Abstract
After the successful roll out of MenAfriVac, Nigeria has experienced sequential meningitis outbreaks attributed to meningococcus serogroup C (NmC). Zamfara State in North-western Nigeria recently was at the epicentre of the largest NmC outbreak in the 21st Century with 7,140 suspected meningitis cases and 553 deaths reported between December 2016 and May 2017. The overall attack rate was 155 per 100,000 population and children 5–14 years accounted for 47% (3,369/7,140) of suspected cases. The case fatality rate (CFR) among children 5–9 years was 10%, double that reported among adults ≥ 30 years (5%). NmC and pneumococcus accounted for 94% (172/184) and 5% (9/184) of the laboratory-confirmed cases, respectively. The sequenced NmC belonged to the ST-10217 clonal complex (CC). All serotyped pneumococci were PCV10 serotypes. The emergence of NmC ST-10217 CC outbreaks threatens the public health gains made by MenAfriVac, which calls for an urgent strategic action against meningitis outbreaks.
Similar content being viewed by others
Introduction
The “meningitis belt” spans twenty-six contiguous countries across Africa and is characterized by large recurrent meningitis epidemics and frequent seasonal outbreaks1. Within the “meningitis belt”, Neisseria meningitidis (meningococcus) accounts for almost all recurrent epidemics. The entire northern region of sub-Saharan Africa’s most populous country, Nigeria, falls within the meningitis belt. Nigeria has a long history of large-scale meningitis outbreaks dating as far as the early 1900s; in 1921, an outbreak in northern Nigeria caused 46,000 deaths2. The most recent largest meningitis outbreak occurred in 1996, with over 80,000 suspected cases3,4.
The bulk of epidemic meningococcal disease outbreaks in Nigeria and across the “meningitis belt” were previously attributed to meningococcus serogroup A (NmA)5. MenAfriVac is a conjugate vaccine that is very immunogenic and, provides effective protection against NmA6. MenAfriVac campaigns have prevented NmA epidemics and reduced the incidence of suspected meningitis by nearly two-thirds7. In 2011, nearly 16 million people in five high priority states (Zamfara, Katsina, Jigawa Bauchi and Gombe) in northern Nigeria received MenAfriVac8.
In 1975, Nigeria experienced a large NmC epidemic associated with relatively high rates of meningococcaemia and high case fatality rates as seen in the recent outbreaks2,9. Before 2013, the latest outbreak of NmC in Africa had occurred in 1979 in Burkina Faso10. Sequential outbreaks of meningococcus serogroup C (NmC) occurred in Nigeria in 2013 and 2014 in Nigeria11,12 despite the roll out of MenAfriVac campaigns. These outbreaks were followed by a larger outbreak in Niger in 201513. All the recent outbreaks were attributed to a novel strain of NmC sequence type (ST)-10217 whose emergence threatens the public health gains made by the MenAfriVac14,15,16.
Monovalent (C) and quadrivalent (A,C,W,Y) formulations of the meningococcal conjugate vaccine are available and The World Health Organization (WHO) recommends that countries with endemic or frequent epidemics of meningococcal disease should implement large-scale vaccination campaigns17,18. However, the rollout of vaccines that protect against NmC has been slow and tends to be reactive during outbreaks. The major constraints to preventive vaccination campaigns include high vaccine cost, limited vaccine availability and competing public health interests15. During epidemics, reactive vaccination campaigns are further hampered by low lumbar puncture rates among suspected cases and inadequate laboratory characterisation of causative pathogens that should guide response19.
Between December 2016 and May 2017, a total of 14,280 suspected meningitis cases were reported across 23 of the 36 states in Nigeria. The descriptive characterization of the 2016–2017 cerebrospinal meningitis outbreak in Nigeria is reported elsewhere20. There were 1,145 deaths (8% case fatality rate) amongst suspected cases. Zamfara and Sokoto states were at the epicenter of the meningitis epidemic in Nigeria. The World Health Organization Collaborating Center (WHO CC) for New Vaccines hosted at the Medical Research Council Unit The Gambia at London School of Hygiene & Tropical Medicine, supports surveillance of invasive bacterial diseases (IBD) in West and Central Africa. The WHO CC with its partners, supported the Nigeria Center for Disease Control (NCDC) to confirm the causative agent of the outbreak in Zamfara state. The WHO CC also trained and supported the laboratory teams at regional and district hospitals in Zamfara for bacteriologic analysis and processing of CSF specimens for molecular analysis.
Results
A total of 7,140 suspected meningitis cases were reported in Zamfara State between 12th December 2016 and 31st May 2017. The attack rate was 155 per 100,000 population. A total of 553 deaths were reported among suspected cases and the case fatality ratio (CFR) was 8% in Zamfara state.
CSF specimens were collected from 404 (6%) suspected meningitis cases and almost all, 383 (95%), had at least one or all laboratory tests performed including bacteriologic culture, Gram stain, Rapid Test (Pastorex) and qPCR. The laboratory processing of CSF specimens is shown in Fig. 1. A pathogen was detected in 184 (48%) of the CSF specimens tested by PCR, rapid test and culture. Meningococcus was the dominant pathogen accounting for 173 (94%) of the confirmed cases. The characteristics of the suspected and confirmed meningitis cases are summarised in Table 1. Of the 112 CSF specimens tested by both PCR and Rapid Test, the concordance was 86 (77%). PCR detected an additional 19 Nm meningitis cases in addition to the 22 cases detected by both methods and 3 cases detected by Rapid Test only.
NmC accounted for 172 (99%) of the meningococci detected. There was one case of meningococcus serogroup W (NmW) meningitis detected by latex rapid test. MLST performed directly from two CSF specimens that tested positive for NmC showed that they belonged to the ST-10217 CC. Unfortunately, the meningococcal isolates cultured from CSF specimens during the outbreak were lost due to the lack of appropriate storage conditions in Zamfara state. It was not possible to perform MLST on more NmC positive CSF specimens due to the limited availability of funds.
Streptococcus pneumoniae, the pneumococcus, accounted for 9 (5%) cases. All five of the serotyped pneumococci were PCV10 vaccine strains; three were serotype 19 F strains, one serotype 1 and one serotype 5. There were also single cases of confirmed Group B Streptococcus (GBS) and H. influenzae.
Spatial and temporal characteristics of the outbreak
The epicentre of the outbreak in Zamfara state (Fig. 2A) was Shinkafi Local Government Area (LGA), which had an attack rate of 669 per 100,000 population and bore a fifth of all the suspected cases (Fig. 2B). Other LGAs with high attack rates were Bakura and Birnin Magaji, which had 210 and 255 suspected cases per 100,000 population, respectively. Gusau, the state capital, accounted for 12% of the suspected cases reported, although many of these suspected cases may have been referrals from other LGAs (Fig. 2B). Shinkafi, Maradun and Gusau had the highest confirmed cases, over 8 per 100,000 population. Meningococcus was the dominant pathogen across all the LGAs in Zamfara state. Pneumococcal meningitis was reported in Bakura, Gusau, Magaji and Bimiri (Fig. 2C).
The geographical distribution of the meningitis outbreak in Zamfara State. The location of Zamfara state in Nigeria is shown in (A). Suspected cases per 100,000 population (B), laboratory-confirmed cases per 100,000 population (C) and case fatality rates (D) across LGAs are shown by colour gradient. The pie charts represent the pathogens detected in confirmed cases (C).
Although Tsafe had a relatively low attack rate of 44.4 per 100,000, this LGA had the highest CFR of 17% (Fig. 2D). Other LGAs with high CFRs were Kaura Namoda (12%), Bukkuyum (13%) and Bakura (11%). Suspected meningitis cases from Tsafe LGA were more than three times likely to die compared to suspected meningitis cases from Gusau (p < 0.01) (Table 2). Likewise, suspected meningitis cases from Bakura and Kaura Namoda LGAs had significantly higher odds of dying than suspected meningitis cases from Gusau LGA (Table 2). Suspected meningitis cases from Maradun and Bungudu had CFRs less than 5% (Fig. 2D) and were significantly less likely to die than suspected from the state capital, Gusau.
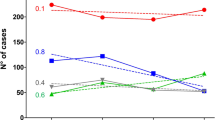
The first suspected meningitis cases in Zamfara state occurred in December 2016 but were only reported at the national level in February 2017. By the 31st of May 2017 the number of cases had increased to 7140. The highest attack rates exceeding 20 per 100,000 population were reported between epidemiologic weeks 16 and 18 (Fig. 3A). The bulk of the suspected meningitis cases 6,205 (86%) occurred between March and April 2017, which corresponds to the peak of hot dry harmattan winds.
Although fewer suspected cases were reported in the earlier weeks, the likelihood of death amongst suspected cases was highest then (Fig. 3B). Although the CFR declined to 10% or less after epidemiologic week 8, the absolute number of deaths increased substantially over the following weeks as the total number of suspected cases also increased. Half the deaths among suspected cases in Zamfara state occurred between epidemiologic weeks 9 and 12 (Fig. 3B). The odds of dying amongst suspected meningitis cases declined significantly over time (Table 2).
The first confirmed case was identified five weeks after the start of the outbreak, with meningococcus as the causing pathogen (Fig. 3C). All confirmed cases thereafter were attributed to meningococcus until the 17th week of the outbreak when pneumococcus, GBS and H. influenzae were detected. Most pneumococcal meningitis cases, 7 (77%) were reported between weeks 20 and 23 (Fig. 3C).
Demographic characteristics of the outbreak
Half of the suspected cases and 56% of the confirmed cases were males (Table 1). Male suspected cases died more frequently with a CFR of 8.7 compared to 6.8 among females. Males accounted for 311 (56%) of all deaths. Older children (5–14 years) and young adults (15–29 years old) accounted for 5,458 suspected cases, more than three-quarters of all suspected cases (Table 1). Older children between 5 and 14 years accounted for two-thirds (322/553) of the deaths reported. Likewise, the highest CFRs of 9.7 and 9.3 were reported among children 5–9 and 10–14 years old, respectively (Fig. 4A). Adults (≥30 years old) had the lowest CFR (4%). Suspected meningitis cases between the ages of 5 and 14 years old were nearly twice as likely to die than adults older than 30 years old (P < 0.01) (Table 2). Two of the three confirmed meningitis cases among infants were attributed to pneumococcus. There was at least one case of pneumococcal meningitis in all the age groups, except among the 15–29 year olds (Fig. 4B). The only cases of H. influenzae and GBS were reported in children aged 4 and 6 years. Two-thirds, of the confirmed meningococcus confirmed cases were among children between 5 and 14 years old (Fig. 4B).
Case fatality rates and laboratory-confirmed cases by age group in Zamfara State. The case fatality rates and total suspected cases are shown by age group (A). The distribution of pathogens among laboratory-confirmed cases by age-group (B). The suspected case counts by age group are presented in Table 1.
Discussion
We report the largest NmC meningitis outbreak to hit a single state in Nigeria to date. Zamfara state in North-western Nigeria accounted for more than half of the 14,280 suspected meningitis cases reported across the country during the reported period of the outbreak20. This outbreak followed three previous outbreaks that occurred in North-western Nigeria after the successful MenAfriVac mass vaccination campaigns. Prior to the recent outbreaks, the last NmC outbreak in sub-Saharan Africa was reported in 1979 in Burkina Faso10. The emergence and expansion of NmC ST-10217 CC with the propensity to cause large outbreaks threatens the public health gains made by the MenAfriVac campaigns7,16. This reinforces the need for strategic vision and action towards controlling meningitis epidemics in the meningitis belt.
Funk and colleagues reported NmC meningitis outbreaks that occurred sequentially in Sokoto (2013) and Kebbi (2014). These outbreaks were relatively small, with less than a thousand suspected cases and localised to specific regions in the affected states12. In 2015, larger outbreaks with 6,394 suspected cases were reported in Sokoto, Kebbi and Niger states11. Sidikou and colleagues also reported a large meningococcal meningitis outbreak with almost ten thousand suspected cases and 549 deaths in 201513. The 2016–2017 outbreak in Nigeria was much larger with 553 deaths in Zamfara state alone. All previous outbreaks were attributed to a novel strain of NmC ST-10217 CC. As with previous outbreaks, sequenced NmC also belonged to the ST-10217 CC. In 2016, NmC meningitis localised outbreaks were reported in Niger and Nigeria along with sporadic cases reported in Cote d’Ivore, Burkina Faso and Ghana21. Brynildsrud and colleagues performed whole-genome sequencing on 150 meningococcal isolates collected during outbreaks in Nigeria and Niger between 2013 and 201716. Their analysis which included four NmC isolates collected from meningitis cases from Zamfara State in 2017 confirmed the dominance of ST-10217. Brynildsrud and colleagues demonstrated that this hypervirulent NmC ST-10217 emerged through the acquisition of virulence factors and capsule genes16.
At a meeting on meningitis outbreak preparedness convened by WHO in 2015, global experts predicted that NmC outbreaks would continue to occur across the meningitis belt17. It may be possible that the current increase in NmC outbreaks is due to serogroup replacement following the mass MenAfriVac campaigns and this warrants further investigation. A key driver of this expansion is poor population immunity as carriage rates of NmC have historically been very low22. Furthermore, since the last NmC outbreak that occurred in the 20th Century, there have only been a few mass vaccination campaigns targeted at NmC17. This is further compounded by the emergence of a novel virulent strain of NmC genetically distinct from previously described strains11,14. In light of this, the expansion in magnitude and spatial spread of NmC culminating in a large countrywide outbreak in Nigeria was predictable23.
There are several polysaccharide and conjugate vaccine formulations that include NmC. However, these vaccines have limited availability and are prohibitively expensive even at subsidized cost. The WHO currently recommends that the limited vaccine stocks be reserved for epidemics24. However, reactive vaccination is hugely dependent on strong and effective in-country surveillance systems with the capacity to rapidly and accurately detect, report and respond to meningitis outbreaks13. The revised WHO alert threshold is 3 confirmed cases per 100,000 per week for meningitis24. Unfortunately, many countries in the meningitis belt, including Nigeria, have weak primary health care and national disease surveillance systems. The first cases of meningitis in Zamfara state occurred in December 2016 but only reported to the national level in February 2017. The NmC vaccination campaigns did not start until April of 2017 at which point the outbreak had gone over its peak (Fig. 3).
One major constraint that delayed adequate and timely responses to the explosive meningitis outbreak was the lack of physicians to diagnose suspected meningitis cases and perform lumbar punctures. A small proportion, 6% of the suspected meningitis cases had lumber puncture performed (Fig. 1). Another important challenge was the poor logistical support for specimen transport. In the few LGAs where the doctors were skilled enough to perform LPs, there were no systems in place for specimen transport to the microbiology laboratories. As a consequence, culture was performed on only a third of CSF specimens collected and the culture positivity was also low at 9%. The requisite TI transport media was not always available and most laboratories in this state did not have the capacity to detect and characterize bacterial agents of meningitis. Strengthening of national disease surveillance systems and laboratories is key in minimizing vaccine-preventable deaths due to meningococcal meningitis in the future.
In previous NmC outbreaks in northern Nigeria and Niger, the highest proportions of cases (39–48%) were among children 5 to 14 years old11,12,13. Likewise, in this outbreak, children 5 to 14 years old accounted for nearly half of suspected meningitis cases and for an even higher proportion (60%) of deaths. The CFR among children 5 to 9 years old was 9.7, more than double the CFR among adults 30 years and older. These findings suggest that older children (5–14 years) may not only be more vulnerable to NmC meningitis but also more likely to die from it. WHO recommends vaccination of individuals 2–29 years during meningitis outbreaks17. However, targeted preventative mass vaccination campaigns for children 5–14 years in the most vulnerable communities may curtail NmC outbreaks and markedly reduce deaths.
An epidemiological curve similar to the 2015 Niger NmC outbreak13 was observed. The number of new suspected meningitis cases remained relatively stable until the 15th week after which there was a sharp rise in new suspected cases. In contrast, outbreaks caused by NmA, NmW and NmX tend to increase steadily peaking earlier in the epidemic season19. Slow initial progression followed by rapid acceleration to the peak of the outbreak, towards the end of the epidemic season may be a unique feature of NmC outbreaks. This pattern reinforces the need to have in place adequate outbreak preparedness plans alongside stockpiling medical and laboratory supplies including NmC-containing vaccines.
All five serotyped pneumococci belonged to serotypes included in the 10-valent pneumococcal conjugate vaccine (PCV10) implemented in Nigeria. Large pneumococcal outbreaks have been reported in Burkina Faso, Togo and northern Ghana in the past25,26,27,28 and more recently in the Brong Ahafo Region of Ghana29. West African pneumococcal outbreaks have characteristically high case-fatality rates (up to 40%) and appear to mimic meningococcal meningitis outbreaks. Although pneumococcus accounted for only 5% of confirmed cases in this outbreak, this finding highlights the need for surveillance and monitoring pneumococcal invasive disease in Nigeria.
An important limitation of this study is that only a very small proportion (6%) of the 7140 suspected cases had lumbar puncture performed and an even smaller fraction (5%) were tested in the laboratory. In addition, highly sensitive quantitative PCR testing was not performed on nearly half of the CSF specimens collected (Fig. 1). As a consequence, we probably missed a significant number of positive specimens. The meningococcal isolates were lost due to poor storage conditions in Zamfara state, therefore more extensive antibiotic resistance and genomic characterisation of the strains was not performed. Genotyping by MLST of the strains had to be performed directly from CSF specimens and this could only be done for two specimens due to limited funds. This report would have been enriched by the inclusion of more detailed clinical and vaccination history data of the suspected meningitis cases. For instance, we could not assess the clinical features such as meningococcaemia that could be associated with high mortality. Unfortunately, clinical and demographic data were not recorded routinely among suspected meningitis cases.
The NmC outbreak in Zamfara with over 500 hundred deaths is strong evidence of the on-going expansion of NmC epidemics. To curtail the spread of NmC outbreaks, governments and partner agencies should consider investing in targeted vaccination of the most vulnerable children (5–14 years old) and communities. However, sustainable control and elimination of meningitis outbreaks in the Africa meningitis belt may depend on the extension of the highly successful preventive MenAfriVac campaign framework to include multivalent conjugate meningococcal vaccine that covers the serogroups with the potential to cause outbreaks7,30. Licensed NmC conjugate vaccines remain largely unaffordable to countries facing the biggest risk of outbreaks. A polyvalent vaccine containing serogroups C and X conjugates has been developed by the Serum Institute of India and is currently undergoing clinical trials in Mali31. As the threat of large meningitis outbreaks looms with every harmattan season, a global action plan that will mobilise resources is urgently needed to strengthen surveillance and preparedness systems in Africa. Without such an action plan, it is likely that many more lives will be lost.
Materials and Methods
Study Area
Zamfara State is located in North-western Nigeria and has an estimated population of 3.85 million. The largest referral hospital, Ahmad Sani Yariman Bakura Specialist Hospital Gusau is in the state capital, Gusau. The state is divided into fourteen LGA. All LGAs, between December 2016 and May 2017, reported suspected meningitis cases. Zamfara has a Sahel Climate, mostly hot and dry, with temperatures rising as high as 40 °C between March and May. The Harmattan, hot and dry winds, lasts from February until the onset of the rains in June.
Case definitions
Suspected meningitis was defined as sudden onset of fever (>38 °C) in combination with stiff neck, altered consciousness and/or petechial rash among patients older than one year. Among infants, suspected meningitis was defined as sudden onset of fever with bulging fontanel and/or petechial rash. Cerebrospinal fluid (CSF) was collected from suspected meningitis patients. Patients with suspected meningitis were treated with ceftriaxone as recommended by WHO17. Cerebrospinal fluid (CSF) was collected from patients meeting the case definition. Suspected cases were reported to the LGA and the State Disease Surveillance Team (DST).
Bacteriologic analysis of CSF specimens
For bacteriologic analysis, 0.5–1.0 ml of patients’ CSF specimen were inoculated upon collection into each of Trans-Isolate (TI) medium when available, a plain tube and a calcium oxalate bottle and transported to the Ahmed Sani Yariman Bakura Specialist Hospital, Gusau within 24 hours. Upon arrival in the laboratory, an aliquot of the CSF specimen was centrifuged at 1,000 × g for 10 minutes and the supernatant was boiled for three minutes at 100 °C, cooled and used for Pastorex according to manufacturer’s instructions. The sediment was inoculated onto a plate 5% sheep blood agar and chocolate agar and used to prepare a smear for gram stain. White blood cell (WBC) count and CSF protein testing were done from the plain tube, and CSF glucose tested using the calcium oxalate samples. The improved Neubauer counting chamber was used for the WBC counts.
Following overnight incubation at 37 °C and in 5% CO2, media plates were examined for growth characteristic of pneumococcus, Haemophilus influenzae and meningococcus. Alpha-haemolytic colonies were subjected to optochin (5 μg) testing; and smooth, glistening and moist colonies positive for the oxidase test subjected to Analytical Profile Index (API; BioMérieux SA, France) for confirmation as pneumococcus and meningococcus, respectively.
Serotyping of pneumococcal isolates was done using the latex agglutination method developed at MRCG as described previously32. Meningococcal serogroups were confirmed by slide latex agglutination serogrouping using Pastorex (Bio-Rad, UK) according to manufacturer’s protocol33.
Molecular detection and serotyping
Molecular detection of pneumococcus, H. influenzae and meningococcus in CSF specimens was performed at the NCDC Laboratory, Abuja, Nigeria as previously described29. All CSF specimens analysed were also subjected to an RNaseP gene assay to confirm they were of human origin and to ascertain the integrity of the nucleic acids in the specimens. Specimens positive for H. influenzae and meningococcus were serotyped/serogrouped as previously described29.
CSF specimens positive for pneumococcus were shipped to the WHO CC at MRCG for molecular pneumococcal serotyping using the African scheme as described previously34. CSF specimens with CT values >32 were further subjected to conventional multiplex PCRs also described elsewhere35,36.
Meningococcal Genotyping
MLST PCRs were carried out directly on only two of the CSF specimens that tested positive for NmC as previously described37,38. More specimens could not be tested due to limited funds. Nucleic acids were sent to Macrogen, Amsterdam Netherlands for amplification and sequencing of the seven housekeeping genes (abcZ, adk, aroE, fumC, gdh, pdhC and pgm).
Data collection and analysis
Data collection was carried out using a customized version of the WHO immediate case-based report Integrated Disease Surveillance Report (IDSR) form and the Invasive Bacterial Vaccine Preventable Disease (IB-VPD) surveillance excel line list template. Demographic and clinical data were entered into the Microsoft Excel WHO approved IB-VPD surveillance line list template at the first reporting health facility. The completed ward line lists were collated and verified by the LGA by Disease Surveillance Notification Officers (DSNO) who in turn reported to the State Disease Surveillance Office. All CSF specimens were accompanied with a completed case-based report form in which laboratory results were appended. The Excel data sets from all LGAs were imported and merged into a Microsoft Access database. Pathogen detection and serotyping data were imported and merged with demographic and clinical data in the customised Microsoft Access database. Geographical Position System (GPS) coordinates of the Zamfara government levels (federal, state and LGAs) areas were downloaded from the United States of America Geographical Survey ((USGS), https://www.usgs.gov). Data analysis was carried out using R statistics tool (version 3.3.1) and Microsoft Excel. The maps were generated using QGIS v2.18.
A logistic regression model was applied to estimate odds ratios and their 95% confidence intervals for the associations between mortality and potential risk factors including age, timing (week) of disease onset and LGA of suspected cases. Sex was used as predefined potential confounder in the model. All analyses were carried out using Stata/SE 14.1 (Stata Corp. Texas, USA). P-values < 0.05 have been taken to indicate statistical significance.
Ethics approval and consent to participate
All cases of suspected meningitis were defined following WHO protocol. The study was approved by the scientific coordinating committee of MRC Unit The Gambia and the joint MRC Unit/Gambian Government Ethics Committee (reference number SCC1188). All surveillance participants including parents/guardians of all children gave informed consent prior to enrolment.
The MRC Unit The Gambia, molecular microbiology laboratory submits to the external quality assurance programme of the UK National External Quality Assessment Service (http://www.ukneqas.org.uk) and is a World Health Organization (WHO) Regional Reference Laboratory for invasive bacterial pathogens.
All the methods in this study, including clinical and laboratory were carried out using WHO protocols.
References
Lapeyssonnie, L. Cerebrospinal Meningitis in Africa. Bulletin of the World Health Organization 28(Suppl), 1–114 (1963).
Whittle, H. C. & Greenwood, B. M. Meningococcal meningitis in the northern savanna of Africa. Tropical doctor 6, 99–104, https://doi.org/10.1177/004947557600600303 (1976).
Court, C. Meningitis epidemic sweeps northern Nigeria. Bmj 312, 598 (1996).
Cerebrospinal meningitis: Nigeria. Releve epidemiologique hebdomadaire 71, 80 (1996).
al-Gahtani, Y. M., el Bushra, H. E., al-Qarawi, S. M., al-Zubaidi, A. A. & Fontaine, R. E. Epidemiological investigation of an outbreak of meningococcal meningitis in Makkah (Mecca), Saudi Arabia, 1992. Epidemiology and infection 115, 399–409 (1995).
Sow, S. O. et al. Immunogenicity and safety of a meningococcal A conjugate vaccine in Africans. The New England journal of medicine 364, 2293–2304, https://doi.org/10.1056/NEJMoa1003812 (2011).
Trotter, C. L. et al. Impact of MenAfriVac in nine countries of the African meningitis belt, 2010-15: an analysis of surveillance data. The Lancet. Infectious diseases. https://doi.org/10.1016/S1473-3099(17)30301-8 (2017).
LaForce, F. M. & Okwo-Bele, J. M. Eliminating epidemic Group A meningococcal meningitis in Africa through a new vaccine. Health Aff (Millwood) 30, 1049–1057, https://doi.org/10.1377/hlthaff.2011.0328 (2011).
Evans-Jones, L. G., Whittle, H. C., Onyewotu, I. I., Egler, L. J. & Greenwood, B. M. Comparative study of group A and group C meningococcal infection. Arch Dis Child 52, 320–323 (1977).
Broome, C. V. et al. Epidemic group C meningococcal meningitis in Upper Volta, 1979. Bulletin of the World Health Organization 61, 325–330 (1983).
Chow, J. et al. Invasive Meningococcal Meningitis Serogroup C Outbreak in Northwest Nigeria, 2015 - Third Consecutive Outbreak of a New Strain. PLoS currents 8, https://doi.org/10.1371/currents.outbreaks.06d10b6b4e690917d8b0a04268906143 (2016).
Funk, A. et al. Sequential outbreaks due to a new strain of Neisseria meningitidis serogroup C in northern Nigeria, 2013-14. PLoS currents 6, https://doi.org/10.1371/currents.outbreaks.b50c2aaf1032b3ccade0fca0b63ee518 (2014).
Sidikou, F. et al. Emergence of epidemic Neisseria meningitidis serogroup C in Niger, 2015: an analysis of national surveillance data. The Lancet. Infectious diseases 16, 1288–1294, https://doi.org/10.1016/S1473-3099(16)30253-5 (2016).
Kretz, C. B. et al. Whole-Genome Characterization of Epidemic Neisseria meningitidis Serogroup C and Resurgence of Serogroup W, Niger, 2015. Emerging infectious diseases 22, 1762–1768, https://doi.org/10.3201/eid2210.160468 (2016).
Mustapha, M. M. & Harrison, L. H. Vaccine prevention of meningococcal disease in Africa: Major advances, remaining challenges. Hum Vaccin Immunother 14, 1107–1115, https://doi.org/10.1080/21645515.2017.1412020 (2018).
Brynildsrud, O. B. et al. Acquisition of virulence genes by a carrier strain gave rise to the ongoing epidemics of meningococcal disease in West Africa. Proc Natl Acad Sci USA 115, 5510–5515, https://doi.org/10.1073/pnas.1802298115 (2018).
Preparedness for outbreaks of meningococcal meningitis due to Neisseria meningitidis serogroup C in Africa: recommendations from a WHO expert consultation. Releve epidemiologique hebdomadaire 90, 633–636 (2015).
Obaro, S. K. & Habib, A. G. Control of meningitis outbreaks in the African meningitis belt. The Lancet. Infectious diseases 16, 400–402, https://doi.org/10.1016/S1473-3099(16)00121-3 (2016).
Borrow, R. et al. The Global Meningococcal Initiative: global epidemiology, the impact of vaccines on meningococcal disease and the importance of herd protection. Expert review of vaccines 16, 313–328, https://doi.org/10.1080/14760584.2017.1258308 (2017).
Nnadi, C. et al. Large Outbreak of Neisseria meningitidis Serogroup C - Nigeria, December 2016-June 2017. MMWR Morb Mortal Wkly Rep 66, 1352–1356, https://doi.org/10.15585/mmwr.mm6649a3 (2017).
Epidemic meningitis control in countries of the African meningitis belt, 2016. Releve epidemiologique hebdomadaire 92, 145–154 (2017).
The Diversity of Meningococcal Carriage Across the African Meningitis Belt and the Impact of Vaccination With a Group A Meningococcal Conjugate Vaccine. The Journal of infectious diseases 212, 1298—1307, 10.1093/infdis/jiv211 (2015).
Continuing risk of meningitis due to Neisseria meningitidis serogroup C in Africa: revised recommendations from a WHO expert consultation. Wkly Epidemiol Rec 92, 612–617 (2017).
World Health, O. WHO position paper, Meningococcal A conjugate vaccine: Updated guidance, February 2015. Vaccine, 10.1016/j.vaccine.2017.07.063 (2017).
Yaro, S. et al. Epidemiological and molecular characteristics of a highly lethal pneumococcal meningitis epidemic in Burkina Faso. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 43, 693–700, https://doi.org/10.1086/506940 (2006).
Traore, Y. et al. Incidence, seasonality, age distribution, and mortality of pneumococcal meningitis in Burkina Faso and Togo. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 48(Suppl 2), S181–189, https://doi.org/10.1086/596498 (2009).
A Study of the Meningococcus. British medical journal 1, 210–211 (1929).
Leimkugel, J. et al. An outbreak of serotype 1 Streptococcus pneumoniae meningitis in northern Ghana with features that are characteristic of Neisseria meningitidis meningitis epidemics. The Journal of infectious diseases 192, 192–199, https://doi.org/10.1086/431151 (2005).
Kwambana-Adams, B. A. et al. An outbreak of pneumococcal meningitis among older children (>/=5 years) and adults after the implementation of an infant vaccination programme with the 13-valent pneumococcal conjugate vaccine in Ghana. BMC Infect Dis 16, 575, https://doi.org/10.1186/s12879-016-1914-3 (2016).
Obaro, S. K. Surveillance in Africa’s meningitis belt: time for new targets? The Lancet. Global health 4, e514–515, https://doi.org/10.1016/S2214-109X(16)30095-X (2016).
LaForce, F. M., Djingarey, M., Viviani, S. & Preziosi, M. P. Successful African introduction of a new Group A meningococcal conjugate vaccine: Future challenges and next steps. Hum Vaccin Immunother 14, 1098–1102, https://doi.org/10.1080/21645515.2017.1378841 (2018).
Hill, P. et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Gambian infants: a longitudinal study. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America 46, 807–814 (2008).
CLSI. Performance Standards for Antimicrobial Susceptibility Testing. Twenty-Fourth Information Supplement (M100-S24); January 2014.
Pimenta, F. C. et al. Sequential triplex real-time PCR assay for detecting 21 pneumococcal capsular serotypes that account for a high global disease burden. Journal of clinical microbiology 51, 647–652, https://doi.org/10.1128/JCM.02927-12 (2013).
Pai, R., Gertz, R. E. & Beall, B. Sequential multiplex PCR approach for determining capsular serotypes of Streptococcus pneumoniae isolates. Journal of clinical microbiology 44, 124–131, https://doi.org/10.1128/JCM.44.1.124-131.2006 (2006).
Dias, C. A., Teixeira, L. M., Carvalho Mda, G. & Beall, B. Sequential multiplex PCR for determining capsular serotypes of pneumococci recovered from Brazilian children. J Med Microbiol 56, 1185–1188, https://doi.org/10.1099/jmm.0.47347-0 (2007).
Birtles, A. et al. Multilocus sequence typing of Neisseria meningitidis directly from clinical samples and application of the method to the investigation of meningococcal disease case clusters. Journal of clinical microbiology 43, 6007–6014, https://doi.org/10.1128/JCM.43.12.6007-6014.2005 (2005).
Kriz, P., Kalmusova, J. & Felsberg, J. Multilocus sequence typing of Neisseria meningitidis directly from cerebrospinal fluid. Epidemiology and infection 128, 157–160 (2002).
Acknowledgements
We would like to thank the Nigeria Federal Ministry of Health and the Nigeria Centre for Disease Control (NCDC), the health care workers, disease surveillance officers, data managers and biomedical scientists who worked relentlessly during the outbreak. We would also like to thank our partners at Public Health England (PHE) and the Centre for Disease Control (CDC), USA for supporting the outbreak response. WHO sponsored the outbreak response, technical mission and donated consumables and equipment. MRCG hosts the WHO CC and supported the outbreak response. The Research Molecular Microbiology Group at MRCG provided technical support for sample shipment, storage and analysis. We also thank Miss Miriam Wathuo for providing statistical advice and support. Finally, we would like to acknowledge the contributions of all the patients and their families.
Author information
Authors and Affiliations
Contributions
B.A.K.A., A.C.R., C.O., E.F., E.B., M.S. and M.A. led the writing of the manuscript. A.C.R., C.O., E.F., R.M., A.G., B.Y., S.R., Y.A., B.H., A.B., processed C.S.F. samples by Gram staining, rapid diagnostic tests (Pastorex) and culture at the Ahmad Sani Yariman Bakura Specialist Hospital Gusau, Zamfara State, Nigeria. C.O., A.C.R., P.M., E.E., S.B., A.A., O.A. and M.A. performed molecular analysis of C.S.F. samples at the N.C.D.C. National Reference Laboratory, Abuja Nigeria. B.A.K.A., C.O., A.W., S.A.S. and M.A. performed M.L.S.T. on C.S.F. samples and determined S.T. of N.m. C. strains. A.W. and B.A.K.A. carried out data management and data management analyses. L.O., K.A., M.I., B.A., O.I., S.M., R.O., M.H.J., A.M., G.S., D.H., W.A., D.U., E.B., U.A., U.R., A.A., A.O. and M.A. led and coordinated the outbreak response. I.C. and M.A. conceived the project and contributed equally to data acquisition, analyses, interpretation and writing of the manuscript. All authors have read and approved the article for submission.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kwambana-Adams, B.A., Amaza, R.C., Okoi, C. et al. Meningococcus serogroup C clonal complex ST-10217 outbreak in Zamfara State, Northern Nigeria. Sci Rep 8, 14194 (2018). https://doi.org/10.1038/s41598-018-32475-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32475-2
Keywords
This article is cited by
-
Meningococcal Disease and Immunization Activities in Hajj and Umrah Pilgrimage: a review
Infectious Diseases and Therapy (2022)
-
An overview of bacterial meningitis epidemics in Africa from 1928 to 2018 with a focus on epidemics “outside-the-belt”
BMC Infectious Diseases (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.