Abstract
Vertebrate sex differentiation follows a conserved suite of developmental events: the bipotential gonads differentiate and shortly thereafter sex specific traits become dimorphic. However, this may not apply to squamates, a diverse vertebrate lineage comprising of many species with thermosensitive sexual development. Of the three species with data on the relative timing of gonad differentiation and genital dimorphism, the females of two (Niveoscincus ocellatus and Barisia imbricata) exhibit a phase of temporary pseudohermaphroditism or TPH (gonads have differentiated well before genital dimorphism). We report a third example of TPH in Pogona vitticeps, an agamid with temperature-induced male to female sex reversal. These findings suggest that for female squamates, genital and gonad development may not be closely synchronised, so that TPH may be common. We further observed a high frequency of ovotestes, a usually rare gonadal phenotype characterised by a mix of male and female structures, exclusively associated with temperature-induced sex reversal. We propose that ovotestes are evidence of a period of antagonism between male and female sex-determining pathways during sex reversal. Female sexual development in squamates is considerably more complex than has been appreciated, providing numerous avenues for future exploration of the genetic and hormonal cues that govern sexual development.
Similar content being viewed by others
Introduction
Sex determination and differentiation in amniotes is widely accepted to follow a well-defined sequence1,2,3. Early in development, the bipotential gonads differentiate, then secrete sex-specific steroid hormones, which are thought to prompt the development of sex-specific traits, such as the male Wolffian or female Müllerian ducts (and regression of the opposing sex ducts), and the external genitalia (e.g. hemipenes/hemiclitores)1,2,4,5,6. Among reptiles the primary sex-determining cue can be either temperature or genetic3. In temperature-dependent sex determination (TSD), incubation temperature determines the sex of the individual during the thermosensitive period, which usually occurs in the middle-third of development7,8. The mechanism by which temperature influences sexual development in squamates (snakes and lizards) is not fully understood but is likely to involve epigenetic re-modelling via altered expression and/or splicing of chromatin modifying genes9,10,11. In contrast, gonadal differentiation is controlled in other squamates, by the presence, absence or dosage of as yet unidentified genes on sex chromosomes (genetic sex determination or GSD)12,13,14. Regardless of whether sex is controlled by TSD or GSD, the downstream molecular processes of gonad differentiation appear to be highly conserved15,16,17,18.
Although organisms tend to be classified as either TSD or GSD in the literature, in some species sex can be determined via gene–environment interactions19,20. This can occur when GSD is overridden by high or low incubation temperatures. In most cases of sex reversal in nature, the phenotype of the homogametic sex (ZZ or XX) becomes discordant with the sex chromosomes, though there are rare, mostly experimental examples of heterogametic (XY or ZW) sex reversal3,21,22. Such gene-environment interactions are possibly more common than assumed20, occurring in at least three squamate species - the spotted skink (Niveoscincus ocellatus), the three-lined skink (Bassiana duperreyi), and the central bearded dragon (Pogona vitticeps)7,19,23. The best-studied of these, P. vitticeps, exhibits GSD when eggs are incubated at moderate temperatures (ZW females, ZZm males)24. At high temperatures, ZZm males reverse their sex and develop as phenotypic females (ZZf herein). This process can lead to a complete transition from GSD to TSD within one generation19.
The sex determination system of P. vitticeps has provided novel insights into the molecular pathways underpinning the genetic and temperature influence of sex in reptiles, making this species an important emergent model organism supported by significant molecular resources9,19,25,26. A recent developmental study27 showed that body and genital development do not differ under TSD and GSD, but revealed an unexpected developmental trait: both genetic and temperature-induced females initially develop male genitalia (hemipenes), retain them for much of development, and then regress these structures close to hatching27.
The late development of female genitalia through regression of well-developed hemipenes is at odds with the general consensus that vertebrate gonad differentiation rapidly triggers sex-specific genital formation6,28,29. However, this assumption remains to be fully tested in squamates as there are only three studies examining the degree of synchronisation of gonad and genital development in this order. Of these examples, two phylogenetically disparate species (the imbricate alligator lizard, Barisia imbricata, and N. ocellatus; Fig. 1) display a developmental phase where differentiated ovaries exist alongside male genitalia across multiple developmental stages, which is a form of temporary pseudohermaphroditism (TPH)30,31. The third squamate species for which there are comparable developmental data, the Carolina anole (Anolis carolinensis), develops genitalia almost immediately after gonad differentiation in both males and females, so that TPH is functionally absent in this species32,33.
The timing and duration of temporary pseudohermaphroditism (TPH) in squamates. White bars indicate indeterminate sex, dark grey shows the TPH phase, and light grey indicates dimorphic sexes. The asterisk symbol for Anolis carolinensis indicates that there is a short delay (half a stage) between ovarian differentiation and genital dimorphism but is functionally lacking TPH. The dagger symbol on the dotted section of developmental stage axis indicates the approximate post-hatch timing of hemipenis regression in Barisia imbricata31. The timing of events are approximations standardised to the staging system described for Pogona vitticeps27. The reproductive mode (O/V) and sex determination mode (SDM) is reported for each species (O = oviparity, V = viviparity, G + T = genetic with thermal influence, GSD = genetic sex determination). The dashed vertical line at stage 18 denotes approximate time of hatching/birth. Phylogeny adapted from70, branch length is for illustrative purposes only.
In this study, we demonstrate that sexual development of P. vitticeps is considerably more complex than expected based on current understanding of squamate sex determination and differentiation. In particular, we show histologically that asynchronous sexual development of the gonads and external genitalia of P. vitticeps under both normal and sex-reversing temperatures arises from a form of temporary pseudohermaphroditism, as in B. imbricata and N. ocellatus. We also show that temperature-induced sex reversal is characterised by the presence of ovotestes, a rare gonadal phenotype suggestive of a period of antagonism between genetic and thermal influences during sex determination.
Results
Timing of gonad differentiation
Early in development (approximately stages 4 to 8), the bipotential gonads were loosely attached to the posterior end of the mesonephros and exhibited an elongated shape as they progressively moved to an anterior position (Fig. 2a). Gonad-mesonephric attachment increased once the gonads were at the anterior-most portion of the mesonephros, and the gonads developed a rounder shape. Defined cortex and medullary layers were present just before gonad differentiation. This process was observed in all specimens regardless of genotype (ZZ or ZW), and whether or not they underwent sex reversal. Testes differentiation occurred by approximately stage 9 (Figs 2d and 3a) and was characterised by reduction of the cortex and proliferation of the medulla, within which seminiferous tubules formed (Fig. 2d). Two ZZm offspring of sex reversed mothers incubated at 28 °C exhibited differentiated testes at stages 6 and 7 respectively (Fig. 3a), which is earlier than what was observed in ZZm offspring of concordant mothers (ZW). Ovarian differentiation (begins approximately stage 8; Fig. 3a,b) was characterised by a reduced medulla and a proliferating cortex with oogonia (Fig. 2c).
Histological sections of embryonic Pogona vitticeps urogenital systems stained with haematoxylin and eosin (H & E). (a) Bipotential gonads with developing cortex and medullary regions during migration towards the anterior mesonephros. (b) Ovotestes from an embryo incubated at 36 °C undergoing sex reversal showing a proliferating cortex with oogonia, a medulla with numerous rudimentary seminiferous tubules. (c) Differentiated ovary with a reducing medulla, cortex proliferating with oogonia. (d) Differentiated testes with a reducing cortex, and medulla with developing seminiferous tubules. B. P = bipotential gonad, Mes. = mesonephros, C = cortex, M = medulla, black arrows = seminiferous tubules.
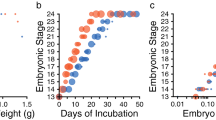
Timing of gonad (a,b) and genital (c,d) development for Pogona vitticeps at normal (28 °C; a,c) and sex-reversing (36 °C; b,d) incubation temperatures. Sexual phenotype is indicated by colour as per the legend, and sexual genotype is indicated by shape (squares = ZZ specimens, triangles = ZW specimens, circles = unknown). The grey shading defines the period of temporary pseudohermaphroditism during female development, persisting for approximately 9 stages. The black asterisks denote approximate time of hatching (stage 18, ~73 dpo at 28 °C and ~47 dpo at 36 °C; Holleley et al.19). Genital development data was re-analysed from Whiteley et al.27.
Asynchronous internal and external female sexual development
Regardless of whether sex was determined by genotype or temperature, the development of sexual phenotypes (genitalia described in Whiteley et al.27; gonads, and accessory ducts; Fig. S1) in female P. vitticeps followed the same pattern described for other squamates. However, there was a delay in relative timing of gonad and genital differentiation in both concordant (ZW) and sex reversed (ZZf) females. Ovaries began differentiating at stage 8 (Fig. 3a,b), whereas the genitalia continued to masculinise until distinctly bilobed hemipenes formed, typically by stage 11 (Fig. 3c,d). Later in development (approximately stages 14–15), long after ovarian differentiation, the mature female genital phenotype (pedicel) began to develop as the hemipenes regressed (Fig. 3c,d). This period of asynchronous development was characteristic of a temporary pseudohermaphroditism phase, or TPH (grey shading Fig. 3). We estimated the TPH phase to occur from stage 8 to stage 15, equating to approximately 45% of total development, with some inter-individual variation in the timing of events (see Figs 1, 3, and File S1).
Sex reversal specific occurrence of ovotestes
We observed ovotestes (Fig. 2b) in 4 of 10 ZZf individuals exposed to sex-reversing temperatures (36 °C) at stages 9–9.5 of development (Fig. 2b). The occurrence of ovotestes coincided with the transition from bipotential to committed gonad. Ovotestes were characterised by a typical ovarian cortex with oogonia. However, instead of the medulla consisting of loose, randomly arranged connective tissues, the cells had condensed into rudimentary seminiferous tubules, occasionally with lumen, akin those seen in normal testes. We did not observe ovotestes in ZW individuals incubated at 36 °C at this stage (4 individuals), or in ZZ or ZW individuals incubated at 28 °C (5 and 3 individuals respectively).
Hemipenal ultrastructures
Morphological comparison of genital phenotypes between males (ZZm), concordant (ZW), and sex reversed females (ZZf) at stage 14 using SEM showed conserved ultrastructural characteristics. The hemipenes of the ZW (Fig. 4b,c) and the ZZf female (Fig. 4f) were very similar, and both exhibited a sulcus spermaticus, which extended from the base of each hemipenis, and bifurcated at the bilobes. The hemipenes of two ZZ males (Fig. 4d,e) were more similar to each other than they were to those of the ZW and ZZf females as they displayed a more uniformly shaped sulcus spermaticus and the hemipenes exhibited a smooth surface interspersed with irregular invaginations (Fig. 4b–f). A late stage 17 ZZm male examined (Fig. 4g–i) exhibited a distinctive ultrastructure we have termed the hemipenal lattice. This structure comprised of a furrow that extended along the distal surface of each lobe of the hemipenes, within which was a series of interconnected indentations forming a lattice-like structure (Fig. 4g–i).
Homologous hemipenal structures in male and female Pogona vitticeps. Scanning electron micrographs of stage 14 (a–f) and stage 17 (g–i) embryonic genitalia. (a) Reduced hemipenes of a ZW female showing trilobes. (b) ZW female from the same clutch as specimen in with well-developed bilobed hemipenes each with a sulcus spermaticus. (c) Enhanced view of right hemipenis of specimen in (b) showing the beginnings of the hemipenal lattice. (d) Bilobed hemipenes of a ZZ male with a sulcus spermaticus. (e) Bilobed hemipenes with sulcus spermaticus of a ZZ embryo incubated at 36 °C that did not undergo sex reversal. (f) Bilobed hemipenes with sulcus spermaticus of a sex reversed female. (g) Well developed bilobed hemipenes of a ZZ male showing the sulcus spermaticus and hemipenal lattice. (h) Enhanced view of left hemipenis of specimen shown in (g). (i) Enhanced view of the hemipenal lattice from specimen shown in (g) and (h). C = cloaca, P = pedicel, blue arrows = sulcus spermaticus. The specimens presented in E and F were validated histologically to have testes and ovaries respectively.
Some variability in the timing of hemipenis regression was observed in stage 14 ZW females. Two specimens from the same clutch exhibited differing phenotypes; one female had begun hemipenis regression with each appendage exhibiting a trilobed appearance (Fig. 4a), while the other female possessed well-formed bilobed hemipenes with a sulcus spermaticus (Fig. 4b,c). There was also a texture on the surface of the hemipenes suggestive of a precursor to the hemipenal lattice observed in the late stage male (Fig. 4b).
Discussion
Our results show that late regression of hemipenes in female Pogona vitticeps is part of a prolonged period of temporary pseudohermaphroditism (TPH) during which ovaries and hemipenes are both present (Figs 1 and 3). This TPH phase was observed in both genetically concordant (ZW) and sex-reversed females (ZZf). Consistent with the other two species of squamates displaying TPH, all males (ZZm) of P. vitticeps displayed a fast succession of gonad development and hemipenis differentiation, as is also the case in most other amniotes that have been studied17,18,34,35,36.
The TPH phase in female development of Pogona vitticeps is the third case of TPH in squamates, out of four species that have been examined to date30,31,32. This raises the possibility that TPH might be relatively common in squamates, and that gonad differentiation and genital development are much less tightly linked than currently thought. A phylogenetically widespread TPH phase (Fig. 1) may also explain why many squamate species display very limited sexual dimorphism at hatching37,38. This further raises the possibility that embryonic or hatchling sex identification in squamates using only genitalia may be incorrect if TPH is not recognised. In addition, the variable timing and duration of the TPH phase hints at an evolutionarily flexible relationship between ovary differentiation and genital formation (Fig. 1). This might be a contributing factor to the very fast evolution of squamate genital morphology34, and may explain the evolution of extreme female phenotypes, such as the strongly developed hemipenes seen in females of two snake species, Pseudoficimia frontalis29,39, and Bothrops insularis40. Thus, a better understanding of the frequency of TPH among squamates, and the mechanisms by which genital development is governed by the ovaries, has the potential to provide a substantial advance in the understanding of squamate (and possibly amniote) sexual evolution. It is also interesting to speculate whether TPH is a trait associated with thermosensitive sex determination, since the only species not to display a prolonged TPH phase (A. carolinensis) is also the only strictly GSD species where this has been studied. While the sex determination mode of B. imbricata is unknown, the only other species in the same family (Anguidae) that has been investigated (the southern alligator lizard, Gerrhonotus multicarinatus) may be GSD with a thermal influence38,41. We have further demonstrated that in GSD species with known thermosensitivity, TPH is associated with both male and female heterogamety (ZW/ZZ system of P. vitticeps; XY/XX of N. ocellatus) (Fig. 1)24,42.
It is unclear what mechanisms are behind the asynchronous internal and external sexual development in squamate TPH, but hormone-related processes likely play an important role. In particular, delayed hormonal secretion could be a main factor in determining TPH of P. vitticeps, as a recent study showed that the estrogen inhibitor fadrozole prevents the hemipenes of P. vitticeps from regressing until after hatching43, suggesting that estrogen is required for the formation of the female genital phenotype. Artificial introduction of testosterone in adult female leopard geckoes (Eublepharis macularius) induces the formation of hemipenes, demonstrating that the genitalia can be sensitive to endogenous hormones44. These findings are consistent with research demonstrating that the gonads of some lacertilians gain the ability to synthesize sex steroids only after hatching45,46,47. It is also possible that TPH may be due to a delay in hormone receptiveness of the genitalia. While little is known about this in squamates, in the one species studied that does not exhibit TPH (Anolis carolinensis), steroid hormone receptors are expressed dimorphically during external genital development, such that embryonic hemipenes express more androgen receptors and female hemiclitores express more estrogen receptors48,49. This might indicate that genital tissues are indeed able to display differential receptiveness to hormones throughout development, adding another layer of complexity to the sexual development of squamates.
In addition to asynchronous gonadal and genital sexual development, we also observed a relatively high frequency of ovotestes during early gonad differentiation in genetically male individuals at sex-reversing temperatures (4 out of 10 of ZZf specimens at stages 9–9.5 at 36 °C). Naturally occurring ovotestes have only been observed in reptiles in isolated cases50,51. However, they have been experimentally induced in one lizard and several turtles with TSD that were incubated at their pivotal temperature (the temperature at which the sex ratio is approximately 50:50) or at fluctuating temperature regimes52,53,54,55. This has led to the suggestion that ovotestes arise in situations where the levels of estrogen and testosterone are similar and acting antagonistically52. However, ovotestes can also be indicative of an epigenetic re-programming event, causing the transition from one sex to another, as has been observed in some sequentially hermaphroditic fish56,57. Additionally, new single cell sequencing techniques have shown that despite apparently committing to a fate, cells can change trajectories during development, suggesting that gonadal differentiation may be quite flexible58,59. Ovotestes in P. vitticeps may be triggered by the initiation of environmental sex reversal via the activation of chromatin modifying genes, allowing ovotestes to develop as a result of simultaneous and competing testicular and ovarian tissue hormonal activity during sex reversal9,11,57,60,61. It may be that ovotestes are more broadly indicative of environmental effects on sex determination, and may facilitate the identification of new thermally sensitive species.
Finally, it is interesting that in all three known cases of sexual development asynchrony, the TPH phase is displayed by females only. Even in A. carolinensis, which does not exhibit a prolonged TPH phase, the hemipenes regress quickly (within half a stage) to form the female hemiclitores29,32,48. The hemipenes of female P. vitticeps resemble those of males in every respect, including the general ultrastructure and the presence of a male-specific functional character (sulcus spermaticus). This is consistent with previous suggestions that female genital phenotypes of amniotes arose through a hormonal modification of the male phallus27,49,62.
This study highlights the need to better understand the nuanced influences of temperature on the development of thermally sensitive species, such as P. vitticeps. This is particularly important given that increasing global temperatures can destabilise population sex ratios in temperature sensitive species worldwide63,64,65,66,67. Thermosensitive sex determination systems, which are being found to be increasingly common, may face additional challenges in a rapidly changing climate20,68. We have also highlighted that a tendency to rely on insufficiently tested assumptions, and a bias towards research on male phenotypes, has resulted in an incomplete view of female squamate sexual development. Further research on the interplay between genetic, hormonal, and environmental determinants of squamate development have the potential to reveal a more complete understanding of the drivers of squamate sexual evolution.
Methods
Embryo sampling
Animal breeding and embryo sampling for the embryological staging series is described in Whiteley et al.27. Briefly, embryos from combinations of high (36 °C) and low (28 °C) incubation temperatures, and genotypes (ZZm, ZZf, ZW) were examined. 296 eggs were obtained from the breeding colony established at the University of Canberra, while an additional 33 eggs were obtained from a commercial breeder. The breeding colony contains a mix of wild caught and lab bred adults of known sex (validated by genotyping and hemipenal eversion; File S1). The sex chromosome complement of all specimens was determined using a sex specific PCR test, with DNA extracted from embryonic blood sampled from the interior of the eggshell, as described previously27.
Histology
To determine the timing of gonad differentiation at the two incubation temperatures (36 °C and 28 °C), specimens were staged according to the system presented in Whiteley et al.27 and sampled at three periods throughout development; early (stages 4–8.5, n = 21), middle (stages 9–12, n = 25), and late (stages 13 onwards, n = 16). Once a mature gonadal phenotype was consistently observed, sampling intensity was lowered. A total of 62 specimens were examined histologically (21 at 28 °C and 41 at 36 °C). The larger sample sizes at sex reversing temperatures ensured sufficient data were collected for morphological changes during sex reversal (File S1).
Specimens were processed following standard histological procedures69. Briefly, the urogenital systems were dehydrated through graduations of ethanol (70%, 90%, 100%) and two changes of xylene for 45 minutes each, before being embedded in paraffin wax, and sectioned 6 µm thick using a Leica Rotary Microtome (Leica Microsystems Pty Ltd, Waverley, Australia). The slides were stained with Meyer’s haematoxylin and eosin (H & E), with a staining time of three minutes in haematoxylin, and 10 dips in 0.25% eosin in 80% ethanol, before being mounted in depex. All slides were analysed using standard light microscopes, and the gonads and accessory ducts were defined using established cellular characteristics described previously for other reptile species16,18,45,52. Detail regarding the development of the accessory ducts is provided in the supplement (Fig. S1).
Scanning electron microscopy
We investigated the degree of homology between the genital structures of males (ZZm), and concordant (ZW) and sex reversed (ZZf) females at the same developmental stage using scanning electron microscopy (SEM). A total of 36 ZW female offspring were incubated at either 28 °C (25 specimens) or 36 °C (11 specimens) and sampled at the same developmental stage (stage 14) across treatments (see File S1). An additional stage 17 male (ZZm) was examined to assess the later structural development of the hemipenes. Specimens were genotyped for the sex specific marker (see above) and a subset were processed for SEM using standard techniques. Briefly, after formalin preservation, the whole genitalia were dissected, dehydrated through graduations of ethanol (70%, 90%, 100%). They were critical point dried and coated with gold according to the manufacturer’s instructions and imaged using a Zeiss EVO LS 15 (Carl Zeiss Pty Ltd, North Ryde, Australia). The late stage 17 ZZm male was critical point dried using hexamethyldisilazane and coated with a 10 nm thick layer of iridium and imaged using a Field Emission Scanning Electron Microscope JEOL JSM-7001 F (JOEL Australasia Pty Ltd, Frenchs Forest, Australia). Sex reversal in ZZ specimens incubated at 36 °C was validated using gonadal histology following the methods described above.
Ethics approval
All experimental protocols were conducted with the permission of Animal Ethics Committees at the University of Canberra (CEAE15-21) and the University of Queensland (SBS/295/16). All experiments were conducted in accordance with guidelines and regulations established by these committees.
Data Availability
All data generated and analysed in this study are available in Supplementary File S1. Registered voucher specimens for each Pogona vitticeps embryonic stage are available for inspection or inter-institutional loan from the Australian National Wildlife Collection, CSIRO (Registration numbers R11229 – R11246).
References
Wake, M. H. Hyman’s Comparative Vertebrate Anatomy. (The University of Chicago Press, 1992).
Wibbels, T., Wilson, C. & Crews, D. Mullerian duct development andregression in a turtle with temperature-dependent sex determination. J. Herpetol. 33, 149–152, https://doi.org/10.2307/1565558 (1999).
Capel, B. Vertebrate sex determination: evolutionary plasticity of a fundamental switch. Nat. Rev. Genet. 18, 675–689, https://doi.org/10.1038/nrg.2017.60 (2017).
Norris, D. L. K. Hormones and reproduction of vertebrates reptiles. Vol. 3 (Elsevier Science, 2011).
Greenbaum, E. & Carr, J. L. Sexual differentiation in the spiny softshell turtle (Apalone spinifera), a species with genetic sex determination. J. Exp. Zool. 290, 190–200 (2001).
Barske, L. A. & Capel, B. Blurring the edges in vertebrate sex determination. Curr. Opin. Genet. Dev. 18, 499–505, https://doi.org/10.1016/j.gde.2008.11.004 (2008).
Shine, R., Warner, D. A. & Radder, R. Windows of embryonic sexual lability in two lizard species with environmental sex determination. Ecology 88, 1781–1788 (2007).
Pieau, C. Temperature variation and sex determination in reptiles. Bioessays 18, 19–26, https://doi.org/10.1002/bies.950180107 (1996).
Deveson, I. W. et al. Differential intron retention in Jumonji chromatin modifier genes is implicated in reptile temperature-dependent sex determination. Science Advances 3 https://doi.org/10.1126/sciadv.1700731 (2017).
Ge, C. et al. The histone demethylase KDM6B regulates temperature-dependent sex determination in a turtle species. Science 360(6389), 645–648 (2018).
Georges, A. & Holleley, C. E. How does temperature determine sex? Science 360, 601–602 (2018).
Bachtrog, D. et al. Sex determination: Why so many ways of doing it? PLoS Biol. 12, e1001899, https://doi.org/10.1371/journal.pbio.1001899 (2014).
Quinn, A. E., Sarre, S. D., Ezaz, T., Marshall Graves, J. A. & Georges, A. Evolutionary transitions between mechanisms of sex determination in vertebrates. Biol. Lett. 7, 443–448, https://doi.org/10.1098/rsbl.2010.1126 (2011).
Rupp, S. M. et al. Evolution of dosage compensation in Anolis carolinensis, a reptile with xx/xy chromosomal sex determination. Genome Biology and Evolution 9, 231–240, https://doi.org/10.1093/gbe/evw263 (2017).
Raman, R. Sex determination and gonadal differentiation in vertebrates: A case for unity in diversity. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 6, 529–546 (2002).
Doddamani, L. S. Differentiation and development of testis in the oviparous lizard, Calotes versicolor (Daud.). J. Exp. Zool. A Comp. Exp. Biol. 305, 299–308 (2006).
DeFalco, T. & Capel, B. Gonad morphogenesis in vertebrates: divergent means to a convergent end. Annu. Rev. Cell. Dev. Biol. 25, 457–482, https://doi.org/10.1146/annurev.cellbio.042308.13350 (2009).
Antonio-Rubio, N. R., Villagrán-SantaCruz, M., Santos-Vázquez, A. & Moreno-Mendoza, N. Gonadal morphogenesis and sex differentiation in the oviparous lizard, Sceloporus aeneus (Squamata: Phrynosomatidae). Zoomorphology 134, 279–289, https://doi.org/10.1007/s00435-015-0259-6 (2015).
Holleley, C. E. et al. Sex reversal triggers the rapid transition from genetic to temperature-dependent sex. Nature 523, 79, https://doi.org/10.1038/nature14574 (2015).
Holleley, C. E., Sarre, S. D., O’Meally, D. & Georges, A. Sex reversal in reptiles: Reproductive oddity or powerful driver of evolutionary change? Sex. Dev. 10, 279–287, https://doi.org/10.1159/000450972 (2016).
Wallace, H., Badawy, G. M. I. & Wallace, B. M. N. Amphibian sex determination and sex reversal. Cell. Mol. Life Sci. 55, 901–909, https://doi.org/10.1007/s000180050343 (1999).
Pandian, T. J. & Sheela, S. G. Hormonal induction of sex reversal in fish. Aquaculture 138, 1–22, https://doi.org/10.1016/0044-8486(95)01075-0 (1995).
Cunningham, G. D., While, G. M. & Wapstra, E. Climate and sex ratio variation in a viviparous lizard. Biol. Lett. 13 (2017).
Ezaz, T. et al. The dragon lizard Pogona vitticeps has ZZ/ZW micro-sex chromosomes. Chromosome Research 13, 763–778 (2005).
Deakin, J. E. et al. Anchoring genome sequence to chromosomes of the central bearded dragon (Pogona vitticeps) enables reconstruction of ancestral squamate macrochromosomes and identifies sequence content of the Z chromosome. BMC Genomics 17, 447, https://doi.org/10.1186/s12864-016-2774-3 (2016).
Georges, A. et al. High-coverage sequencing and annotated assembly of the genome of the Australian dragon lizard Pogona vitticeps. Gigascience 4, 45, https://doi.org/10.1186/s13742-015-0085-2 (2015).
Whiteley, S. L. et al. Sex determination mode does not affect body or genital development of the central bearded dragon (Pogona vitticeps). EvoDevo 8 (2017).
Andrews, R. M. Patterns of embryonic development. (Nottingham University Press, 2004).
Gredler, M. L. et al. Evolution of external genitalia: Insights from reptilian development. Sex. Dev. 8, 311–326, https://doi.org/10.1159/000365771 (2014).
Neaves, L., Wapstra, E., Birch, D., Girling, J. E. & Joss, J. M. Embryonic gonadal and sexual organ development in a small viviparous skink, Niveoscincus ocellatus. J. Exp. Zool. A Comp. Exp. Biol. 305, 74–82, https://doi.org/10.1002/jez.a.249 (2006).
Martínez‐Torres, M., Rubio‐Morales, B., Piña‐Amado, J. J. & Luis, J. Hemipenes in females of the mexican viviparous lizard Barisia imbricata (Squamata: Anguidae): an example of heterochrony in sexual development. Evol. Dev. 17, 270–277, https://doi.org/10.1111/ede.12134 (2015).
Gredler, M. L., Sanger, T. J. & Cohn, M. J. Development of the cloaca, hemipenes, and hemiclitores in the Green Anole. Anolis carolinensis. Sex. Dev. 9, 21–33, https://doi.org/10.1159/000363757 (2015).
Sanger, T. J., Losos, J. B. & Gibson‐Brown, J. J. A developmental staging series for the lizard genus Anolis: A new system for the integration of evolution, development, and ecology. J. Morphol. 269, 129–137, https://doi.org/10.1002/jmor.10563 (2008).
Klaczko, J., Ingram, T. & Losos, J. Genitals evolve faster than other traits in Anolis lizards. J. Zool. 295, 44–48, https://doi.org/10.1111/jzo.12178 (2015).
Cohn, M. J. Development of the external genitalia: Conserved and divergent mechanisms of appendage patterning. Dev. Dyn. 240, 1108–1115, https://doi.org/10.1002/dvdy.22631 (2011).
Valenzuela, N. Sexual development and the evolution of sex determination. Sex. Dev. 2, 64–72, https://doi.org/10.1159/000129691 (2008).
Botterill-James, T. et al. Family aggression in a social lizard. Sci. Rep. 7, 3502, https://doi.org/10.1038/s41598-017-03531-0 (2017).
Telemeco, R. Sex determination in southern alligator lizards (Elgaria multicarinata; Anguidae). Herpetologica 71, 8–11, https://doi.org/10.1655/Herpetologica-D-14-00033 (2015).
Hardy, L. M. Intersexuality in a mexican colubrid snake (Pseudoficimia). Herpetologica 26, 336–343 (1970).
Hoge, A. R., Belluomini, E., Schreiber, G. & Penha, A. Sexual abnormalities in Bothrops insularis (Amaral) 1921 (Serpentes). Mem. Inst. Butantan 29, 17–88 (1959).
The Tree of Sex, C. et al. Tree of Sex: A database of sexual systems. Scientific Data 1 140015, https://doi.org/10.1038/sdata.2014.15 (2014).
Hill, P. L., Burridge, C. P., Ezaz, T. & Wapstra, E. Conservation of sex-linked markers among conspecific populations of a viviparous skink, Niveoscincus ocellatus, exhibiting genetic and temperature-dependent sex determination. Genome Biol Evol 10, 1079–1087, https://doi.org/10.1093/gbe/evy042 (2018).
Ehl, J., Vukić, J. & Kratochvíl, L. Hormonal and thermal induction of sex reversal in the bearded dragon (Pogona vitticeps, Agamidae). Zool. Anz. 271, 1–5, https://doi.org/10.1016/j.jcz.2017.11.002 (2017).
Holmes, M. M., Putz, O., Crews, D. & Wade, J. Normally occurring intersexuality and testosterone induced plasticity in the copulatory system of adult leopard geckos. Horm. Behav. 47, 439–445, https://doi.org/10.1016/j.yhbeh.2004.11.020 (2005).
Doddamani, L. S. Histoenzymological studies on embryonic and posthatching development of the ovary in the tropical oviparous lizard. Calotes versicolor. J. Morphol. 222, 1–10, https://doi.org/10.1002/jmor.1052220102 (1994).
Gaitonde, S. G. & Gouder, B. Y. M. The structure and steroidogenic potential of the developing gonad and interrenals in the tropical oviparous lizard, Calotes versicolor (Daud.). Reprod. Nutr. Dev. 24, 915–926 (1984).
White, R. B. & Thomas, P. Adrenal-kidney and gonadal steroidogenesis during sexual differentiation of a reptile with temperature-dependent sex determination. Gen. Comp. Endocrinol. 88, 10–19, https://doi.org/10.1016/0016-6480(92)90189-Q (1992).
Beck, L. A. & Wade, J. Steroid receptor expression in the developing copulatory system of the green anole lizard (Anolis carolinensis). Gen. Comp. Endocrinol. 157, 70–74, https://doi.org/10.1016/j.ygcen.2008.03.019 (2008).
Holmes, M. M. & Wade, J. Sexual differentiation of the copulatory neuromuscular system in green anoles (Anolis carolinensis): Normal ontogeny and manipulation of steroid hormones. J. Comp. Neurol. 489, 480–490, https://doi.org/10.1002/cne.20645 (2005).
Crespo, J. L. et al. Two cases of pseudohermaphroditism in loggerhead sea turtles Caretta caretta. Dis Aquat Organ 105, 183–191, https://doi.org/10.3354/dao02622 (2013).
Fox, H. In Biology of the Reptilia Vol. 6 (ed C. Gans, Parsons, T. S.) (Academic Press, 1977).
Pieau, C., Dorizzi, M., Richard-Mercier, N. & Desvages, G. Sexual differentiation of gonads as a function of temperature in the turtle Emys orbicularis: Endocrine function, intersexuality and growth. J. Exp. Zool. A Comp. Exp. Biol. 281, 400–408 (1998).
Crews, D. & Bergeron, J. M. Role of reductase and aromatase in sex determination in the red-eared slider (Trachemys scripta), a turtle with temperature-dependent sex determination. J. Endocrinol. 143, 279–289 (1994).
Sreenivasulu, K., Ganesh, S. & Raman, R. Evolutionarily conserved, DMRT1, encodes alternatively spliced transcripts and shows dimorphic expression during gonadal differentiation in the lizard. Calotes versicolor. Mech. Dev. 119, S55–S64, https://doi.org/10.1016/S0925-4773(03)00092-3 (2002).
Inamdar Doddamani, L. S., Vani, V. & Seshagiri, P. B. A tropical oviparous lizard, Calotes versicolor, exhibiting a potentially novel FMFM pattern of temperature-dependent sex determination. J Exp Zool A Ecol Genet Physiol 317, 32–46, https://doi.org/10.1002/jez.718 (2012).
Liu, J. et al. Dynamic evolution and biogenesis of small RNAs during sex reversal. Sci. Rep. 5, 9999, https://doi.org/10.1038/srep09999 (2015).
Zhang, Y., Zhang, S., Liu, Z., Zhang, L. & Zhang, W. Epigenetic modifications during sex change repress gonadotropin stimulation of cyp19a1a in a teleost ricefield eel (Monopterus albus). Endocrinology 154, 2881–2890, https://doi.org/10.1210/en.2012-2220 (2013).
Farrell, J. A. et al. Single-cell reconstruction of developmental trajectories during zebrafish embryogenesis. Science 360(6392), 3131 (2018).
Wagner, D. E. et al. Single-cell mapping of gene expression landscapes and lineage in the zebrafish embryo. Science 360(6392), 981–987 (2018).
Kuroki, S. et al. Epigenetic Regulation of Mouse Sex Determination by the Histone Demethylase Jmjd1a. Science 341, 1106 (2013).
Navarro-Martin, L. et al. DNA methylation of the gonadal aromatase (cyp19a) promoter is involved in temperature-dependent sex ratio shifts in the European sea bass. PLoS Genet. 7, e1002447, https://doi.org/10.1371/journal.pgen.1002447 (2011).
Yang, J. H., Menshenina, J., Cunha, G. R., Place, N. & Baskin, L. S. Morphology of mouse external genitalia: implications for a role of estrogen in sexual dimorphism of the mouse genital tubercle. J. Urol. 184, 1604–1609, https://doi.org/10.1016/j.juro.2010.03.079 (2010).
Jensen, M. P. et al. Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr. Biol. 28, 154–159.e154, https://doi.org/10.1016/j.cub.2017.11.057 (2018).
Hulin, V., Delmas, V., Girondot, M., Godfrey, M. & Guillon, J.-M. Temperature-dependent sex determination and global change: are some species at greater risk? Oecologia 160, 493–506, https://doi.org/10.1007/s00442-009-1313-1 (2009).
Mitchell, N. J. & Janzen, F. J. Temperature-dependent sex determination and contemporary climate change. Sex. Dev. 4, 129–140, https://doi.org/10.1159/000282494 (2010).
Neuwald, J. L. & Valenzuela, N. The lesser known challenge of climate change: Thermal variance and sex-reversal in vertebrates with temperature-dependent sex determination (thermal fluctuations reverse turtle sex ratios). PLoS ONE 6, e18117, https://doi.org/10.1371/journal.pone.0018117 (2011).
Refsnider, J. M. & Janzen, F. J. Temperature-dependent sex determination under rapid anthropogenic environmental change: evolution at a turtle’s pace? J. Hered. 107, 61–70, https://doi.org/10.1093/jhered/esv053 (2016).
Bókony, V., Kövér, S., Nemesházi, E., Liker, A. & Székely, T. Climate-driven shifts in adult sex ratios via sex reversals: the type of sex determination matters. Philosophical Transactions of the Royal Society B: Biological Sciences 372 (2017).
Cardiff, R. D., Miller, C. H. & Munn, R. J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc 2014, 655–658, https://doi.org/10.1101/pdb.prot073411 (2014).
Pyron, R. A., Burbrink, F. T. & Wiens, J. J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 13, 93, https://doi.org/10.1186/1471-2148-13-93 (2013).
Acknowledgements
We thank Dr Rosemary White for her assistance in producing scanning electron micrographs at CSIRO’s Black Mountain Microimaging Centre. We thank Anne Prins of the Imaging and Cytometry Facility, John Curtin School of Medical Research, ANU for expert histology advice and sample and slide preparation. We also thank Dr Wendy Ruscoe at the University of Canberra’s Animal House Facility for her animal husbandry expertise. This research was funded by CSIRO strategic funding to CEH, UQ start-up funding to VW, Australian Research Council Discovery Grants DP110104377 and DP170101147 led by AG.
Author information
Authors and Affiliations
Contributions
S.L.W. carried out all experiments, collected and analysed the data, conducted all photographic imaging and interpreted histology and SEM results. S.W. and C.E.H. prepared the figures. V.W. and C.E.H. contributed equally to the study by conceiving of the experiments and co-analysing the data. A.G. oversaw the collection of animals, established egg incubation protocols, and made available the necessary facilities. A.R.G.G. assisted with optimising histology and SEM protocols and interpreting the data. D.W. assisted with specimen preparation, histological processing, and made available the necessary facilities. S.L.W., C.E.H., V.W. and A.G. wrote the manuscript with input from all other authors. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Whiteley, S.L., Weisbecker, V., Georges, A. et al. Developmental asynchrony and antagonism of sex determination pathways in a lizard with temperature-induced sex reversal. Sci Rep 8, 14892 (2018). https://doi.org/10.1038/s41598-018-33170-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-33170-y
Keywords
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.