Abstract
Antigens (Ags) in Mycobacterium tuberculosis (Mtb) that are constitutively expressed, overexpressed during growth, essential for survival, and highly conserved may be good vaccine targets if they induce the appropriate anti-Mtb Th1 immune response. In this context, stress response-related antigens of Mtb might serve as attractive targets for vaccine development as they are rapidly expressed and are up-regulated during Mtb infection in vivo. Our group recently demonstrated that GrpE, encoded by rv0351 as a cofactor of heat-shock protein 70 (HSP70) in the DnaK operon, is a novel immune activator that interacts with DCs to generate Th1-biased memory T cells in an antigen-specific manner. In this study, GrpE was evaluated as a subunit vaccine in comparison with the well-known HSP70 against the hyper-virulent Mtb Beijing K-strain. Both HSP70- and GrpE-specific effector/memory T cells expanded to a similar extent as those stimulated with ESAT-6 in the lung and spleen of Mtb-infected mice, but GrpE only produced a similar level of IFN-γ to that produced by ESAT-6 stimulation during the late phase and the early phase of Mtb K infection, indicating that GrpE is highly-well recognised by the host immune system as a T cell antigen. Mice immunised with the GrpE subunit vaccine displayed enhanced antigen-specific IFN-γ and serum IgG2c responses along with antigen-specific effector/memory T cell expansion in the lungs. In addition, GrpE-immunisation markedly induced multifunctional Th1-type CD4+ T cells co-expressing IFN-γ, TNF-α, and IL-2 in the lungs of Mtb K-infected mice, whereas HSP70-immunisation induced mixed Th1/Th2 immune responses. GrpE-immunisation conferred a more significant protective effect than that of HSP70-immunisation in terms of bacterial reduction and improved inflammation, accompanied by the remarkable persistence of GrpE-specific multifunctional CD4+ T cells. These results suggest that GrpE is an excellent vaccine antigen component for the development of a multi-antigenic Mtb subunit vaccine by generating Th1-biased memory T cells with multifunctional capacity, and confers durable protection against the highly virulent Mtb K.
Similar content being viewed by others
Introduction
Although the prevention of tuberculosis (TB) is the most effective control measure to reduce the incidence of TB, the protection efficacy of bacillus Calmette-Guerin (BCG), the only currently available licensed TB vaccine1, is thought to be insufficient to protect against pulmonary TB and latent infection. In addition, variable results of BCG vaccine efficacy for different geographical locations have been reported because Mycobacterium tuberculosis (Mtb) genotypes with different virulence levels may be dominant in different regions2,3,4. Importantly, the Mtb Beijing genotype is highly prevalent in East Asian countries including China, Korea, and Japan and the isolation rate of strains belonging to the Mtb Beijing family has increased worldwide, indicating that BCG vaccine provides a relatively low level of protection against this Mtb genotype5,6. Furthermore, epidemiological studies have recently suggested that continuous BCG vaccination may have driven the emergence of the Beijing genotype6. Thus, the control of Mtb Beijing strains is a major challenge and is urgently needed globally.
To develop new prophylactic vaccines capable of replacing and/or boosting the BCG vaccine, many vaccine candidates have entered into the clinical7,8. In particular, prophylactic vaccines require Ag targets that are expressed during the early phase of infection and are recognised by the host immune system to immediately initiate host defense mechanisms9,10.
It is well documented that host defense against Mtb infection is strongly associated with the expansion and generation of Mtb-specific multifunctional Th1-type T cell subsets, in concert, to activate macrophages, thereby limiting mycobacterial replication during the early infection period11,12. Thus, the identification of Ags triggering protective T cell subsets at the early infection course is a goal of TB vaccine development. In this regard, Ags in Mtb that are constitutively expressed, overexpressed during growth, essential for survival and growth, and highly conserved may be good vaccine targets if they induce a prompt anti-Mtb Th1 immune response13,14. Following this rationale, many Mtb vaccine Ag targets, including Ag85 complex antigens, ESAT-6 (early secreted anti-genic target-6), and heat shock proteins (HSPs), have been evaluated as target vaccine antigens because they are abundantly expressed and induce a strong cell-mediated immune defense response by evoking T-cell proliferation and IFN-γ production in an antigen-specific dependent manner, especially during the early phase of Mtb infection15,16.
Accordingly, stress response-related antigens of Mtb are attractive targets for vaccine development, as they are rapidly expressed and up-regulated during Mtb infection17,18. HSPs are essential molecular chaperones for the maintenance of cellular functions in normal as well as stress conditions19. The regulation of the expression of HSPs plays an important role in the pathogenesis of Mtb20. Among mycobacterial HSPs, Mtb HSP70 is the most well-known Ag linking innate and adaptive immune responses with potent adjuvant activity21. In Mtb, the hsp70 operon consists of the dnaK-grpE-dnaJ (rv0350-rv0351-rv0352) genes and is co-expressed and negatively regulated by the repressor hspR (rv0353)22. The Dnak operon in mycobacterial species is essentially required for cell growth, even in the absence of stress, indicating that it is a promising target for the development of antibiotics and vaccines against mycobacteria23. Notably, due to the host immune response to HSPs, the constitutive over-expression of Mtb HSP70 results in the reduced survival of Mtb during prolonged infection24, indicating that it may be possible to design interventions capable of promoting an immediate and durable host immune response to control Mtb.
Although the role of HSP70 in the immune response to Mtb infection has been studied extensively, relatively little is known about its cofactor, GrpE, especially with respect to its immunological functions and TB vaccine potential. In addition, HSP70 and GrpE were recently identified as potential vaccine candidates in an in silico analysis of open reading frames in the Mtb genome25. Importantly, our group recently showed that Th1-type T-cell immunity-biasing effect of GrpE by intercommunicating with dendritic cells26.
In the present study, the immunogenicity and the potential protective efficacy of stress antigens in HSP70 and its co-factor GrpE in the DnaK operon were assessed in a head-to-head format in a Mtb Beijing strain challenge model by delivery as subunit vaccines. We found that GrpE is rapidly recognised by the host immune system and produces a similar level of IFN-γ to that produced by ESAT-6-stimulation during Mtb K infection. Following the challenge with Mtb K, the GrpE-subunit vaccine showed an equivalent protective efficacy to BCG immunisation against Mtb K by rapidly recruiting and activating GrpE-specific multi-functional CD4+ T cells in the lungs pre- and post-challenge, along with the expansion of antigen-specific Th1 and effector/memory T cells in the lungs of Mtb-infected mice. Thus, our results suggest that GrpE is a possible component of future multi-antigenic vaccines and therefore is a candidate for a novel TB vaccine.
Results
Purification of recombinant proteins
For the purification of recombinant proteins, HSP70 and GrpE were expressed with a 6× histidine-tag in E. coli BL21 (Fig. 1a). Both recombinant proteins were purified as soluble forms; SDS-PAGE revealed that the molecular weights of HSP70 and GrpE were around 70 kDa and 32 kDa, respectively. The purified protein showed the expected molecular mass based on SDS-PAGE. Protein purification was ultimately confirmed by Western blotting. The endotoxin contents were measured by an LAL assay and were undetectable by showing below 10 pg/mL (<0.1 EU/mL) in both antigen preparations. These results indicate that HSP70 and GrpE were successfully purified for subsequent experiments.
IFN-γ production and memory T cell phenotypes induced by recombinant antigen stimulation in the lung and spleen of Mtb K-infected mice. (a) HSP70 and GrpE antigens were successfully induced in E. coli BL21 by supplementation with 1 mM isopropyl β-d-thiogalactoside (IPTG) for 12 h. (a, 1 and 4) The expression of N-terminal His-tagged antigens was identified by SDS-PAGE (M, molecular weight markers; Lane 1, uninduced; Lane 2, induced). (a, 2 and 5) SDS–PAGE analysis of purified antigens by Ni-nitrilotriacetic acid (NTA). (a, 3 and 6) Western blot analysis of purified antigens using mouse anti-His antibodies. (b) HSP70 (10 μg/mL)- and GrpE (10 μg/mL)-specific IFN-γ production were measured in the spleen and lung cells of individual mice 4 and 8 weeks after challenge with aerosolised Mtb K strain (n = 4 mice/group). NI: Non-infected mice, K: Mtb K-infected mice. ESAT-6 (1 μg/mL) was used as a positive control. Data from one of two independent experiments are shown. (c) At the same time point, spleen and lung cells from Mtb K-infected mice (n = 4 mice) were stimulated in vitro with antigens, and numbers of CD4+ effector/memory and effector T cells were analysed by flow cytometry. Data from one of two independent experiments are shown. All results are expressed as means ± SD and statistical significance (*p < 0.05, **p < 0.01, or ***p < 0.001) is shown for treatments compared to the non-treated each T cells (non; effector or effector/memory T cells, respectively). The value of n.s. was defined as no significant effect.
HSP70 and GrpE induced an antigen-specific IFN-γ response and effector/memory T cell expansion in Mtb K-infected mice upon ex vivo re-stimulation
Many intracellular bacterial infections induce memory T cell and antigen-specific T cell responses by bacterial antigens27. Prior to evaluating the efficacy of the two vaccine antigen candidates, we investigated whether HSP70 and GrpE recognition by the host immune system can induce an immunological T cell activation along with memory response during the course of Mtb infection9. We therefore analysed the antigen-specific IFN-γ response (Fig. 1b) and the generation of effector or effector/memory CD4 T cells (Fig. 1c) by HSP70, GrpE, or ESAT-6 (as a positive control) stimulation in T cells of Mtb-infected mice. Single cell suspensions from the lung and spleen of Mtb K-infected mice were prepared at 4 and 8 weeks post-infection. The cells were stimulated with HSP70 and GrpE for 24 h, and IFN-γ production was analysed by ELISA (Fig. 1b). As shown in Fig. 1b, lung and spleen cells from the Mtb K-infected mice show higher levels of IFN-γ in response to GrpE stimulation compared to those for HSP70 stimulation. Also, we measured effector (CD44+CD127+CD62L− cells) and effector/memory (CD44+CD127+CD62L+ cells) via the surface expression of CD44 and CD127 on CD3+CD4+-gated CD62L− by flow cytometry (Fig. 1c; top panel). Both HSP70 and GrpE stimulation substantially increased effector/memory T cells, but there was no difference between HSP70 and GrpE stimulation (Fig. 1c; bottom panel). Taken together, HSP70 and GrpE are immunologically recognised during Mtb K infection; thus, Ag-specific memory T cells are generated.
Immunogenicity and immunological memory induced by HSP70 and GrpE immunisation
Incomplete Freund’s adjuvant (IFA), which lacks mycobacterial components, was used in this study. We first investigated the IFN-γ response of T cells after BCG and IFA-based antigen immunisation. After the final immunisation, we assessed IFN-γ responses (Fig. 2b,c), as well as the up-regulation of antigen-specific IFN-γ-producing memory Th1 cells (Fig. 2d,e) upon cognate antigen stimulation in the spleen and lung cells of immunised mice. Purified protein derivative (PPD) was used as a stimulant for the T cells of the BCG vaccine-immunised mice. Antigen-specific IFN-γ+ T cells were evaluated by T cell specific markers (CD3+CD4+ and CD3+CD8+) and activated or a memory Th1 cell marker (CD44high) using flow cytometry (Fig. 2d). BCG-, HSP70- and GrpE-immunisation induced IFN-γ release (Fig. 2b,c) and the number of IFN-γ+CD44highCD4+ and CD8+ T cells (Fig. 2e) in response to re-stimulation with PPD, HSP70, and GrpE were measured. A further analysis of the antigen-specific IgG1 response, an indicator of the Th2 response, showed that the HSP70-immunised groups exhibited a HSP70-specific IgG1 response. Additionally, all groups exhibited an elevated IgG2c response, an indicator of the Th1 response, compared with that of adjuvant control groups (Fig. 2f).
Immunogenicity in the lung and spleen of HSP70- and GrpE-immunised mice. (a) Schematic diagram of the subunit vaccine experimental design. (b,c) Mice (n = 5 mice/group) were immunised with BCG (Red bars), HSP70 (Black bars), GrpE (Blue bars), or adjuvant alone (Green bars), and their spleen and lung cells were stimulated in vitro with no antigen, PPD, HSP70, or GrpE 4 weeks after the last vaccination. IFN-γ secretion by spleen and lung cells in response to PPD (b,c), HSP70 (b) and GrpE (c) stimulation was analysed by ELISA. (d) An example of the gating strategy for the analysis of antigen-specific IFN-γ producing T cells among lung T cells of one representative mouse from the GrpE-immunised group is shown. (e) The percentages of PPD-, HSP70- or GrpE-specific IFN-γ-producing CD3+CD4+CD44high and CD3+CD8+CD44high T cells were analysed in cells isolated from the spleen and lungs of BCG- and antigen-immunised mice (n = 5 mice/group). (f) Serum levels of antigen-specific IgG1 and IgG2c at 4 weeks after the final immunisation (n = 5 mice/group). Data from one of two independent experiments are shown. *p < 0.05, **p < 0.01, or ***p < 0.001, compared to the adjuvant control group (Green bars).
Protective efficacy of the antigen vaccine in the Mtb K-infected mouse model
Four weeks after the last immunisation, the ability of protein vaccines to protect against Mtb K was analysed by aerosol challenges. For the histopathological analysis, lungs were isolated and stained with H&E at 4 and 8 weeks post-infection. The lungs of adjuvant alone-, HSP70-, GrpE-, and BCG vaccine-immunised groups (as a positive control for the efficacy testing of TB vaccines in this study) were analysed with respect to histology (Fig. 3a,b) and bacterial burden (Fig. 3c). Lung tissues from GrpE- and BCG vaccine-immunised mice 4 and 8 weeks after Mtb K challenge clearly showed reduced granulomatous inflammation compared to the lung tissues of the adjuvant control group, along with the microscopic appearance (Fig. 3b). However, a reduction of granulomatous inflammation via HSP70 immunisation was only observed at 4 weeks post-infection. In addition, with respect to bacterial burden, the group immunised with GrpE showed a significant reduction of CFUs in the lung and spleen at 4 and 8 weeks after Mtb K challenge compared to the adjuvant alone group. HSP70 immunisation had a minor effect on the bacterial burden in lung and spleen (Fig. 3c). However, 8 weeks post the Mtb K challenge, the degree of pathological improvement observed after GrpE vaccination was lesser than that observed after BCG immunisation (Fig. 3b).
Protective efficacy of antigen immunisation against Mtb K infection. (a) H&E-stained sections (n = 5 mice/group) of the lung for each immunised mouse (Adjuvant alone, BCG, HSP70, or GrpE) at 4 and 8 weeks after challenge with the aerosolised Mtb K strain. One BCG vaccinated group was included as a positive control. (b) Lung inflammation values are presented as the mean percentages of the area of inflammation from lung sections of infected mouse groups. (c) The differences in bacterial numbers between vaccinated groups and adjuvant control groups at 4 and 8 weeks after challenge are shown. Data from one of two independent experiments are shown. *p < 0.05, **p < 0.01, or ***p < 0.001, when compared to adjuvant control group; green bars or plots (a one-way ANOVA followed by Dunnett’s test). #p < 0.05 (BCG vs. GrpE; unpaired t-test).
GrpE immunisation dominantly increases the Th1 immune response during the course of Mtb K infection, while HSP70 immunisation simultaneously induces mixed Th1/Th2 responses
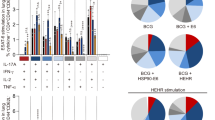
Because a decrease in multifunctional T cells is associated with increased bacterial growth in active Mtb-infection models28, both the Th1-type immune response and multifunctional T cells may be potential correlates of an effective host-protective immune response in TB29. Base on established the methodology from the previous study for multiufnctionality of T cells30, we next assessed the T cell phenotype and immune responses that are induced following antigen vaccination. At 4 and 8 weeks after the challenge, lung cells (top panel of Figs 4a and 5a) and splenocytes (bottom panel of Figs 4a and 5a) were stimulated in vitro with PPD, HSP70 or GrpE and the phenotypes of CD4+ (Fig. 4) and CD8+ (Fig. 5) antigen-specific T cells were evaluated by multi-colour intracellular cytokine staining and flow cytometry (Supplementary Fig. S1). Culture supernatants containing the secreted cytokines were collected from splenocytes and lung cells after antigen re-stimulation and analysed by ELISA (Fig. 6). As a consequence, the groups immunised with BCG, HSP70, or GrpE exhibited robust responses against their respective antigens after re-stimulation. Interestingly, increased levels of triple-positive (IFN-γ+TNF-α+IL-2+) and double-positive (IFN-γ+TNF-α+) antigen-specific CD4+CD44high T cells were observed in the BCG-, GrpE-immunised group compared to the control groups in the spleen and lung at 4 and 8 weeks post-infection (Fig. 4), and triple-positive (IFN-γ+TNF-α+IL-2+) and double-positive (IFN-γ+TNF-α+ and TNF-α+IL-2+) antigen-specific CD8+CD44high T cells were observed in the lung at 4 weeks post-infection. In the BCG-immunised groups, the cells were well-maintained for up to 8 weeks post infection (Fig. 5). However, in the case of the HSP70-immunised group, cytokine-secreting CD4+ and CD8+ T cells were significantly decreased in the lung and spleen compared with those of the BCG- or GrpE-immunised group (Figs 4 and 5). Furthermore, the lung and spleen of mice immunised with HSP70 induced Th1-related cytokines together with Th2-related cytokines, such as IL-5 and IL-10 (Fig. 6a), whereas BCG and GrpE-immunisation induced a significantly higher frequency of Th1-related cytokines, including TNF-α, IFN-γ, and IL-2 (Fig. 6b). Interestingly, antigen-specific IgG1 and IgG2c responses did not differ significantly between adjuvant control groups and antigen-immunised groups after Mtb infection (data not shown). These findings suggest that GrpE immunisation induces a greater Th1 response than HSP70 immunisation, but not a humoral response.
Antigen-specific multifunctional CD4+ T cell responses in antigen-immunised mice after challenge with Mtb K. BCG-, HSP70-, GrpE-, or adjuvant alone-immunised mice were infected by aerosol exposure to Mtb K. At different time points after infection, as indicated, mice (n = 5 mice/group at 4 and 8 weeks post-infection) were euthanised and their lung cells (a and b; top panel) and splenocytes (b; bottom panel) were stimulated in vitro with PPD (10 μg/mL), HSP70 (10 μg/mL) or GrpE (10 μg/mL). (a) The percentage of antigen-specific CD4+CD44high T cells producing IFN-γ, TNF-α, or IL-2 was measured by use of the gating strategy shown in Supplementary Fig. S1, in cells isolated from lung cells and splenocytes at 4 and 8 weeks after challenge with Mtb K strain. (b) The pie charts present the mean frequencies of cells co-expressing IFN-γ, TNF-α, and/or IL-2. The cytokine profiles for individual cells were analysed by multi-colour flow cytometry by gating for lymphocytes, CD3+CD4+CD44high. Data from one of two independent experiments are shown. *p < 0.05, **p < 0.01, or ***p < 0.001 relative to the control groups, respectively.
Antigen-specific multifunctional CD8+ T cell responses in antigen-immunised mice after Mtb K challenge. (a) The percentage of antigen-specific CD8+CD44high T cells producing IFN-γ, TNF-α, or IL-2 measured in cells isolated from the lung (a and b; top panel) and splenocytes (a and b; bottom panel) of vaccinated mice (n = 5 mice/group at 4 and 8 weeks post-infection) at 4 and 8 weeks after challenge with Mtb K. The pie charts present the mean frequencies of cells co-expressing IFN-γ, TNF-α, and/or IL-2. The cytokine profiles for individual cells were analysed by multi-colour flow cytometry by gating for lymphocytes, CD3+CD8+CD44high. Data from one of two independent experiments are shown. *p < 0.05, **p < 0.01, or ***p < 0.001 relative to the control groups, respectively.
T cell immunity induced by antigen immunization. (a,b) Spleen and lung cells from BCG-, adjuvant alone-, HSP70-, and GrpE-immunised mice (n = 5 mice/group at 4 and 8 weeks post-infection in each group) were stimulated with PPD, HSP70, or GrpE, and the supernatant was collected and assayed for cytokines by ELISA. Antigen-specific cytokine responses are shown for spleen and lung cells collected post-infection (4 and 8 weeks). Data from one of two independent experiments are shown. *p < 0.05, **p < 0.01, or ***p < 0.001 by unpaired t-tests.
Discussion
Inside host cells such as macrophages and dendritic cells, Mtb may experience many harsh conditions. In particular, Mtb Ags play important roles in cell-to-cell communication, detoxification, eliciting host immune responses, and essentially aiding in Mtb growth. They are of great interest as vaccine antigen targets because their expression is obligatory for Mtb survival and they are likely to be recognised by host immune cells.
Overall, effective Ag targets for the rational design of Mtb vaccines should satisfy the following requirements: constitutive expression, accessibility (cell wall-associated or secreted) to Ag-presenting cells (APCs), recognised by the immune system during the early infection phase in vivo, ability to induce a Th1-biased memory immune response, and eventual efficacy against hyper-virulent Mtb strains. In this study, we evaluated antigens in the DnaK operon as vaccine candidates that fulfil these criteria and are effective against challenge with the highly virulent Mtb K strain in a head-to-head comparison format.
In vaccine design, early protection by the rapid recruitment of IFN-γ-producing CD4+ effector T cells to the infected site is crucial because once acquired immunity is initiated, Mtb growth is inhibited31. For example, ESAT-6 is recognised promptly by T cells with strong immunogenicity at the early phase of infection and is a major antigen component of many prophylactic vaccine candidates16,32. However, it is believed that ESAT-6 is a key factor in diagnostic tools33,34, indicating that ESAT-6-based vaccines may be difficult for differentiation between naturally infected subjects and vaccinated people. Thus, a novel T cell antigen recognised by the host immune system at the early phase of infection is required for TB vaccine development, without interfering with diagnosis. In the present study, we first investigated whether HSP70 and GrpE are recognised by the host immune system during the early (4 weeks) and late (8 weeks) phases of Mtb infection by comparisons with ESAT-6. In our study, IFN-γ production (Fig. 1b) and CD4+ memory T cell expansion (Fig. 1c) were observed in spleen and lung cells isolated from mice treated with HSP70 and GrpE at various time points after infection with Mtb K, indicating that both antigens are recognised by the host immune system as immunogenic antigens. In particular, GrpE exhibited a comparable IFN-γ response to that of ESAT-6, the most well-characterised immunogenic Mtb Ag at the active phase of Mtb infection35, suggesting that GrpE is promptly recognised by the host immune system.
In fact, grpE is an essential gene, as determined by Himar1-based transposon mutagenesis, in the H37Rv strain and is highly conserved in all Mtb strains, with only a single gene copy36,37,38. In addition to the constitutive overexpression of grpE, basal grpE expression is essentially required for the survival and growth of Mtb, indicating that the obligatory expression of GrpE occurs during Mtb infection. Moreover, the over-expression of GrpE was identified in the most prevalent clinical Mtb strains from South India under hypoxic culture conditions compared to aerated cultures, while other HSPs, including HSPX, GroEL2, and GroES, are more highly expressed than the expression in the laboratory-adapted strain H37Rv, indicating that GrpE is up-regulated during clinical Mtb infection and may have vaccine potential39.
In general, high CD44 expression on T cell surfaces indicates that the T cells are antigen-experienced (i.e., they exhibit an antigen-specific memory phenotype)30,40. In addition, a recent study has shown that CD44 is essential for the generation and maintenance of memory T helper 1 (Th1) cells41. Interestingly, HSP70- and GrpE-immunised mice showed increased populations of IFN-γ+CD44high T cells in both the lung and spleen (Fig. 2e). Notably, both serum IgG1 and IgG2c, which are representative antibody isotypes for Th2 and Th1 responses42, respectively, increased in HSP70-immunised mice, but only the IgG2c antibody response increased significantly in GrpE-immunised mice (Fig. 2f). The C-terminal portion of the heat shock protein Hsp70 has an adjuvant function by stimulating Th1-polarizing cytokines in human monocytes to produce IL-12, TNF-α, NO, and C-C chemokines43. Furthermore, Mtb HSP70 induces the expression of IL-10 and inhibits T-cell proliferation in vitro, indicating that HSP70 has immunomodulatory properties, rather than inflammatory potential44.
Based on these results, we evaluated and compared the protective effects of HSP70 and GrpE subunit vaccines against the Mtb K strain in a mouse model. Interestingly, GrpE immunisation displayed a protective effect comparable to that of the BCG vaccine in terms of CFU reduction, while HSP70 showed marginal protection, indicating that GrpE showed better protection than HSP70 after challenge with the Mtb K strain based on assessments of lung pathology (Fig. 3a,b) and the reduction of bacterial CFUs (Fig. 3c). However, the mice in the BCG vaccination group showed a more substantial CFU reduction (spleen, 8 weeks post infection, p < 0.05) and attenuated lung inflammation, compared to the single GrpE-immunised mice (Fig. 3; lungs, 8 weeks post infection, p < 0.05). These results suggest that the live BCG vaccine contains many Ags that may augment the functions of a variety of agents of the protective T cell repertoire, including the antigen 85 complex45,46.
Recently, Forbes et al. showed that multifunctional abilities of T cells are associated with protection against Mtb infection in a mouse model47. In addition, a continuous decrease in the multifunctionality of T cells corresponds with a decrease in protection against challenge with Mtb in the mouse model29. Thus, we used multi-parameter flow cytometry to analyse CD4+ and CD8+ T cells at infectious site after immunisation. In this study, the GrpE immunisation remarkably induced a high frequency of multifunctional CD4+CD44high T cells (IFN-γ+TNF-α+IL-2+, IFN-γ+TNF-α+, and TNF-α+IL-2+) in the spleen and lung, while less induction was observed in the HSP70-immunised group (Figs 4 and 5). In addition, in GrpE-immunised mice, significantly higher levels of Th1-related cytokines were induced, including TNF-α, IFN-γ, and IL-2, while HSP70-immunised mice exhibited the induction of Th1-related cytokines along with Th2-related cytokines, such as IL-5 and IL-10 (Fig. 6). In general, the Th2 type immune response is reported to be harmful for immunity against Mtb infection. Aron et al. reported that the adoptive transfer of Th2 cells leads to an increase in CFUs of Mtb in the lung48. In addition, mixed Th1/Th2 immune responses occur in patients with active tuberculosis49. Thus, the mixed Th1/Th2 immune response and IgG1/IgG2c antibody responses and immune responses may be responsible for a relatively lower level of protection in the HSP70-immunised group; the robust Th1 immune response induced by GrpE immunisation was associated with a high level of protection against Mtb K infection.
Taken together, GrpE is recognised by the immune system at the early phase of infection and has the ability to induce a robust and durable Ag-specific multifunctional Th1-type T-cell memory response, conferring protective immunity and imparting significant protection in a murine model against the Mtb K strain. These results indicate that GrpE is a potential Ag target for the development of future multi-antigenic vaccines against Mtb, especially against highly virulent Mtb Beijing strains. Furthermore, the efficacy of a BCG priming vaccination combined with a GrpE subunit vaccine should be investigated to determine whether the GrpE subunit vaccine can boost the efficacy of the BCG vaccine.
Methods
Ethics statement and animals
All animal experiments were conducted following the guidelines provided by the Korean Food and Drug Administration (KFDA). The experimental procedures were evaluated and approved by the Institutional Animal Care and Use Committee (Permit Number: 2015-0041) of Yonsei University Health System (Seoul, Korea). After obtaining permission for the experiments, specific pathogen-free (SPF) female C57BL/6J mice at 6–7 weeks of age were purchased from Japan SLC, Inc. (Shizuoka, Japan) and maintained under barrier conditions in a ABSL-3 facility at the Avison Biomedical Research Center in Yonsei College of Medicine. The animals were fed a sterile commercial mouse diet and provided water ad libitum.
Expression and purification of recombinant proteins
Cloning was conducted in Escherichia coli (E. coli) DH5α. rv0350 and rv0351 were amplified from Mtb H37Rv (ATCC 27294) genomic DNA by polymerase chain reaction (PCR). The rv0350 primers were as follows: 5′-GGGCCCCATATGGCTCGTGCGGTCGGGATC-3′ (forward primer; the NdeI site is underlined) and 5′-GGGCCCAAGCTTCTTGGCCTCCCGGCCGTCGTC-3′ (reverse primer; the HindIII site is underlined). The rv0351 primers were as follows: 5′-CGCCATATGGTGACGGACGGAAATCAAAAGC-3′ (forward primer; the NdeI site is underlined) and 5′-CCCAAGCTTACTGCCCGACGGTTCTGATTC-3′ (reverse primer; the HindIII site is underlined). The amplified PCR products were inserted into the pGEMT Easy Vector and sequences of the plasmids with rv0350 and rv0351 insertions were analysed. Finally, these genes were ligated into pET22b (Novagen, Madison, WI, USA) and transferred to E. coli BL21 cells.
Expression and purification of proteins
The expression and purification of recombinant proteins were performed as described previously50. For protein expression, transformed E. coli (pET22b-rv0350 and pET22b-rv0351) and vector controls (pET22b in E. coli BL21 cells) were incubated in LB broth supplemented with 50 μg/mL ampicillin and 1 mM isopropyl-β-d-thio-galactoside (IPTG) at 37 °C for 12 h. To confirm the protein expression and purification, each step was evaluated by 12% SDS-PAGE with Coomassie brilliant blue staining and immunoblotting using an anti-His antibody (Lab Vision, Fremont, CA, USA). To remove endotoxin contamination, the dialyzed proteins were incubated with polymyxin B-agarose (Sigma, St. Louis, MO, USA) and analysed using the limulus amebocyte lysate (LAL) test (Lonza, Lockland, ME, USA), according to the manufacturer’s instructions. The purity of antigens was quantified using Quantity One (Bio-Rad, Hercules, CA, USA); values were obtained by dividing the intensity per square millimeter of the HSP70- and GrpE-specific bands by that of all protein bands in the preparation lane.
Bacteria and culture condition
Mtb H37Rv (ATCC 27294) was purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA), and the Mtb K strain was obtained from the strain collections at the Korean Institute of Tuberculosis (KIT, Osong, Chungchungbuk-do, Korea). BCG (Pasteur 1173P2) was kindly provided by the Pasteur Institute (Paris, France). The mycobacterial strains used in this study were cultured as described previously51. Enumerated mycobacteria were used for subsequent experiments.
Analysis of memory responses induced by antigen stimulation in T cells during the course of Mtb infection
C57BL/6 mice were aerogenically infected with Mtb K as previously described52. The bacteria were counted 1 day after aerosol exposure; approximately 150 viable bacteria were found to be delivered to the lungs. 4 and 8 weeks post infection, single-cell suspensions from the spleen and lungs of Mtb K-infected mice were prepared as described previously14. The single-cell suspensions were stimulated with antigens (HSP70, GrpE, and ESAT-6) for 24 h at 37 °C, and then, the cells and culture supernatants were examined for IFN-γ production and effector/memory T cell responses using ELISA assay and flow cytometric analysis, respectively.
Immunisation and evaluation of vaccine efficacy in a murine model
For the adjuvant control and antigen vaccination groups, C57BL/6 J mice were immunised subcutaneously 3 times at 3-week intervals with incomplete Freund’s adjuvant (Sigma) (Adjuvant; 20 μg), HSP70 (antigen; 10 μg, Adjuvant; 20 μg), and GrpE (antigen; 10 μg, Adjuvant; 20 μg). Groups vaccinated with BCG (as positive control for the efficacy testing experiment of TB vaccine) were subcutaneously vaccinated with 3 × 105 CFU of BCG Pasteur 1173 P2 at the time of the 2nd immunisation using the antigens. Four weeks after the last immunisation, 5 mice per group were euthanized to analyse the immune responses, and adjuvant controls and vaccinated mice were aerogenically infected with Mtb K as previously described52. Briefly, mice were exposed to Mtb K for 60 min in the inhalation chamber of an airborne infection apparatus calibrated to deliver a predetermined dose (Glas-Col, Terre Haute, IN, USA). To confirm the initial bacterial burden, four mice were euthanised one day later, and approximately 150 viable bacteria were delivered to the lungs of each mouse. This in vivo protective efficacy test was conducted twice. At 4 and 8 weeks after the Mtb K challenge, single-cell suspensions from the spleen and lungs of BCG-, HSP70-, and GrpE-immunised mice were stimulated with antigens (PPD, HSP70, and GrpE; 10 μg/mL concentrations) for 12 h at 37 °C in the presence of GolgiStop (BD Biosciences, San Diego, CA, USA), and then, the populations of antigen-specific CD44high T cells producing IFN-γ, TNF-α, and/or IL-2 were analysed by flow cytometry. Additionally, the cytokines released into in the culture supernatants 24 h after antigen stimulation at 37 °C in the absence of GolgiStop were quantified using ELISA.
ELISA for serum IgG titres
Antigen-specific IgG1 (Sigma) and IgG2c (Southern Biotech, Birmingham, AL, USA) responses in serum from antigen-immunised mice were analysed by sandwich ELISA, as described previously14. Briefly, antigen-specific Abs were detected on 96-well plates coated with 1 μg/mL antigens, and blocked with PBST supplemented with 5% BSA. Serum samples diluted from 1/1000 were incubated for 24 h at 4 °C. To detect antigen-specific IgG1 and IgG2C, incubation with HRP-conjugated Abs was performed for 1 h at 37 °C. Optical densities (OD) were determined at 495 nm within 15 min of stopping the reaction.
Colony forming units (CFU) and histopathology
At 4 and 8 weeks after the Mtb K challenge, the numbers of viable bacteria in the lung and spleen were obtained for CFU counts. Briefly, each bacterial count was determined by plating serial dilutions of the organ homogenates onto Middlebrook 7H10 agar (Becton Dickinson, Franklin Lakes, NJ, USA) supplemented with 10% OADC enrichment medium until the late-exponential phase. The numbers of colonies were counted after 3 weeks of incubation at 37 °C. The resultant values were reported as the mean log10CFU ± SD per gram of lung and spleen tissues. Lung samples collected for histopathology were preserved overnight in 10% normal buffered formalin, embedded with paraffin, cut into 4–5-mm sections, and stained with hematoxylin-eosin (H&E). A specialised pathologist evaluated the inflammation level in the tissue sections from the lung in a blinded manner.
Intracellular cytokine staining and analysis of T cell subpopulations
Single cell suspensions from the spleen and lung were prepared as described preciously14. Single-cell suspensions (2 × 106 cells) from adjuvant-, BCG- and antigen-immunised mice were stimulated with PPD (10 μg/mL), HSP70 (10 μg/mL), or GrpE (10 μg/mL) for 12 h at 37 °C in the presence of GolgiStop. Cells were first blocked with Fc Block (anti-CD16/32; eBioscience, San Diego, CA, USA) for 15 min at 4 °C and then stained with fluorochrome-conjugated Abs to Live/Dead (InvivoGen, San Diego, CA, USA), CD3 (BD Biosciences), CD4 (eBioscience), CD8 (eBioscience), CD62L (eBioscience), CD44 (eBioscience), and CD127 (eBioscience) for 30 min at 4 °C. Cells stained with the appropriate isotype-matched immunoglobulin were used as negative controls. After they were washed with PBS twice, cells were fixed and using a Cytofix/Cytoperm Kit (BD Biosciences) according to the manufacturer’s instructions. Intracellular TNF-α (eBioscience), IL-2 (eBioscience), and IFN-γ (eBioscience) were detected with fluorescein-conjugated Abs in a permeation buffer. Cells were analysed using a FACSverse flow cytometer and commercially available software (FlowJo).
Cytokine measurements
The levels of cytokines, such as IL-17, IL-5, TNF-α, IL-10, IL-2, and IFN-γ, were measured by ELISA, according to the manufacturer’s instructions (eBioscience).
Statistical analysis
All analyses were repeated at least two times with consistent results. The levels of significance for comparisons between samples were determined by Dunnett’s post-test or unpaired t-tests using statistical software (GraphPad Prism, version 5; San Diego, CA, USA). Results are expressed as means. Values of *p < 0.05, **p < 0.01, and ***p < 0.001 were considered statistically significant.
References
McShane, H. Tuberculosis vaccines: beyond bacille Calmette-Guerin. Philos Trans R Soc Lond B Biol Sci 366, 2782–2789, https://doi.org/10.1098/rstb.2011.0097 (2011).
Pitt, J. M., Blankley, S., McShane, H. & O’Garra, A. Vaccination against tuberculosis: how can we better BCG? Microbial pathogenesis 58, 2–16, https://doi.org/10.1016/j.micpath.2012.12.002 (2013).
Tameris, M. D. et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet 381, 1021–1028, https://doi.org/10.1016/S0140-6736(13)60177-4 (2013).
McShane, H. & Williams, A. A review of preclinical animal models utilised for TB vaccine evaluation in the context of recent human efficacy data. Tuberculosis 94, 105–110, https://doi.org/10.1016/j.tube.2013.11.003 (2014).
Jeon, B. Y. et al. Mycobacterium bovis BCG immunization induces protective immunity against nine different Mycobacterium tuberculosis strains in mice. Infect Immun 76, 5173–5180, https://doi.org/10.1128/IAI.00019-08 (2008).
Abebe, F. & Bjune, G. The emergence of Beijing family genotypes of Mycobacterium tuberculosis and low-level protection by bacille Calmette-Guerin (BCG) vaccines: is there a link? Clin Exp Immunol 145, 389–397, https://doi.org/10.1111/j.1365-2249.2006.03162.x (2006).
Voss, G. et al. Progress and challenges in TB vaccine development. F1000Research 7, 199, https://doi.org/10.12688/f1000research.13588.1 (2018).
Zhu, B., Dockrell, H. M., Ottenhoff, T. H. M., Evans, T. G. & Zhang, Y. Tuberculosis vaccines: Opportunities and challenges. Respirology 23, 359–368, https://doi.org/10.1111/resp.13245 (2018).
Cayabyab, M. J., Macovei, L. & Campos-Neto, A. Current and novel approaches to vaccine development against tuberculosis. Frontiers in cellular and infection microbiology 2, 154, https://doi.org/10.3389/fcimb.2012.00154 (2012).
Aguilo, N. et al. Reactogenicity to major tuberculosis antigens absent in BCG is linked to improved protection against Mycobacterium tuberculosis. Nature communications 8, 16085, https://doi.org/10.1038/ncomms16085 (2017).
Bhatt, K., Verma, S., Ellner, J. J. & Salgame, P. Quest for correlates of protection against tuberculosis. Clinical and vaccine immunology: CVI 22, 258–266, https://doi.org/10.1128/CVI.00721-14 (2015).
Lyadova, I. V. & Panteleev, A. V. Th1 and Th17 Cells in Tuberculosis: Protection, Pathology, and Biomarkers. Mediators of inflammation 2015, 854507, https://doi.org/10.1155/2015/854507 (2015).
Kim, W. S. et al. Mycobacterium tuberculosis Rv3628 drives Th1-type T cell immunity via TLR2-mediated activation of dendritic cells and displays vaccine potential against the hyper-virulent Beijing K strain. Oncotarget 7, 24962–24982, https://doi.org/10.18632/oncotarget.8771 (2016).
Kwon, K. W. et al. Novel vaccine potential of Rv3131, a DosR regulon-encoded putative nitroreductase, against hyper-virulent Mycobacterium tuberculosis strain K. Sci Rep 7, 44151, https://doi.org/10.1038/srep44151 (2017).
van Eden, W., van der Zee, R. & Prakken, B. Heat-shock proteins induce T-cell regulation of chronic inflammation. Nat Rev Immunol 5, 318–330, https://doi.org/10.1038/nri1593 (2005).
Aagaard, C. et al. A multistage tuberculosis vaccine that confers efficient protection before and after exposure. Nature medicine 17, 189–194, https://doi.org/10.1038/nm.2285 (2011).
Shekhawat, S. D., Purohit, H. J., Taori, G. M., Daginawala, H. F. & Kashyap, R. S. Evaluation of heat shock proteins for discriminating between latent tuberculosis infection and active tuberculosis: A preliminary report. Journal of infection and public health 9, 143–152, https://doi.org/10.1016/j.jiph.2015.07.003 (2016).
Wilkinson, K. A. et al. Infection biology of a novel alpha-crystallin of Mycobacterium tuberculosis: Acr2. J Immunol 174, 4237–4243 (2005).
Zugel, U. & Kaufmann, S. H. Immune response against heat shock proteins in infectious diseases. Immunobiology 201, 22–35 (1999).
Stewart, G. R. et al. The heat shock response of Mycobacterium tuberculosis: linking gene expression, immunology and pathogenesis. Comparative and functional genomics 3, 348–351, https://doi.org/10.1002/cfg.183 (2002).
Bulut, Y. et al. Mycobacterium tuberculosis heat shock proteins use diverse Toll-like receptor pathways to activate pro-inflammatory signals. The Journal of biological chemistry 280, 20961–20967, https://doi.org/10.1074/jbc.M411379200 (2005).
Bandyopadhyay, B., Das Gupta, T., Roy, D. & Das Gupta, S. K. DnaK dependence of the mycobacterial stress-responsive regulator HspR is mediated through its hydrophobic C-terminal tail. Journal of bacteriology 194, 4688–4697, https://doi.org/10.1128/JB.00415-12 (2012).
Fay, A. & Glickman, M. S. An essential nonredundant role for mycobacterial DnaK in native protein folding. PLoS genetics 10, e1004516, https://doi.org/10.1371/journal.pgen.1004516 (2014).
Stewart, G. R. et al. Overexpression of heat-shock proteins reduces survival of Mycobacterium tuberculosis in the chronic phase of infection. Nature medicine 7, 732–737, https://doi.org/10.1038/89113 (2001).
Zvi, A., Ariel, N., Fulkerson, J., Sadoff, J. C. & Shafferman, A. Whole genome identification of Mycobacterium tuberculosis vaccine candidates by comprehensive data mining and bioinformatic analyses. BMC medical genomics 1, 18, https://doi.org/10.1186/1755-8794-1-18 (2008).
Kim, W. S. et al. Mycobacterium tuberculosis GrpE, a heat-shock stress responsive chaperone, promotes Th1-biased T cell immune response via TLR4-mediated activation of dendritic cells. Frontiers in cellular and infection microbiology 8, 95 (2018).
MacLeod, M. K., Kappler, J. W. & Marrack, P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology 130, 10–15, https://doi.org/10.1111/j.1365-2567.2010.03260.x (2010).
Qiu, Z. et al. Multifunctional CD4 T cell responses in patients with active tuberculosis. Sci Rep 2, 216, https://doi.org/10.1038/srep00216 (2012).
Nandakumar, S., Kannanganat, S., Posey, J. E., Amara, R. R. & Sable, S. B. Attrition of T-cell functions and simultaneous upregulation of inhibitory markers correspond with the waning of BCG-induced protection against tuberculosis in mice. PLoS One 9, e113951, https://doi.org/10.1371/journal.pone.0113951 (2014).
Lindenstrom, T., Knudsen, N. P., Agger, E. M. & Andersen, P. Control of chronic mycobacterium tuberculosis infection by CD4 KLRG1- IL-2-secreting central memory cells. J Immunol 190, 6311–6319, https://doi.org/10.4049/jimmunol.1300248 (2013).
Winslow, G. M., Cooper, A., Reiley, W., Chatterjee, M. & Woodland, D. L. Early T-cell responses in tuberculosis immunity. Immunological reviews 225, 284–299, https://doi.org/10.1111/j.1600-065X.2008.00693.x (2008).
Pym, A. S. et al. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nature medicine 9, 533–539, https://doi.org/10.1038/nm859 (2003).
Hoff, S. T. et al. Sensitivity of C-Tb: a novel RD-1-specific skin test for the diagnosis of tuberculosis infection. The European respiratory journal 47, 919–928, https://doi.org/10.1183/13993003.01464-2015 (2016).
Dominguez, J. et al. Comparison of two commercially available gamma interferon blood tests for immunodiagnosis of tuberculosis. Clinical and vaccine immunology: CVI 15, 168–171, https://doi.org/10.1128/CVI.00364-07 (2008).
Ulrichs, T., Anding, P., Porcelli, S., Kaufmann, S. H. & Munk, M. E. Increased numbers of ESAT-6- and purified protein derivative-specific gamma interferon-producing cells in subclinical and active tuberculosis infection. Infect Immun 68, 6073–6076 (2000).
Cole, S. T. et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544, https://doi.org/10.1038/31159 (1998).
Lupoli, T. J., Fay, A., Adura, C., Glickman, M. S. & Nathan, C. F. Reconstitution of a Mycobacterium tuberculosis proteostasis network highlights essential cofactor interactions with chaperone DnaK. Proceedings of the National Academy of Sciences of the United States of America 113, E7947–E7956, https://doi.org/10.1073/pnas.1617644113 (2016).
Sassetti, C. M., Boyd, D. H. & Rubin, E. J. Genes required for mycobacterial growth defined by high density mutagenesis. Molecular microbiology 48, 77–84 (2003).
Devasundaram, S., Gopalan, A., Das, S. D. & Raja, A. Proteomics Analysis of Three Different Strains of Mycobacterium tuberculosis under In vitro Hypoxia and Evaluation of Hypoxia Associated Antigen’s Specific Memory T Cells in Healthy Household Contacts. Frontiers in microbiology 7, 1275, https://doi.org/10.3389/fmicb.2016.01275 (2016).
Baaten, B. J., Tinoco, R., Chen, A. T. & Bradley, L. M. Regulation of Antigen-Experienced T Cells: Lessons from the Quintessential Memory Marker CD44. Front Immunol 3, 23, https://doi.org/10.3389/fimmu.2012.00023 (2012).
Baaten, B. J. et al. CD44 regulates survival and memory development in Th1 cells. Immunity 32, 104–115, https://doi.org/10.1016/j.immuni.2009.10.011 (2010).
Cetre, C. et al. Profiles of Th1 and Th2 cytokines after primary and secondary infection by Schistosoma mansoni in the semipermissive rat host. Infect Immun 67, 2713–2719 (1999).
Wang, Y. et al. Stimulation of Th1-polarizing cytokines, C-C chemokines, maturation of dendritic cells, and adjuvant function by the peptide binding fragment of heat shock protein 70. J Immunol 169, 2422–2429 (2002).
Motta, A. et al. Mycobacterium tuberculosis heat-shock protein 70 impairs maturation of dendritic cells from bone marrow precursors, induces interleukin-10 production and inhibits T-cell proliferation in vitro. Immunology 121, 462–472, https://doi.org/10.1111/j.1365-2567.2007.02564.x (2007).
Metcalfe, H. J. et al. Protection associated with a TB vaccine is linked to increased frequency of Ag85A-specific CD4(+) T cells but no increase in avidity for Ag85A. Vaccine 34, 4520–4525, https://doi.org/10.1016/j.vaccine.2016.07.055 (2016).
Dockrell, H. M. & Smith, S. G. What Have We Learnt about BCG Vaccination in the Last 20 Years? Front Immunol 8, 1134, https://doi.org/10.3389/fimmu.2017.01134 (2017).
Forbes, E. K. et al. Multifunctional, high-level cytokine-producing Th1 cells in the lung, but not spleen, correlate with protection against Mycobacterium tuberculosis aerosol challenge in mice. J Immunol 181, 4955–4964 (2008).
Wangoo, A. et al. Contribution of Th1 and Th2 cells to protection and pathology in experimental models of granulomatous lung disease. J Immunol 166, 3432–3439 (2001).
Amelio, P. et al. Mixed Th1 and Th2 Mycobacterium tuberculosis-specific CD4 T cell responses in patients with active pulmonary tuberculosis from Tanzania. PLoS neglected tropical diseases 11, e0005817, https://doi.org/10.1371/journal.pntd.0005817 (2017).
Kim, J. S. et al. Mycobacterium tuberculosis RpfB drives Th1-type T cell immunity via a TLR4-dependent activation of dendritic cells. Journal of leukocyte biology 94, 733–749, https://doi.org/10.1189/jlb.0912435 (2013).
Rachman, H., Lee, J. S., Angermann, J., Kowall, J. & Kaufmann, S. H. Reliable amplification method for bacterial RNA. Journal of biotechnology 126, 61–68, https://doi.org/10.1016/j.jbiotec.2006.02.020 (2006).
Jeon, B. Y. et al. In vivo characteristics of Korean Beijing Mycobacterium tuberculosis strain K1 in an aerosol challenge model and in the Cornell latent tuberculosis model. J Med Microbiol 61, 1373–1379, https://doi.org/10.1099/jmm.0.047027-0 (2012).
Acknowledgements
This research was supported by the Basic Science Research Program and the International Research & Development Program through the National Research Foundation of Korea (NRF) funded by the Ministry of science, ICT & Future Planning (NRF-2016R1A2A1A05005263 and NRF-2014K1A3A7A03075054).
Author information
Authors and Affiliations
Contributions
W.S.K., J.-S.K. and S.J.S. designed and performed experiment, analyzed data, and wrote the manuscript. W.S.K., H.M.K., K.W.K. and S.Y.E. contributed to conduct immunological experiments and interpret data.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kim, W.S., Kim, JS., Kim, H.M. et al. Comparison of immunogenicity and vaccine efficacy between heat-shock proteins, HSP70 and GrpE, in the DnaK operon of Mycobacterium tuberculosis. Sci Rep 8, 14411 (2018). https://doi.org/10.1038/s41598-018-32799-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32799-z
Keywords
This article is cited by
-
GrpE and ComD contribute to the adherence, biofilm formation, and pathogenicity of Streptococcus suis
Archives of Microbiology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.