria parasites, for example members of the LCCL protein family. In rodent parasites most LCCL members are expressed after fertilisation, in the ookinete stage, and play a role in sporozoite formation25,26, whereas in P. falciparum these proteins are expressed in gametocytes and are part of protein complexes on the surface of gametocytes/gametes27,28.
The lack of RBC binding of PfΔp230p male gametes is similar to the phenotype of P. falciparum mutants lacking expression of its paralog, P2307. These observations suggest that both proteins have a similar, but not interchangeable, function in RBC binding. We provide evidence that PfΔp230p male and female gametes retain expression of both P230 and P48/45, indicating that the lack of RBC binding is not due to the absence of expression of P230 and/or P48/45. These observations would suggest that RBC binding of male gametes is not due to direct interactions of P230 to RBC receptors as was also demonstrated in the studies using males lacking expression of P2307. Moreover, P230 unlike P230p is also expressed at the surface of female gametes8,9,10,23, which makes it less likely that P230 interacts directly with RBC.
P230 and P48 form complexes with several other proteins at the surface of female gametes and zygotes23,29,30. Given that of P48/45, P230 and P230p are expressed in male gametes, it is conceivable that comparable complexes that may include additional proteins, are also formed at the surface of male gametes. The absence of either P230 or P230p may affect the correct formation of such protein complexes at the gamete surface, which may in turn lead to the same loss of RBC binding phenotype observed in mutants that lack either paralog. This would indicate that neither P230 nor P230p but rather other parasite proteins/factors are directly responsible for binding to RBC receptors. Future studies are needed to unravel in more detail the molecular interactions between male gametes and RBC and the Plasmodium ligand(s) that bind to the putative proteins, sialic acid and/or glycophorin receptors on the RBC surface21.
Whether the reduction in fertilisation and ookinete formation within the mosquito midgut of parasites lacking either P230 or P230p is directly due to the inability of male gametes to bind to RBC or whether these proteins have an additional role in fertilisation remains unknown. Studies on male gametes lacking P230 showed that the inability to form exflagellation centres did not affect the release of male gametes from activated gametocytes7 and we also observed in vitro that PfΔp230p male gametes were released after gametocyte activation. It has been suggested that the RBC interactions may trigger changes in the gamete that are required for fertilisation such as the release of additional proteins, which through a process analogous to sperm capacitation, permit the male gamete to be able to bind to molecules in the zona pellucida of the oocyte and thereby initiating the process of male penetration of the female gamete21. Unfortunately, efficient in vitro assays for P. falciparum fertilisation are absent31 and fertilised female gametes cannot easily be distinguished from unfertilized female gametes, thereby hampering more detailed analyses and quantification of fertilisation events.
Our study expands the role of the 6-Cys proteins in fertilisation and specifically demonstrates that P230p, like P230 and P48/45, has a clear and vital role in P. falciparum male fertility, zygote formation and parasite transmission through mosquitoes.
Materials and Methods
Parasites and culture
We analysed wild type (WT) P. falciparum NF54 parasites and two mutant lines PfΔp230p-1 (GFP@cam clones 0022cl1 and 0022cl5) and PfΔp230p-2 (GFP@hsp70 clone 0035cl4) with a disrupted p230p gene locus (PF3D7_0208900). These mutants were generated in NF54 parasites by introducing a GFP-reporter cassette into the p230p gene locus using CRISPR/Cas9 methodology20. In the two mutants, GFP is either under the control of the promoter from calmodulin (cam; PF3D7_1434200) or from heat shock protein 70 (hsp70; PF3D7_0818900). The genotype and phenotype of (asexual) blood stages of these mutants have been reported previously20. WT NF54 parasites32 had been obtained from the Radboud University Medical Center (Nijmegen, The Netherlands). NF54 parasites were cultured following the standard conditions in RPMI-1640 culture medium supplemented with L-Glutamine 25 mM HEPES (Gibco Life Technologies) and 50 mg/L hypoxanthine (Sigma). Culture medium was supplemented with 10% human serum and 0.225% NaHCO3. Parasites were cultured at a 5% hematocrit under 4% O2, 3% CO2 and 93% N2 gas-conditions at 75 rpm at 37 °C in a semi-automated culture system33. Fresh human serum and human red blood cells (RBC) were obtained from the Dutch National Blood Bank (Sanquin Amsterdam, the Netherlands; permission granted from donors for the use of blood products for malaria research and microbiology safety-tests).
In addition, a P. falciparum (3D7) transgenic line that expresses a GFP-tagged version of Pfp230p (Pfp230p-GFP) was analysed for P230p expression and localisation. This line has been engineered to express endogenous PfP230p fused to GFP to its carboxyl terminal and was generated using a single cross-over recombination strategy9.
Two different P. berghei ANKA mutants were analysed that have been previously generated. One with a p230p gene disruption (line 676m1cl1; PbΔp230p; RMgm-29; www.pberghei.eu)34 and the other with a p230 gene disruption (line 310cl1; PbΔp230; RMgm-350; www.pberghei.eu)5. In addition, we generated a double gene deletion P. berghei ANKA mutant with both the p230p (PBANKA_0306000) and p230 (PBANKA_0306100) gene loci disrupted (see below).
Animal ethics statement
Female OF1 mice (6–8 weeks old; Charles River/Janvier) were used. All animal experiments of this study were in accordance with relevant guidelines and regulations approved by the Animal Experiments Committee of the Leiden University Medical Center (DEC 12042). The Dutch Experiments on Animal Act is established under European guidelines (EU directive no. 86/609/EEC regarding the Protection of Animals used for Experimental and Other Scientific Purposes).
Generation of the P. berghei double knock-out mutant PbΔp230Δp230p
To generate a P. berghei mutant lacking expression of both P230 and P230p we disrupted the p230 locus in the existing PbΔp230p mutant (676m1cl1; see above) which has a disrupted p230p locus. To disrupt p230 we used a DNA construct that had been used to create the mutant PbΔp230 (310cl1; see above). This construct (pL1139) integrates by double cross integration and replaces (part of) the p230 locus with the selectable marker cassette containing Toxoplasma gondii dihydrofolate reductase/thimidylate synthase (tgdhfr/ts)5. Parasites of line 676cl1 were transfected with this construct (exp. 2764) using standard transfection technologies and selection with pyrimethamine35. Selected parasites were cloned by limiting dilution and mutant 2764cl3 was used for genotype and phenotype analysis.
Genotyping and phenotyping P. berghei mutant PbΔp230Δp230p
Correct disruption of the p230p and p230 gene loci was performed by diagnostic PCR-analysis and Southern analysis of pulsed field gel (PFG) separated chromosomes as described previously35. Briefly, for the PCR-analysis confirmation of disruption of p230 was performed using the primers p13/p14 for 5′ integration and p17/p18 for 3′ integration of the construct and for p230p, 5′ and 3′ integration with primer pairs p21/p22 and p25/p26, respectively (see Supplementary Fig. S2 and Supplementary Table S1 for details of the primers and the PCR fragments). For Southern analysis, diagnostic probes against the 3′UTR of pbdhfr/ts and the tgdhfr/ts selectable marker were used.
In vitro activation of gametocytes to determine exflagellation, formation of exflagellation centres and formation of ookinetes were performed as described5,35. In brief, 10–20 µl of tail blood from infected mice containing gametocytes was diluted in 1 ml of activation medium. Within 12–20 min after activation, exflagellating male gametocytes and exflagellation centres were quantified in a Bürker cell counter and 18–24 h later the number of zygotes/ookinetes formed was quantified.
Genotyping P. falciparum mutants PfΔp230p-1 and PfΔp230p-2
Supplementary TablesSouthern analysis of restricted genomic DNA to confirm disruption of p230p in mutants PfΔp230p-1 and PfΔp230p-2 have been reported20. We performed additional Southern analysis to confirm disruption of p230p and to confirm that the neighbouring p230 gene locus (PF3D7_0209000) remained unmodified. Total DNA was isolated from infected red blood cells (iRBC) obtained from 10 ml cultures (parasitemia 5–10%, 5% hematocrit), pelleted by centrifugation (400 g; 5 min). RBC were lysed with 5–10 ml of cold (4 °C) erythrocyte lysis buffer (10x stock solution 1.5 M NH4Cl, 0.1 M KHCO3, 0.01 M Na2EDTA; pH 7.435; and parasites pelleted by centrifugation (400 g during 5 min) and treated with RNAse and proteinase-K before DNA isolation by standard phenol-chloroform methods. Genomic DNA was digested with SpeI and SphI restriction enzymes (4 h at 37 °C) to confirm the specific disruption of Pfp230p locus. Restricted DNA was hybridized with 2 probes: one targeting the p230p homology region 2 (HR2) and one targeting the 5′ p230 open reading frame (5′-p230) amplified from WT NF54 genomic DNA by PCR using the primers P3/P4 for HR2 and P1/P2 for 5′-p230, respectively (see Supplementary Table S1 for details of the primers).
Transcriptional analyses of 6-cys family proteins in the mutants PfΔp230p-1 and PfΔp230p-2
To analyse transcription of 6-cys family proteins P. falciparum gametocytes were generated using standard culture conditions (see above) with some modifications33. Briefly, parasites from asexual stage cultures were diluted to a final parasitemia of 0.5% and cultures were followed during 14 days without refreshing RBC. After 9 days these cultures were treated with 50 mM of N-acetyl-D-glucosamine (Sigma) to kill asexual stages and to enrich for gametocytes. At day 14 the cultures were harvested and infected RBC (iRBC), enriched for gametocytes, pelleted by centrifugation (400 g during 5 min), washed three times with 1X PBS and the iRBC lysed with saponin following standard procedures36. Total RNA was isolated from the pelleted parasites using the Kit RNA Pure Link TM RNA Mini kit (Invitrogen) according to the manufactures instructions. Northern blot analysis on the isolated RNA, was performed as previously described36 using probes amplified from genomic DNA from WT NF54 parasites; one targeting an internal fragment (259 bp) of p230p with primers P5/P6 and the other targeting a fragment (754 bp) of the 5′ p230 open reading frame with primers P1/P2 (see Supplementary Table S1 for details of the primers). RNA (1–5 µg) isolated from the iRBC was further purified for RT-PCR analysis by adding 1X DNase I digestion buffer (Promega), 20 U of RNase inhibitor (RNasin, Promega) and 20 U of DNase I (Promega); this was incubated for 45 min at 37 °C followed by chloroform/isoamyl alcohol purification and RNA precipitated in absolute ethanol36 Subsequently, RT-PCR was performed using standard methods36. Briefly, 1–3 µg of RNA was collected for first strand cDNA synthesis using the kit SuperScript III (Invitrogen) and PCR amplification (annealing temperatures ranging 50–57 °C) was performed with KOD polymerase (Invitrogen). For amplification of the Pf48/45 gene (PF3D7_1346700) the primers P7/P8 were used, for Pfp230 primers P1/P2, for Pfp230p primers P5/P6 and for 18 S rRNA primers P9/P10 (see Supplementary Table S1 for details of the primers).
Expression analysis of 6-cys family proteins in P. falciparum gametocyes by immunofluorescence assay (IFA)
To analyse the expression of PfP48/45 and PfP230 in live gametocytes by immunofluorescence microscopy, 500 µl of the gametocyte culture was pelleted (400 g 30 s) and gametocytes activated in 1 ml of fetal calf serum (FCS) for 1 h at room temperature and samples collected for live fluorescence microscopy. To analyse PfP48/45 and PfP230 expression in fixed (male) gametocytes, 20 µl of the activated cells were collected 15–20 min after activation. This gamete enriched solution was placed on a microscope slide, dried for 10 min, and fixed with ice-cold methanol for 5 min. After fixation the slides were blocked with 10% of FCS in 1X PBS for 1 h. Live and fixed cells were washed with 1X PBS and incubated with monoclonal antibodies against PfP48/45 (rat MAb 85RF45.1; 1:200 dilution of 5 µg/ml stock solution37), PfP230 (mouse MAb 63F2A2; 1:200 dilution of 5 µg/ml stock solution38) for 30 min at 4 °C for live imaging and 1 h at room temperature for fixed slides. Subsequently, cells were rinsed 3 times with 1X PBS and incubated with the secondary antibodies Alexa FLuor®488/594-conjugated chicken anti-rat and anti-mouse (Invitrogen Detection technologies), respectively (both at 1:200). Finally, the cells were stained with the DNA-specific dye Hoechst-33342 at a final concentration of 10 µM. Fixed cells were covered with 1–2 drops of an anti-fading agent (Vectashield), and a coverslip placed onto the cells and sealed with nail polish. Stained cells (live and fixed) were analysed for fluorescence using a Leica fluorescence MDR microscope (100x magnification). Pictures were recorded with a DC500 digital camera microscope using Leica LAS X software with the following exposure times: Alexa: 0.7 s; Hoechst 0.136 s; bright field 0.62 s (1x gain). To analyse PfP230p expression in stage V gametocytes by immunofluorescence slides for microscopy analysis were prepared as follows: 20 µl of the cell suspension containing activated gametocytes was placed per well of a 8-well black cell-line diagnostic microscope slide (Thermo Scientific), that was air dried, fixed with ice-cold absolute methanol (2 min) and subsequently washed 3 times with 1X PBS. Cells were permeabilized with 0.5% of Triton X-100 in 1X PBS for 1 h and blocked with 10% FCS in 1X PBS. 20 µl of polyclonal serum raised in mice against recombinant PfP230p (1:200; anti-rMBP.PfB0400w 1:200 dilution17) was incubated with the fixed gametocytes for 1 h at room temperature and slides were washed 3 times with 1XPBS. Subsequently each well was incubated with 20 µl goat-anti-mouse secondary IgG monoclonal antibody conjugated to Alexa FLuor®594- (Supplier; 1:200 dilution) for 1 h at room temperature. Slides were then washed 3 times with 1X PBS and stained with 20 µl of Hoechst-33342 in 1XPBS (10 µM) for 30 min at 37 °C. The slides were washed 3 times in 1X PBS and the cells were analysed for fluorescence using a Leica fluorescence MDR microscope (see above for details).
Further analysis of PfP230p expression was performed by detecting the GFP pattern in the live or fixed gametocytes of Pfp230p-GFP line. Rabbit anti-GFP IgG (Invitrogen; 4 µg/ml) and Goat anti-rabbit IgG conjugated to Alexa FLuor®488 (Invitrogen; 4 µg/ml) were used for detection of GFP in fixed activated mature gametocytes.
Phenotype analysis of gametocytes/gametes and mosquito stages of mutants PfΔp230p-1 and PfΔp230p-2
Gametocyte development was analysed in gametocyte cultures, established as described above. Exflagellation was determined after activation of P. falciparum stage V gametocytes with FCS. To activate gametocytes 20 µl of the gametocyte cultures at day 14 were diluted 1:1 with FCS at room temperature. Gametes and exflagellation centres were examined and quantified 10–20 min after activation using a Bürker cell counter.
The number of male gametocytes per 105 red blood cells (RBC) was determined in stage V gametocyte cultures by analysing Giemsa stained slides. Quantification of exflagellating males of these cultures was performed in triplicate, using a Bürker chamber (at 40X magnification). The number of exflagellating males is given as the number of exflagellating males observed per 1 × 105 of total red blood cells (RBC).
Exflagellation center formation was determined by counting the number exflagellating males adhering to multiple red blood cells and forming characteristic dense clusters of RBC. Exflagellating males, which did not adhere to RBC and failed to form a characteristic dense cluster of RBC were scored as ‘non-adhering’ males. +++ denotes that more than 90% of the exflagellating males formed the dense RBC clusters and − denotes that less than 1% of exflagellating males formed the dense RBC clusters. For analysis of mosquito stages (ookinetes, oocysts and sporozoites) A. stephensi were infected using the standard membrane feeding assay (SMFA)39,40. Ookinetes were analysed and counted 22 h after feeding. Oocyst and salivary gland sporozoites were counted at day 6 and day 14 post feeding, respectively. For counting sporozoites, salivary glands from 10 mosquitoes were dissected and homogenized in a homemade glass grinder in 100 µl of RPMI-1640 (pH 7.2) and sporozoites were analysed in a Bürker cell counter using phase-contrast microscopy.
Cross-fertilisation of WT and PfΔp230p gametocytes was performed by mixing gametocytes obtained from enriched gametocyte cultures (see above) from WT and PfΔp230p and feeding these mixtures to mosquitoes using SMFA. In different experiments WT and PfΔp230p gametocytes were mixed in different ratios (1:1, 1:2, and 1:3) based on exflagellating male gametocytes counts per ml of gametocyte culture after activation with FCS. At day 10 after feeding oocyst development was analysed with a fluorescence stereomicroscope Leica MZ16 FA and GFP-fluorescence was visualized using GFP filter settings (GFP exposure time: 4,2 s). Pictures were recorded using a DM2500 digital camera.
Statistics
All data were analyzed using the GraphPad Prism software package 5.04 (GraphPad Software, Inc). To calculate significant levels for ookinete and oocyst numbers the unpaired Student’s t-test was used.
Change history
03 May 2019
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Annoura, T. et al. Two Plasmodium 6-Cys family-related proteins have distinct and critical roles in liver-stage development. FASEB J 28, 2158–2170, https://doi.org/10.1096/fj.13-241570 (2014).
Theisen, M., Jore, M. M. & Sauerwein, R. Towards clinical development of a Pfs48/45-based transmission blocking malaria vaccine. Expert Rev Vaccines 16, 329–336, https://doi.org/10.1080/14760584.2017.1276833 (2017).
Draper, S. J. et al. Recent advances in recombinant protein-based malaria vaccines. Vaccine 33, 7433–7443, https://doi.org/10.1016/j.vaccine.2015.09.093 (2015).
Wu, Y., Sinden, R. E., Churcher, T. S., Tsuboi, T. & Yusibov, V. Development of malaria transmission-blocking vaccines: from concept to product. Adv Parasitol 89, 109–152, https://doi.org/10.1016/bs.apar.2015.04.001 (2015).
van Dijk, M. R. et al. Three members of the 6-cys protein family of Plasmodium play a role in gamete fertility. PLoS Pathog 6, e1000853, https://doi.org/10.1371/journal.ppat.1000853 (2010).
van Dijk, M. R. et al. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–164 (2001).
Eksi, S. et al. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol Microbiol 61, 991–998, https://doi.org/10.1111/j.1365-2958.2006.05284.x (2006).
Tao, D. et al. Sex-partitioning of the Plasmodium falciparum stage V gametocyte proteome provides insight into falciparum-specific cell biology. Mol Cell Proteomics 13, 2705–2724, https://doi.org/10.1074/mcp.M114.040956 (2014).
Miao, J. et al. Sex-Specific Biology of the Human Malaria Parasite Revealed from the Proteomes of Mature Male and Female Gametocytes. Mol Cell Proteomics 16, 537–551, https://doi.org/10.1074/mcp.M116.061804 (2017).
Lasonder, E. et al. Integrated transcriptomic and proteomic analyses of P. falciparum gametocytes: molecular insight into sex-specific processes and translational repression. Nucleic Acids Res 44, 6087–6101, https://doi.org/10.1093/nar/gkw536 (2016).
van Schaijk, B. C. et al. Pfs47, paralog of the male fertility factor Pfs48/45, is a female specific surface protein in Plasmodium falciparum. Mol Biochem Parasitol 149, 216–222, https://doi.org/10.1016/j.molbiopara.2006.05.015 (2006).
Ukegbu, C. V. et al. Plasmodium berghei P47 is essential for ookinete protection from the Anopheles gambiae complement-like response. Sci Rep 7, 6026, https://doi.org/10.1038/s41598-017-05917-6 (2017).
Molina-Cruz, A. et al. The human malaria parasite Pfs47 gene mediates evasion of the mosquito immune system. Science 340, 984–987, https://doi.org/10.1126/science.1235264 (2013).
Ramphul, U. N., Garver, L. S., Molina-Cruz, A., Canepa, G. E. & Barillas-Mury, C. Plasmodium falciparum evades mosquito immunity by disrupting JNK-mediated apoptosis of invaded midgut cells. Proc Natl Acad Sci USA 112, 1273–1280, https://doi.org/10.1073/pnas.1423586112 (2015).
Lin, J. W. et al. A novel ‘gene insertion/marker out’ (GIMO) method for transgene expression and gene complementation in rodent malaria parasites. PLoS One 6, e29289, https://doi.org/10.1371/journal.pone.0029289 (2011).
Hart, R. J., Lawres, L., Fritzen, E., Ben Mamoun, C. & Aly, A. S. Plasmodium yoelii vitamin B5 pantothenate transporter candidate is essential for parasite transmission to the mosquito. Sci Rep 4, 5665, https://doi.org/10.1038/srep05665 (2014).
Eksi, S. & Williamson, K. C. Male-specific expression of the paralog of malaria transmission-blocking target antigen Pfs230, PfB0400w. Mol Biochem Parasitol 122, 127–130 (2002).
Santolamazza, F. et al. Detection of Plasmodium falciparum male and female gametocytes and determination of parasite sex ratio in human endemic populations by novel, cheap and robust RTqPCR assays. Malar J 16, 468, https://doi.org/10.1186/s12936-017-2118-z (2017).
Schneider, P. et al. Quantification of female and male Plasmodium falciparum gametocytes by reverse transcriptase quantitative PCR. Mol Biochem Parasitol 199, 29–33, https://doi.org/10.1016/j.molbiopara.2015.03.006 (2015).
Mogollon, C. M. et al. Rapid Generation of Marker-Free P. falciparum Fluorescent Reporter Lines Using Modified CRISPR/Cas9 Constructs and Selection Protocol. PLoS One 11, e0168362, https://doi.org/10.1371/journal.pone.0168362 (2016).
Templeton, T. J., Keister, D. B., Muratova, O., Procter, J. L. & Kaslow, D. C. Adherence of erythrocytes during exflagellation of Plasmodium falciparum microgametes is dependent on erythrocyte surface sialic acid and glycophorins. J Exp Med 187, 1599–1609 (1998).
Kumar, N. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol 9, 321–335 (1987).
Simon, N., Kuehn, A., Williamson, K. C. & Pradel, G. Adhesion protein complexes of malaria gametocytes assemble following parasite transmission to the mosquito. Parasitol Int 65, 27–30, https://doi.org/10.1016/j.parint.2015.09.007 (2016).
Khan, S. M. et al. Proteome analysis of separated male and female gametocytes reveals novel sex-specific Plasmodium biology. Cell 121, 675–687, https://doi.org/10.1016/j.cell.2005.03.027 (2005).
Saeed, S., Carter, V., Tremp, A. Z. & Dessens, J. T. Plasmodium berghei crystalloids contain multiple LCCL proteins. Mol Biochem Parasitol 170, 49–53, https://doi.org/10.1016/j.molbiopara.2009.11.008 (2010).
Saeed, S., Carter, V., Tremp, A. Z. & Dessens, J. T. Translational repression controls temporal expression of the Plasmodium berghei LCCL protein complex. Mol Biochem Parasitol 189, 38–42, https://doi.org/10.1016/j.molbiopara.2013.04.006 (2013).
Simon, N. et al. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. J Biol Chem 284, 14537–14546, https://doi.org/10.1074/jbc.M808472200 (2009).
Pradel, G., Wagner, C., Mejia, C. & Templeton, T. J. Plasmodium falciparum: Co-dependent expression and co-localization of the PfCCp multi-adhesion domain proteins. Exp Parasitol 112, 263–268, https://doi.org/10.1016/j.exppara.2005.11.010 (2006).
Kuehn, A., Simon, N. & Pradel, G. Family members stick together: multi-protein complexes of malaria parasites. Med Microbiol Immunol 199, 209–226, https://doi.org/10.1007/s00430-010-0157-y (2010).
Bennink, S., Kiesow, M. J. & Pradel, G. The development of malaria parasites in the mosquito midgut. Cell Microbiol 18, 905–918, https://doi.org/10.1111/cmi.12604 (2016).
Delves, M. J. et al. Failure of in vitro differentiation of Plasmodium falciparum gametocytes into ookinetes arises because of poor gamete fertilisation. bioRxiv, https://doi.org/10.1101/216721 (2017).
Ponnudurai, T., Leeuwenberg, A. D. & Meuwissen, J. H. Chloroquine sensitivity of isolates of Plasmodium falciparum adapted to in vitro culture. Trop Geogr Med 33, 50–54 (1981).
Ponnudurai, T., Lensen, A. H., Meis, J. F. & Meuwissen, J. H. Synchronization of Plasmodium falciparum gametocytes using an automated suspension culture system. Parasitology 93(Pt 2), 263–274 (1986).
Janse, C. J. et al. High efficiency transfection of Plasmodium berghei facilitates novel selection procedures. Mol Biochem Parasitol 145, 60–70, https://doi.org/10.1016/j.molbiopara.2005.09.007 (2006).
Janse, C. J., Ramesar, J. & Waters, A. P. High-efficiency transfection and drug selection of genetically transformed blood stages of the rodent malaria parasite Plasmodium berghei. Nat Protoc 1, 346–356, https://doi.org/10.1038/nprot.2006.53 (2006).
Kristen Moll, A. K. Arthur, S. & Mats, W. Methods in malaria research. 6 edn (2013).
Outchkourov, N. S. et al. Correctly folded Pfs48/45 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in mice. Proc Natl Acad Sci USA 105, 4301–4305, https://doi.org/10.1073/pnas.0800459105 (2008).
Roeffen, W. et al. Transmission blockade of Plasmodium falciparum malaria by anti-Pfs230-specific antibodies is isotype dependent. Infect Immun 63, 467–471 (1995).
Ponnudurai, T. et al. Infectivity of cultured Plasmodium falciparum gametocytes to mosquitoes. Parasitology 98(Pt 2), 165–173 (1989).
Ponnudurai, T., van Gemert, G. J., Bensink, T., Lensen, A. H. & Meuwissen, J. H. Transmission blockade of Plasmodium falciparum: its variability with gametocyte numbers and concentration of antibody. Trans R Soc Trop Med Hyg 81, 491–493 (1987).
Acknowledgements
C. M. Mogollon was supported by Colciencias Ph.D. fellowship (Call 568 from 2012 Resolution 01218 Bogotá, Colombia). A.S. Othman Othman is supported by a Skim Latihan Akademik IPTA - SLAI (Ministry of Higher Education, Malaysia). Jun Miao and Liwang Cui were partially supported by grant (R01AI104946) from National Institute of Allergy and Infectious Diseases, NIH.
Author information
Authors and Affiliations
Contributions
Study concept and design: C.M.M., C.J.J., S.M.K. Acquisition of data: C.M.M., M.v.d.V.-B., G.-J.v.G., F.J.A.P., J.R., A.S.O., H.K., J.M., L.C., C.J.J. Analyses and interpretation of data: C.M.M., C.J.J., S.M.K. Drafting the manuscript: C.M.M., C.J.J., S.M.K. and all authors reviewed the manuscript. Critical revision of the manuscript for important intellectual content: C.M.M., J.M., L.C., K.C.W., R.W.S., C.J.J., S.M.K. Technical or material support: M.v.d.V.-B., G.-J.v.G., F.J.A.P., J.R., A.S.O., H.K., J.M., L.C., K.C.W., R.W.S., C.J.J. Study supervision: C.J.J., S.M.K.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marin-Mogollon, C., van de Vegte-Bolmer, M., van Gemert, GJ. et al. The Plasmodium falciparum male gametocyte protein P230p, a paralog of P230, is vital for ookinete formation and mosquito transmission. Sci Rep 8, 14902 (2018). https://doi.org/10.1038/s41598-018-33236-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33236-x
Keywords
This article is cited by
-
A dual-antigen malaria vaccine targeting Pb22 and Pbg37 was able to induce robust transmission-blocking activity
Parasites & Vectors (2023)
-
A versatile Plasmodium falciparum reporter line expressing NanoLuc enables highly sensitive multi-stage drug assays
Communications Biology (2023)
-
Characterization of PSOP26 as an ookinete surface antigen with improved transmission-blocking activity when fused with PSOP25
Parasites & Vectors (2022)
-
Malaria parasites utilize two essential plasma membrane fusogens for gamete fertilization
Cellular and Molecular Life Sciences (2022)
-
Are malaria transmission-blocking vaccines acceptable to high burden communities? Results from a mixed methods study in Bo, Sierra Leone
Malaria Journal (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.