Abstract
Ulinastatin has been found to have anti-inflammatory effect for patients with sepsis. However, its clinical effects were conflicting. The study aimed to investigate the cost-effectiveness of ulinastatin and to perform mediation analysis to explore the proportion of the total effects that can be explained by inflammatory responses. This is a retrospective study involving critically ill patients with sepsis from January 2014 to July 2017. A total of 263 patients were included in the study, involving 179 patients in the ulinastatin group and 84 in the control group. Ulinastatin group showed significantly lower 28-day mortality rate than that in the control group (31% vs. 55%; p < 0.001). Both total (46330 [26000,83500] vs. 19870 [8747,41140] RMB; p < 0.01) and drug cost (18210 [9492,31920] vs. 7230 [2675,19270] RMB; p < 0.01) were significantly higher in the ulinastatin group than the control group. In multivariable model, the adjusted odds ratio for ulinastatin was 0.304 (95% CI: 0.152 to 0.592; p = 0.001). The mediation analysis showed that the use of ulinastatin was able to reduce the probability of death by 23.5%. The average causal mediation effect of delta C-reactive protein (CRP) was 8%, accounting for 35% of the total effect.
Similar content being viewed by others
Introduction
Sepsis is common in the intensive care unit (ICU) and imposes a great challenge for clinicians1,2. In recent years, many efforts have been made to increase the awareness of sepsis, as well as to improve the treatment of sepsis3,4,5. While patients with mild sepsis treated outside ICU usually has a good outcome, those requires ICU admission may have mortality rate as high as 50%6,7. Such a high mortality is attributable to the development of multiple organ failures such as renal failure, acute respiratory distress syndrome, coagulation dysfunction and cardiac failure. Uncontrolled inflammatory response is the core to the pathophysiology of severe sepsis, which is also a potential target for its treatment8. Ulinastatin is one of such agents that have been shown to be promising in improving clinical outcomes for critically ill patients with sepsis9,10. However, clinical studies are conflicting. While some studies showed benefits of ulinastatin11,12, others failed to replicate the results13. A recent systematic review showed no statistical difference on mortality between ulinastatin and control groups11. The review included 2 trials with a total sample size of 172, which was underpowered to detect a beneficial effect of ulinastatin. Furthermore, the cost-effectiveness of ulinastatin has never been investigated. In the present study, we aimed to investigate 1) the effectiveness of ulinastatin in reducing mortality for critically ill patients with sepsis; 2) the cost-effectiveness of ulinastatin; and 3) whether the effects of ulinastatin on mortality was mediated by the reduction in the inflammatory biomarker C-reactive protein (CRP).
Methods
Setting and subjects
The study was retrospective in design and was conducted in the ICU of Wuhu No. 2 People’s Hospital from January 2014 to July 2017. All patients fulfilled the criteria of sepsis were screened for potential eligibility. According to the sepsis-3.0 definition, sepsis was defined as life-threatening organ dysfunction caused by a dysregulated host response to infection14. In our study, organ dysfunction was represented by an increase in the Sequential Organ Failure Assessment (SOFA) score of 2 points or more. Infection was defined as the presence of one of ICD-9 diagnoses for infection such as pneumonia, sepsis, urinary tract infection and blood stream infection. Exclusion criteria included: (1) age younger than 18 years; (2) contraindications for ulinastatin such as allergy; (3) patients with do-not-resuscitation order; (4) pregnant women; and (5) patients transferred to our hospital at late stage of sepsis. The study was approved by the ethics committee of Wuhu NO. 2 People’s Hospital. Informed consent was waived because the study was retrospective in design. The study was performed in accordance to the Helsinki declaration. Individual patient data were de-identified and stored in an encrypted computer.
Demographic and clinical variables
Demographic data including age and gender were extracted from the first sheet of medical chart. Laboratory variables including white blood cell (WBC), platelet, IL-6, procalcitonin (PCT), C-reactive protein (CRP), and pro-BNP were obtained on day 1 after ICU admission. If there were more than one measurements, the maximum value was recorded. CRP was also obtained on day 3. Changes in CRP (delta CRP) were calculated as the difference between CRP values on day 1 and 3. There were many other inflammatory biomarkers such as PCT, IL-6 measured in our institution. However, they were not included because they are not measured routinely on a daily basis. Acute physiology and chronic health evaluation (APACHE) II and SOFA scores were calculated based on demographic data, vital signs and laboratory variables15,16. APACHE II score was calculated by using the clinical and laboratory variables within 24 hours after ICU admission. The values with the maximum point score were used. Patient type included surgical, medical patients and patients admitted from emergency room.
Use of ulinastatin
The use of ulinastatin (Guangdong Techpool Bio-pharma Co. Ltd., Guangdong, China) was determined by the attending physician and not all sepsis patients received ulinastatin. Data on the use of ulinastatin were extracted from electronic healthcare records. The typical dosage for the use of ulinastatin was 200,000 U three times a day. Ulinastatin would be discontinued if the general condition of the patient was improved, or if there was suspected allergy. Vasopressors including norepinephrine, epinephrine, dopamine greater than 5 mcg/kg/min were recorded.
Clinical outcomes
Mortality was defined as the vital status at 28 days after ICU admission. Other outcomes included ICU length of stay, duration of mechanical ventilation for the ulinastatin and control groups. ICU length of stay was defined as the period during ICU stay. Readmission to ICU within 48 hours was not considered as successful discharge from ICU. In such a situation, ICU length of stay was calculated as the sum of the two ICU stays. Similarly, reintubation for mechanical ventilation within 48 hours was considered as weaning failure, and the duration of mechanical ventilation was considered as the sum of the two episodes of mechanical ventilation. There were two components of medical cost: 1) total cost was the overall cost during hospital stay; and 2) drug cost was the cost spent on drugs, excluding procedures, laboratory tests, imaging study and other logistic costs.
Statistical analysis
Statistical descriptions were employed for the overall population, and then for the ulinastatin and control groups separately. Firstly, continuous data were examined by tests for skewness and kurtosis. Normal data were expressed as mean and standard deviation, and were compared between ulinastatin and control groups using student t test. Non-normal data were expressed as median and interquartile range, and were compared using Mann-White U test17,18. Logistic regression model was employed to adjust for confounding factors for the effectiveness of ulinastatin on mortality outcome. These factors, as judged by their statistical significance in univariate analysis and subject-matter knowledge, included APACHE II, SOFA, age, use of vasopressors, WBC, neutrophil percent and patient type19. Mediation analysis was performed to investigate the proportion of the effect of ulinastatin that was mediated by the change in CRP20. Specifically, a linear regression was fit by regressing delta CRP on the use of ulinastatin, then a logistic regression model was fit by regressing 28-day mortality on the use of ulinastatin and delta CRP21. The total effects of ulinastatin on mortality was split into two parts, one was the direct effect and the other was the causal mediation effect via delta CRP. All statistical analyses were performed using R (version 3.4.3). Two-tailed p value less than 0.05 were considered as statistically significant.
Results
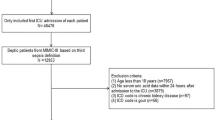
Initially, a total of 297 patients who fulfilled the criteria of Sepsis-3.0 were identified by chart review. In-depth screening excluded 34 patients because 13 had do-not-resuscitate order, 15 were transferred from other hospitals to our ICU at late stage of sepsis, 2 were pregnant women and 4 were younger than 18 years old (Fig. 1). As a result, a total of 263 patients, including 101 non-survivors and 162 survivors, were included for final analysis. The overall 28-day mortality of 38.4%.
Flowchart of patient selection. Initially, a total of 297 patients who fulfilled the criteria of Sepsis-3.0 were identified by chart review. In-depth screening excluded 34 patients because 13 had do-not-resuscitate order, 15 were transferred from other hospitals to our ICU at late stage of sepsis, 2 were pregnant women and 4 were younger than 18 years old. As a result, a total of 263 patients, including 101 non-survivors and 162 survivors, were included for final analysis.
There were 84 patients in the control group and 179 in the ulinastatin group (Table 1). All demographic and clinical variables were not significantly different between the ulinastatin and control groups. Although there was no significant difference in baseline CRP, CRPs measured on day 3 were marginally lower in the ulinastatin group than that in the control group (83.8 [21.35,125.5] vs. 124 [61.9,217]; p = 0.061). However, we found that the patient types were significantly different between the two groups. While there were more medical patients in the control group than that in the ulinastatin group (86% vs. 66%; p = 0.004), there were more surgical patients in the ulinastatin group (19% vs. 8% for elective surgery; 15% vs. 6% for emergency surgery) than the control group.
Ulinastatin group showed significantly lower 28-day mortality rate than that in the control group (31% vs. 55%; p < 0.001). However, ulinastatin group showed significantly longer duration of mechanical ventilation (3 [1,7] vs. 0 [0, 3] days; p < 0.001), longer length of stay in ICU (5 [3, 11] vs. 1 [0, 6]) and hospital (16 [7, 27] vs. 9.5 [2, 21.25] days; p < 0.001). Both total (46330 [26000,83500] vs. 19870 [8747,41140] RMB; p < 0.01) and drug cost (18210 [9492,31920] vs. 7230 [2675,19270] RMB; p < 0.01) were significantly higher in the ulinastatin group than the control group (Table 2).
In multivariable logistic regression model (Table 3), the adjusted odds ratio (OR) and confidence interval (CI) for ulinastatin was 0.304 (95% CI: 0.152 to 0.592; p = 0.001). Other variables associated with 28-day mortality were APACHE II (OR: 1.108; 95% CI: 1.030 to 1.199; p = 0.008), age (OR: 1.033; 95% CI: 1.010 to 1.059; p = 0.006) and vasopressor (OR: 4.636; 95% CI: 2.128 to 10.747; p < 0.01).
The mediation analysis showed that the use of ulinastatin was able to reduce the probability of death by 23.5%. The average causal mediation effect of delta CRP was 8%, accounting for 35% of the total effect. The average direct effect of ulinastatin was 15%, accounting for 65% of the total effect. Since the mediation analysis was performed under the counterfactual framework, the average causal mediation effect and direct effect were also reported for the treated and control groups separately in Table 4. Sensitivity analysis showed that the mediation effect of delta CRP was robust to the violation of ignorability assumption (Fig. 2).
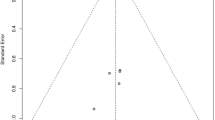
Sensitivity analysis of the causal mediation analysis. Causal mediation analysis is performed under the assumption of sequential ignorability, stating that there was no unmeasured confounder that is related to both the mediator and the outcome. Sensitivity parameter \({\rm{\rho }}\) is the residual correlation and a large value means that there is a large unmeasured confounder. The dashed line indicates the causal mediation effect for the control (upper panel) and treatment groups (lower panel) under assumption that there is no unmeasured confounder (ignorability assumption: ρ = 0). It appears that a \({\rm{\rho }}\) of 0.3 is required to modify the sign of the mediation effect. Judged by subject-matter knowledge, a \({\rm{\rho }}\) value of 0.3 is very large and it is unlikely that the mediation effect doesn’t exist.
Discussion
The study showed that the use of ulinastatin was associated with reduced mortality rate in 28-day after ICU admission. The effect remained unchanged after adjustment for potential confounders. However, ulinastatin was associated with increased medical cost. Interestingly, we observed that 35% of the total effect of ulinastatin was mediated via the reduction in CRP, consistent with the anti-inflammatory effect of ulinastatin. The results of the study can be generalized to sepsis population defined by the sepsis-3.0 definition. Furthermore, it was applicable to sepsis patients with any infection site, as the result showed that the infection sites had no significant impact on the effect.
This study was consistent with several previous studies showing that ulinastatin was associated with reduced risk of mortality22. In a randomized controlled trial, Karnad and colleagues showed that the 28-day all-cause mortality was 7.3% with ulinastatin (4 deaths) versus 20.3% (12 deaths) with placebo (p = 0.045), and the effect was robust to multivariable adjustment (odds ratio 0.26, 95% CI 0.07–0.95; p = 0.042)23. The mortality in Karnad’s study was significantly lower than that in our study, but the beneficial effect of ulinastatin was consistent. This indicates that the beneficial effect of ulinastatin was consistent across a wide range of disease severity. In patients with acute severe pancreatitis, ulinastatin was also found to be able to reduce the mortality rate and the number of new organ dysfunction24. However, the beneficial effect was not replicated in elderly patients with multiple organ failure and sepsis13. In a randomized controlled trial, Wu and colleagues found that ulinastatin was able to improve inflammatory profile of patients with severe sepsis, but the improvement in mortality outcome was not statistically significant25. There are several reasons that are responsible for the discrepancy among these studies. First, the small sample size (n = 60) may explain the failure to obtain a statistically significant result in Wu’s study. The study showed a tendency towards beneficial of ulinastatin on mortality, but the statistical significance was not reached. Second, the selection of study population is important for a study to obtain a positive result. Uchida’s study enrolled elderly patients who had heavy comorbidity burden and poor overall mortality (mean APACHE II = 25). It may be reasonable that ulinastatin alone is not enough to rescue lives of these frail patients. Furthermore, this study enrolled patients with multiple organ failure which was not necessarily caused by sepsis. Third, the evolving definition of sepsis may also explain the conflicting results. While early trials defined sepsis as the infection plus systematic inflammatory response syndrome (SIRS)23, more recent trails and the present study enrolled patients with sepsis-3.0 criteria26. There is large body of evidence that sepsis identified by these two definitions can be quite different1,27.
Medical cost was increased in the ulinastatin group, which can be explained by longer stay in ICU and hospital in this group. Consistent with our study, Abhyankar and colleagues also found the length of stay in ICU and hospital was significantly prolonged for patients receiving ulinastatin28. Possibly, patients in the ulinastatin group can survive the critical phase of the disease and the recovery of organ dysfunction required prolonged duration of organ support (e.g. vasopressor use and mechanical ventilation) and stay in ICU. In the control group, patients died in a short period of time. Although the medical cost in the control group was much less than that in the ulinastatin group, the mortality rate was higher. In other studies, although they found significantly reduced mortality rate by the use of ulinastatin, the length of stay in the hospital was not significantly prolonged22,29. In a systematic review and meta-analysis, treatment with ulinastatin was not able to reduce the duration of vasopressor use and length of stay in ICU12. However, the length of stay in ICU and hospital can be influenced by many factors such as the criteria for ICU discharge, financial insurance of a patient, economic status and culture believes. Thus, direct comparisons between different studies may be problematic due to significant heterogeneity among these trials11.
An interesting finding in our study was that the total effect of ulinastatin on mortality outcome can be split into direct and casual mediation effects. The reduction in CRP accounted for 35% of the total effects. CRP was reported in ours study because this biomarker was routinely measured on a daily basis for patients with sepsis. Also, CRP was widely available in other institutions, making it more useful for clinicians. This clinical result further supports results from bench work that ulinastatin has anti-inflammatory effect30,31,32,33. However, CRP is not a specific biomarker of inflammatory response, and its diagnostic utility is inferior than other inflammatory biomarkers such as interleukins and procalcitonin. Further studies may be needed to investigate whether the remaining 65% direct effect can be explained by these biomarkers, or ulinastatin has other therapeutic effects not involving inflammatory responses. Besides the anti-inflammatory effect of ulinastatin, the direct effect can be explained by its ability to inhibit apoptosis34, protect the endothelial glycocalyx layer35 and vascular endothelium cells36.
Due to retrospective design of the study, some limitations must be acknowledged. First, the selection bias cannot be fully accounted for in observational studies. Although there was no specific indication for the use of ulinastatin in our hospital, the use of ulinastatin cannot be a random process. We have tried to adjust for confounding factors in multivariable regression model, but other unmeasured confounding factors cannot be fully excluded. Second, as mentioned above, CRP is not a specific inflammatory biomarker and it cannot represent the whole process of inflammatory responses. There are hundreds even thousands of inflammatory biomarkers. Thus, the remaining effect of ulinastatin might be explained by these biomarkers. Further studies are needed to address this issue. Third, other agents that have inflammatory modulatory effects were not included for analysis in the present study, making the presence of potential uncontrolled confounding possible. There have been evidence that ulinastain, when combined with other agents such as Xuebijing and thymosin alpha1, can have better effect than used alone12,37. These agents were seldom used in our hospital, and thus cannot be analyzed in the present study.
In conclusion, the study found that the use of ulinastatin was associated with reduced 28-day mortality rate in critically ill patients with sepsis. However, ulinastatin was associated with increased length of stay in ICU and hospital, and the medical cost was also increased. Furthermore, we observed that 35% of the total effect of ulinastatin can be explained by the reduction in CRP, consistent with the anti-inflammatory effect of ulinastatin. Further studies are needed to investigate how the remaining 65% direct effect of ulinastatin takes place.
Declarations
Ethics approval and consent to participate
The study was approved by the ethics committee of Wuhu NO. 2 People’s Hospital. Informed consent was waived because the study was retrospective in design.
References
Zhang, Z. et al. AME evidence series 001-The Society for Translational Medicine: clinical practice guidelines for diagnosis and early identification of sepsis in the hospital. J Thorac Dis 8, 2654–2665 (2016).
Yébenes, J. C. et al. Epidemiology of sepsis in Catalonia: analysis of incidence and outcomes in a European setting. Ann Intensive Care 7, 19 (2017).
Dellinger, R. P. et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock, 2012. Intensive care medicine 39, 165–228 (2013).
Zhang, Z. et al. Early management of sepsis with emphasis on early goal directed therapy: AME evidence series 002. J Thorac Dis 9, 392–405 (2017).
Oda, S. et al. The Japanese guidelines for the management of sepsis. J Intensive Care 2, 55 (2014).
Zhang, Z., Ni, H. & Qian, Z. Effectiveness of treatment based on PiCCO parameters in critically ill patients with septic shock and/or acute respiratory distress syndrome: a randomized controlled trial. Intensive Care Med 41, 444–451 (2015).
Angus, D. C. et al. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 29, 1303–1310 (2001).
Delano, M. J. & Ward, P. A. Sepsis-induced immune dysfunction: can immune therapies reduce mortality? J. Clin. Invest. 126, 23–31 (2016).
Atal, S. S. & Atal, S. Ulinastatin-a newer potential therapeutic option for multiple organ dysfunction syndrome. J Basic Clin Physiol Pharmacol 27, 91–99 (2016).
Luo, Y., Che, W. & Zhao, M. Ulinastatin post-treatment attenuates lipopolysaccharide-induced acute lung injury in rats and human alveolar epithelial cells. International Journal of Molecular Medicine 39, 297–306 (2017).
Feng, Z., Shi, Q., Fan, Y., Wang, Q. & Yin, W. Ulinastatin and/or thymosin α1 for severe sepsis: A systematic review and meta-analysis. J Trauma Acute Care Surg 80, 335–340 (2016).
Liu, D. et al. Effect of ulinastatin combined with thymosin alpha1 on sepsis: A systematic review and meta-analysis of Chinese and Indian patients. J Crit Care 39, 259–266 (2017).
Uchida, M., Abe, T., Ono, K. & Tamiya, N. Ulinastatin did not reduce mortality in elderly multiple organ failure patients: a retrospective observational study in a single center ICU. Acute Med Surg 5, 90–97 (2018).
Singer, M. et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). In 315, 801–810 (American Medical Association, 2016).
Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: A severity of disease classification system. Crit. Care Med. 13, 818–829 (1985).
Minne, L., Abu-Hanna, A. & de Jonge, E. Evaluation of SOFA-based models for predicting mortality in the ICU: A systematic review. Crit Care 12, R161 (2008).
Zhang, Z. Univariate description and bivariate statistical inference: the first step delving into data. Ann Transl Med 4, 91–91 (2016).
Zhang, Z., Gayle, A. A., Wang, J., Zhang, H. & Cardinal-Fernández, P. Comparing baseline characteristics between groups: an introduction to the CBCgrps package. Ann Transl Med 5, 484–484 (2017).
Zhang, Z. Model building strategy for logistic regression: purposeful selection. Ann Transl Med 4, 111–111 (2016).
Zhang, Z. et al. Causal mediation analysis in the context of clinical research. Ann Transl Med 4, 425–425 (2016).
Imai, K., Keele, L. & Tingley, D. A General Approach to Causal Mediation Analysis. Psychological Methods 15, 309–334 (2010).
Lagoo, J. Y., D’Souza, M. C., Kartha, A. & Kutappa, A. M. Role of Ulinastatin, a trypsin inhibitor, in severe acute pancreatitis in critical care setting: A retrospective analysis. J Crit Care 45, 27–32 (2018).
Karnad, D. R. et al. Intravenous administration of ulinastatin (human urinary trypsin inhibitor) in severe sepsis: A multicenter randomized controlled study. Intensive Care Med 40, 830–838 (2014).
Abraham, P. et al. Efficacy and safety of intravenous ulinastatin versus placebo along with standard supportive care in subjects with mild or severe acute pancreatitis. J Assoc Physicians India 61, 535–538 (2013).
Wu, T.-J., Zhang, L.-N. & Kang, C.-C. The effect of ulinastatin on disbalance of inflammation and immune status in patients with severe sepsis. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 25, 219–223 (2013).
Jiang, W. et al. ADJunctive Ulinastatin in Sepsis Treatment in China (ADJUST study): study protocol for a randomized controlled trial. Trials 19, 133 (2018).
Donnelly, J. P., Safford, M. M., Shapiro, N. I., Baddley, J. W. & Wang, H. E. Application of the Third International Consensus Definitions for Sepsis (Sepsis-3) Classification: a retrospective population-based cohort study. Lancet Infect Dis 17, 661–670 (2017).
Abhyankar, S. V. & Vartak, A. M. Impact of Ulinastatin on Outcomes in Acute Burns Patients. J Burn Care Res 1 https://doi.org/10.1097/BCR.0000000000000546 (2017).
Wang, J. et al. Clinical value of the early use of ulinastatin in patients with moderately severe or severe acute pancreatitis. Zhonghua Yi Xue Za Zhi 97, 1252–1255 (2017).
Ren, K.-W., Shen, N., Tang, J.-L., Nong, L.-M. & Gu, Y.-Q. Effects of ulinastatin on inflammatory response and cognitive function after hip arthroplasty for the elderly patients with femoral neck fracture. Eur Rev Med Pharmacol Sci 22, 1126–1132 (2018).
Liu, W. & Chai, J. K. Influences of ulinastatin on acute lung injury and time phase changes of coagulation parameters in rats with burn-blast combined injuries. Zhonghua Shao Shang Za Zhi 34, 32–39 (2018).
Cao, C. et al. Ulinastatin Protects Against LPS-Induced Acute Lung Injury by Attenuating TLR4/NF-κB Pathway Activation and Reducing Inflammatory Mediators. Shock 1, https://doi.org/10.1097/SHK.0000000000001104 (2018).
Li, S.-T. et al. Ulinastatin attenuates LPS-induced inflammation in mouse macrophageRAW264.7 cells by inhibiting the JNK/NF-κB signaling pathway and activating the PI3K/Akt/Nrf2 pathway. Acta Pharmacol. Sin. 8, 41988 (2018).
Long, Y., Zhang, Y., Cai, S.-S., Sun, D.-M. & Li, Y.-H. Ulinastatin inhibits high glucose-induced cardiomyocyte apoptosis through activating Akt signaling. Eur Rev Med Pharmacol Sci 22, 4691–4697 (2018).
Wang, J., Wu, A. & Wu, Y. Endothelial Glycocalyx Layer: A Possible Therapeutic Target for Acute Lung Injury during Lung Resection. Biomed Res Int 2017, 5969657–8 (2017).
Liu, S. et al. Ulinastatin attenuates hyper-permeability of vascular endothelialium cells induced by serum from patients with sepsis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 33, 1600–1604 (2017).
Zheng, J. et al. Xuebijing combined with ulinastation benefits patients with sepsis: A meta-analysis. Am J Emerg Med 36, 480–487 (2018).
Author information
Authors and Affiliations
Contributions
Q.X. conceived the idea and drafted the manuscript; Q.Y. performed data acquisition and analysis; S.C. reviewed the manuscript and helped interpreting the results.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xu, Q., Yan, Q. & Chen, S. Ulinastatin is effective in reducing mortality for critically ill patients with sepsis: a causal mediation analysis. Sci Rep 8, 14360 (2018). https://doi.org/10.1038/s41598-018-32533-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32533-9
Keywords
This article is cited by
-
Immune dysregulation in sepsis: experiences, lessons and perspectives
Cell Death Discovery (2023)
-
The mediating effects of depression, anxiety, and rapid eye movement sleep behavior disorder on the association between dopaminergic replacement therapy and impulse control disorders in Parkinson’s disease
Neurological Sciences (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.