Abstract
An intensified and continuous West Nile virus (WNV) spread across northern Italy has been observed since 2008, which caused more than one hundred reported human infections until 2016. Veneto is one of the Italian regions where WNV is considered endemic, and the greatest intensity of circulation was observed during 2013 and 2016. By using entomological data collected across the region in those years, we calibrated a temperature-driven mathematical model through a Bayesian approach that simulates the WNV infection in an avian population with seasonal demography. We considered two alternative routes of life cycle re-activation of the virus at the beginning of each vector breeding season: in the first one the virus is maintained by infected birds, in the other by diapausing mosquitoes previously infected. Afterwards, we computed seasonal risk curves for human infection and quantified how they translate into reported symptomatic cases. According to our results, WNV is more likely to be re-activated each year via previously infected mosquitoes. The highest probability of human infection is expected to occur in August, consistently with observations. Our epidemiological estimates can be of particular interest for public health authorities, to support decisions in term of designing efficient surveillance plans and preventive measures.
Similar content being viewed by others
Introduction
West Nile virus (WNV) is a neurotropic mosquito-borne virus belonging to the Flavivirus genus and Japanese encephalitis virus serogroup1. WNV is maintained by an enzootic cycle involving birds and ornithophilic mosquitoes such as species of the Culex genus of which the Culex pipiens complex is thought to be one of the most important vectors in Europe2. Some passerine birds are among the most competent amplifier hosts since they develop sufficient serum viremia to infect efficiently mosquitoes feeding upon them3,4. Humans, horses and other mammals are dead-end hosts that may be incidentally involved in the enzootic cycle. While most human infections are asymptomatic, about 25% of the infections develop symptoms such as fever and headache5 and less than 1% more severe neurological diseases6.
WNV is a significant burden for public health in Europe, as it caused hundreds of cases during the last decade7. In Italy, WNV has caused severe illnesses in humans every season in many different regions. Despite its substantial impact, some of WNV epidemiological and ecological features have not completely elucidated yet. For instance, it is not known what proportion of persons develop infection following an infected mosquito bite8. While WNV is considered endemic in many parts of Europe, its persistence mechanism is still under discussion; it might survive from one season to the next in resident avian hosts9,10, mosquitoes11 or it might be reintroduced each year with migratory birds12.
WNV was detected in Italy for the first time in 1998, during an outbreak among horses in Tuscany13, and then re-emerged ten years later in the northeastern part of the country. Though first detections belonged to lineage 1 (the Western Mediterranean clade), WNV lineage 2 (WNV-2) is nowadays the most frequently identified lineage in Italy14. The first human infection due to WNV-2 was reported in Central Italy in 201115 and subsequently WNV-2 became the only strain isolated in humans and mosquitoes in Italy16. Recent phylogenetic investigations show that WNV-2 probably entered Italy in 2007 from the east Adriatic coast and reached the northeastern part of the country in 201117, with a peak of human cases in 201314. WNV cases have been recorded each year across the country, with peaks occurring between August and September18,19.
In this study, we examine WNV transmission in Veneto region (northeastern Italy), where it is now considered endemic. In fact, WNV has been detected in Cx. pipiens mosquitoes20 and a number of cases of human or horse/bird infections are reported every year within the region16. In particular, we focus on determining the most likely route of life cycle re-activation, i.e. whether WNV transmission starts each year with already infectious birds or mosquitoes; in this latter case mosquitoes were possibly infected the previous year and overwintered in a diapausing state. To this aim, we develop a mechanistic temperature-driven model that replicates the avian-vector transmission cycle. While following the structure of previously published studies (e.g21,22.), our modelling effort relies on some important novelties. Indeed, it is calibrated on the vector prevalence recorded in the study area over multiple years and the mosquito dynamics is not modelled explicitly but it is approximated using field observation. Using our modelling results, we estimate the risk of human transmission during the mosquito breeding season (May-October). Finally, we fit the observed human cases to quantify how the human transmission risk predicted by the model translates into reported symptomatic cases.
Methods
Study area and entomological data
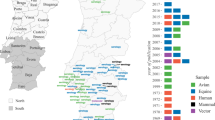
The study area is located in the plain part of Veneto region (northeastern Italy, average altitude 77 m) and includes the provinces of Padova, Rovigo, Treviso, Venezia, Verona and Vicenza. A total of 109 trap locations (orange points, Fig. 1) were chosen among 92 municipalities. Mosquitoes were collected from May to October 2013–2016, using CDC traps baited with CO2. Once every two weeks, each trap was set in the morning and checked after 24 hours. After identification, individuals belonging to the Cx. pipiens species were pooled (average pool size of 50 mosquitoes) to detect the presence of WNV.
We divided the study area in 4 separated clusters (colored regions in Fig. 1) according to their locations by the k-means clustering method23. The four clusters’ extension range from 2,719 km2 to 3,527 km2, with population ranging from 574,000 to 1,696,507 inhabitants.
Temperature data for each cluster, collected with ground stations, was obtained from ARPA Veneto24. As all clusters exhibit very similar landscape characteristics, they also show very similar temperature patterns during the year (see Supplementary Material). The average temperature for the study period (May-October) is 20.3 °C, with maximum and minimum daily records of 30.6 °C in 2015 and 7.2 °C in 2014 respectively.
WNV model
For each cluster, we approximated the average Cx. pipiens weekly captures with a smooth spline. Thus, after rescaling them by the trapping capture rate, α, we obtained for any day t between May 1 and October 31 an average adult mosquito density M(t) for an average trapped area A for every considered cluster, with A = π∙r2, where r is the average Cx. pipiens flight range.
We modelled the transmission of WNV within the average trapped area A among an initially fully susceptible avian community during a typical season (i.e. from May 1 to October 31) according to the compartmental scheme reported in Fig. 2 (model equations can be found in the Supplementary Material). The biological interpretation of model parameters and their values is summarized in Table 1 (see also the Supplementary Material for more details). For the sake of simplicity, we consider all WNV-competent birds as belonging to a single species, whose parameters correspond to those of the House sparrow (Passer domesticus), a common species in the area25 which is competent for WNV transmission4 and is considered to be one of the most important avian reservoirs for the virus26. At the beginning of the season (May 1) the bird community is assumed to consist of adult individuals only, which can breed and reproduce until mid-July, giving birth to juvenile individuals. As maturity age is one year27, they are considered as juveniles throughout the entire season. Susceptible adult (juvenile) birds Bsa(Bsj) contract the virus from bites of infectious mosquitoes. After an intrinsic incubation period, they become infectious and subsequently recover and become immune to reinfections. We did not consider possible deaths due to the infection as laboratory experiments show a low or even no mortality for this species when infected with European WNV strains4 and very limited wild bird mortality due to WNV is usually observed in Europe2. Susceptible mosquitoes (Ms) can become exposed to infection (Me) after biting infectious birds with a temperature-dependent probability (see Table 1); in such a case, they will become infectious to the avian population (Mi) after a temperature-dependent extrinsic incubation period and for the rest of their life. We neglected vertical transmission as it occurs at a very low rate28,29 and is probably relevant only for infection persistence between seasons30,31.
Model scheme. Model flow chart for WNV transmission in birds (squares) and mosquitoes (circles) in an average trapped area. Compartments: Bsa, Bea, Bia, Bra (Bsj, Bej, Bij, Brj): adult (juvenile) susceptible, exposed, infectious and immune birds; Ms, Me, Mi: susceptible, exposed and infectious mosquitoes. Parameters: λB and λM are the force of infection for birds and mosquitoes respectively and are computed as λB = b∙pMB∙Mi/BT and λM = b∙pBM∙(Bia + Bij)/BT, with BT being the total avian population and Ba the number of adult birds. See Table 1 for parameter values and references.
We assume that mosquitoes die at temperature-dependent rate μM, but we do not model completely mosquito population dynamics, as it will depend in a complex way on several environmental variables. Rather we consider the total number of adult mosquitoes, M(t), obtained from the spline, as a known function; to this aim, if ω(t) = M(t) − (Ms(t) + Me(t) + Mi(t)) > 0, then at day t new ω(t) susceptible mosquitoes enter the system.
As stated above, we model independently every breeding season and therefore we need the virus to be already present at its beginning. For the sake of simplicity, we considered two alternative routes of re-activation of the virus: B) the virus is alive in infected birds (Bi(0) > 0, Mi(0) = 0); M) some initial mosquitoes are already infectious (Bi(0) = 0, Mi(0) > 0). To assess which hypothesis explained better the observed data, we compared the goodness of fit of the two modelling scenarios using the Deviance Information Criterion (DIC)32.
The initial number of adult birds B0(y), the initial prevalence p(y) (either in birds or in mosquitoes, according to the considered scenario), which are year-dependent, and the biting rate b, which is assumed to be equal among years, are the set of unknown parameters Ψ (i.e. Ψ = {B0(2013), B0(2016), p(2013), p(2016), b}. The posterior distributions of Ψ were explored by Markov chain Monte Carlo (MCMC) sampling applied to the binomial likelihood of observing the recorded number of positive pool, given the model-predicted mosquito prevalence. Assuming that for each week the number of observed positive follows a binomial distribution B(N, P) where N is the number of tested pool and P the probability that a pool is positive obtained from the model, the likelihood of the observed data over the two simulated years has been defined as
Where y and s run over the considered years and sampling dates respectively, Ns(y) is the number of samplings for year y, K(s, y) is the number of recorded positive pools for sampling s and year y and
where Mi(s, Ψ) and M(s, Ψ) are respectively the number of infected and total mosquitoes predicted by the model for the sampling date s with parameters Ψ and u(s) is the average pool size. The posterior distribution of Ψ was obtained by using random-walk Metropolis-Hastings sampling approach and normal jump distributions.
Finally, we performed a sensitivity analysis by exploring how different values for the model constant parameters, namely pMB, δB, θB, μB, μBj and γ, might affect the model results. We evaluated the effect of these perturbations both on the predicted avian and mosquito infection prevalence and on the estimated posterior distribution of the free model parameters (see Supplementary Material).
Human infections
On average, about f = 6.9% of Cx. pipiens blood meals are taken from human hosts in the study area25. We estimated the probability λH(w) for a human living in a cluster to be bitten by a WNV infected mosquito in any week w during the study season as follows: we divided the estimated number of daily infectious vector bites on humans, obtained as f∙b∙Mi(w) where b is the previously estimated biting rate, by the number of humans living in the study area A. We also allowed the vector to shift the feeding preference during each year33. Specifically, we let f vary between early and late summer as reported in25, where the authors found that the percentage of Cx. pipiens blood meals on human increased from 3.6% to 8.3% after June 30.
Finally, we investigated which scenario (B or M with or without a shifting mosquito feeding preference) better explains the symptomatic human infections observed in a cluster, by predicting the number of reported WNV human infections Nw from a Poisson(H∙ρ∙ λH(w)), where H is the number of human beings living in a cluster and ρ is a free rescaling parameter, estimated with a MCMC approach applied to the Poisson likelihood of observing the recorded infections, given the model predictions, by modelling together the considered years. We can interpret ρ as a product of the probability of virus transmission to humans per mosquito infectious bite times the probability of symptoms development times the reporting rate.
Results
Cluster analysis
The number of WNV positive pools found in the study area greatly varied between years, from a minimum of 3 in 2015 to a maximum of 57 in 2013. We decided to focus our analysis on 2013 and 2016 (27 WNV positive pools), the two years with most positive pools and highest human incidence19. Mosquito trapping season started on May 7 and 20 in 2013 and 2016 respectively. Moreover, we carried out model simulations only for the cluster that includes the province of Verona and partially of Rovigo (in red in Fig. 1, area 3,527 km2, 1,016,138 inhabitants) as the great majority of the positive pools were collected in that area (respectively 50 and 22 for the two years). The interpolated mosquito abundance for this cluster in the two study years is shown in the Supplementary Material.
Model fit
As shown in Fig. 3 (first row) the model fit is qualitatively very similar between the models [B (M): some initial birds (mosquitoes) are already infectious at season start]. The DIC values are 109.23 and 104.03 for scenario B and M respectively, thus the hypothesis that the virus is introduced at the beginning of the season via infected mosquitoes seems significantly more likely. In both cases, we can note that the mosquito prevalence (second row, Fig. 3) starts to increase in June and reaches in maximum in August. Then it declines and remains stable from October onwards. Unsurprisingly, the mosquito prevalence in May is a little higher in scenario M than in B, although it is very low compared to the highest values occurring later in summer. Conversely, the simulated avian prevalence in May is very high for model B (Table 2), and even higher than in summer (Fig. 3, blue lines in panels e and f). These patterns are confirmed by the sensitivity study on model constant parameters (see Supplementary Material).
Model predictions. Predicted number of WNV positive pools (panels a, b) mosquito prevalence (panels c, d) and avian prevalence (panels e, f) for 2013 (first column) and 2016 (second column) according to model assumption: blue: model B; red: model M. Panels a-b: orange points: observed weekly number of WNV positive pools; boxplots (median, quartiles and 95% quantiles) show the predicted distributions of positive pools per week. Panels c-f: solid lines: average values; shaded regions: 95% credible interval.
At the end of the season, the fraction of recovered birds (Bra + Brj)/BT is on average between 0.66 and 0.92 (see Supplementary Material). In particular, it is lower in 2013 than in 2016 regardless of the initial hypothesis. In the case of model M, this fraction starts to increase exponentially in July, as avian prevalence increases, reaching a steady level in September. In the case of model B, recovered birds are about 10% of the total avian community in June already, in line with estimated initial prevalences (Table 2).
The estimated posterior distributions for the biting rate are quite close for the two modelling assumptions (Table 2). Conversely, the estimated number of initial birds is lower for model M and in both models the avian population for 2016 is expected to be smaller than in 2013. We can note that the estimated initial prevalence is much higher for birds than for mosquitoes. Finally, perturbations of the model constant parameters do not produce substantial variations of posterior distributions (see the sensitivity analysis within the Supplementary Material).
Human infections
In the considered cluster, 16 and 8 human WNV symptomatic infections were recorded in 2013 and 2016 respectively, with dates of symptoms onset ranging from mid-July to the end of September.
As shown in Fig. 4 (first row of each panel), the predicted risk λH for a human living in the considered cluster of being bitten by an infectious mosquito in one week varies substantially between the two years (accordingly to the higher proportion of positive pools recorded in 2013 than in 2016), and they are higher when assuming time-dependent feeding preferences. In fact, the highest average probabilities, about 0.011 and 0.007, occur at the beginning of August in 2013 and 2016 respectively by allowing f to take different values before and after June 30 (panels c, d). In general, we can note that from late July to the end of August humans are at greater risk of WNV transmission, consistently with observations. Remarkably, the model predicts a halved risk for 2016 with respect to 2013, following the same pattern observed for the number of cases.
Human transmission risk and infections. Estimated weekly probability per person of being bitten by a WNV infected mosquito (λH, first row of each panel) and notified human symptomatic cases (second row of each panel) in 2013 (first column) and 2016 (second column). Blue: the virus is introduced with birds (model B). Red: the virus is introduced with mosquitoes (model M). Panels a, b: f = 5.9%. Panels c, d: f = 3.6% until June 30, afterwards f = 8.3%. Solid lines: average value. Shaded regions: 95% credible interval. Orange triangles: observed WNV symptomatic cases per week of symptoms onset. Boxplots (median, quartiles and 95% quantiles): predicted symptomatic recorded cases.
Figure 4 (second row of each panel) shows the fit of the observed number of WNV symptomatic cases for the two years. It is clear that different model assumptions produce fits that are qualitatively very similar, and in fact the associated DIC values are very close (Table 3). Anyhow, we can note that the lowest DIC value is achieved assuming the virus is introduced with infected mosquitoes (model M) and f shifts from 3.6% to 8.3% after June 30. ρ estimates concur between different models, being on average between 1.3∙10−3 and 1.9∙10−3, meaning that between one and two out of 1,000 infectious mosquito bites on humans will result in a symptomatic reported case.
Discussion
In this study, we analysed the dynamics of WNV in Veneto region, Northern Italy. In particular, we investigated how the virus life cycle is re-activated every year in the area and we quantified the risk of human spillover.
Our modelling approach included the estimate of some unknown parameters, such as the avian population size and the mosquito biting rate. Assuming our model simulates an area A = π∙r2 = 0.79 km2 (r = 500 m), the computed initial House sparrow density spans between 30 and 140 individuals per km2. Although we do not have a precise estimate on the number of birds living in the area, we note that these estimates are consistent with other ranges obtained in different parts of the world27. Our results for the biting rate indicate Cx. pipiens has a gonotrophic cycle of about 5 days during the breeding season in the study region, very close to the measure published in34 (5.54 ± 1.73 days in laboratory conditions). To the best of our knowledge34, presents the only available data regarding the influence of temperature on Culex pipiens gonotrophic cycle. Since there are no data for different temperatures and many WNV modelling studies assume the biting rate to be temperature independent21,22,31,35, we decided to consider b as a free model parameter which is constant during the season.
According to our results, model fits obtained with different assumptions are qualitatively very similar. We computed DIC values for every model fit we performed, in order to assess quantitatively which assumptions better explained the observed values. The difference between the highest and lowest DIC values was 5.2 when fitting the number of observed mosquito positive pools. As the recommended DIC difference threshold is at least two32, assuming the virus is introduced at the beginning of the season by infected mosquitoes (model M) provides a significantly better fit. Conversely, when fitting the observed number of reported symptomatic human infections, different modelling assumptions produce very similar DIC values. Although there is no striking indication in favour of one particular model, we can note that the lowest DIC is given by model M coupled with shifting mosquito-feeding preferences.
Another observation that makes model B (WNV season starts with infected birds) unlikely is that the highest avian prevalence is predicted to occur in spring; this is contrary to observation, as usually WNV positive birds are found in summer36, and would require that most infection transmission would occur when the mosquito density is relatively very low. Indeed, it has been suggested that in North America WNV might persist in winter in American crow populations, which can transmit the virus with fecal-oral transfer9,10. Moreover, it has been demonstrated that WNV can persist in House sparrow tissues, and this may lead to oral transmission to predatory birds and other animals during times of interrupted mosquito activity37. Thus, winter roost sites might be potential points for vernal amplification of WNV once mosquito activity increases. This might be true also for Italy, but very few studies have been conducted to assess European avian species competence for this virus and the relative contribution of non vectorial transmission. Furthermore, our modelling study suggests that the prevalence in birds in spring should be very high to comply with the overall dynamics, and it seems difficult that it would go undetected. Some modelling studies suggest instead that the virus can be introduced in Europe every year through birds migrating from Africa12,38, but phylogenetic analyses of European and Italian WNV lineages strongly support the hypothesis that the virus overwinters in the area and its introduction occurred years ago14,39,40,41,42. Concerning the hypothesis that the virus overwinters through infected mosquitoes (model M), it is possible that vertically-infected diapausing mosquitoes, emerged at the end of the breeding season, initiate transmission in the following spring11 and the first detection of WNV in overwintering mosquitoes in Europe, occurred in 201743, supports this hypothesis. We did not explicitly consider vertical transmission as it occurs sporadically28,29 and modelling the inter-seasons interval (i.e. November-April) was beyond our scope, also because of the lack of data regarding mosquito and bird survivorship in this period.
The average daily avian prevalence was estimated to be no higher than 5% during the summer months, a result similar to actual observations made in different areas. For instance, about 12% of House sparrow nestlings tested during August 2008 were found to be WNV infected in a rural area in the US44 while WNV prevalence in birds was observed to be as high as about 8% in August 2005 in Chicago45.
The final seroprevalence in birds was estimated between 66 and 92% on average. Reported values vary greatly among locations and years: in Spain in 2004, seroprevalence in blackbirds (Turdus merula) was 5.4%46, while for House sparrows it was estimated to be 1.96% in 2013 in the same study area47. On the other hand, in three close rural House sparrow colonies in the US about 27% of birds tested positive for WNV antibodies in 200844, while seroprevalence was observed to be as high as about 30–40% in Los Angeles in October 2004 and 200948 and 24.4% in Chicago between May and October 2005, with a peak value of more than 60% recorded at the end of September45. In Romania, 33.96% of wild resident birds tested positive between 2011 and 2012 after a big WNV outbreak (50 reported human cases) occurred in 201049,50. The particularly high value obtained in our simulations might depend on our simplifying assumption that there is only one avian competent species. In reality there are several of them3,4,51,52,53, whose competence varies considerably. With more species, possibly less competent, WNV spread would be limited thanks to a dilution effect54,55. Lack of detailed ornithological data prevented us from designing a more realistic model. Moreover, we assumed that the avian population is fully susceptible at the start of the season; however, most immune birds will survive winter and therefore they will be present in the following year, thus reducing the number of potential hosts and of WNV infections during the season. This might be a possible explanation of the low WNV circulation observed in the cluster area in 2014 and 2015 (only seven and two positive pools respectively). This might also explain the lower estimate obtained for the initial number of susceptible birds in 2016.
The sensitivity analysis carried out on the constant parameters set enhances the robustness of our findings and the realism of model predictions. In fact, perturbations of model constant parameters produce very small variations in both model simulations and estimated free model parameters (see Supplementary Material).
After calibrating our WNV model, we estimated the human spillover risk. This probability is higher from mid-July to August, and it can be slightly higher than 0.01, meaning that about one in a hundred people will be bitten by an infectious mosquito in that particular week. The human risk was estimated to be lower for 2016, since fewer positive pools were collected with respect to 2013, in compliance with the smaller number of recorded cases. By fitting the reported WNV human infections, we computed the probability for a mosquito infectious bite to result in a reported human infection. To the best of our knowledge, there are no estimates for the transmission probability from mosquitoes to humans8, nor for the reporting rate for WNV infections, while the proportion of symptomatic infections is about 25%5. Thus, our result might be particularly useful to provide some estimate of the symptomatic human infections also in other similar regions.
In addition, we implemented a simpler fit of the observed human infections by using only the mosquito prevalence observed with the pools and the interpolated vector abundance, without explicitly modelling the avian-vector transmission cycle (see Supplementary Material). However, this simplified method yielded a much worse fit, both qualitatively and quantitatively. This negative result corroborates the relevance of our modelling approach, making our findings more useful and reliable.
Despite its limitations, our study provides new important insights on the ecology of WNV in Southern Europe, in particular regarding its endemism and seasonal dynamics. Estimated human infection risk during the season can be of particular interest for public health authorities, to support decision in designing efficient surveillance and prevention strategies. However, this study highlights the urgency to carry out more detailed eco-epidemiological studies on WNV host and vector interaction in Italy to obtain more precise estimate of the changing hazard and risk of transmission to humans.
Data Availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Colpitts, T. M., Conway, M. J., Montgomery, R. R. & Fikrig, E. West Nile Virus: Biology, Transmission, and Human Infection. Clin Microbiol Rev 25, 635–648 (2012).
Zeller, H. G. & Schuffenecker, I. West Nile virus: An overview of its spread in europe and the Mediterranean Basin in contrast to its spread in the Americas. Eur. J. Clin. Microbiol. Infect. Dis. 23, 147–156 (2004).
Komar, N. et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerging Infect. Dis. 9, 311–322 (2003).
Del Amo, J. et al. Experimental infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro-Mediterranean and North American origins. Vet. Res. 45, 33 (2014).
Zou, S., Foster, G. A., Dodd, R. Y., Petersen, L. R. & Stramer, S. L. West Nile fever characteristics among viremic persons identified through blood donor screening. J. Infect. Dis. 202, 1354–1361 (2010).
Mostashari, F. et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 358, 261–264 (2001).
European Centre for Disease Prevention and Control. West Nile fever in ECDC. Annual Epidemiological Report for 2015. Stockholm: ECDC (2017).
Petersen, L. R., Brault, A. C. & Nasci, R. S. West Nile virus: review of the literature. JAMA 310, 308–315 (2013).
Hinton, M. G., Reisen, W. K., Wheeler, S. S. & Townsend, A. K. West Nile Virus Activity in a Winter Roost of American Crows (Corvus brachyrhynchos): Is Bird-To-Bird Transmission Important in Persistence and Amplification? J. Med. Entomol. 52, 683–692 (2015).
Montecino-Latorre, D. & Barker, C. M. Overwintering of West Nile virus in a bird community with a communal crow roost. Scientific Reports 8, 6088 (2018).
Nelms, B. M. et al. Phenotypic Variation among Culex pipiens Complex (Diptera: Culicidae) Populations from the Sacramento Valley, California: Horizontal and Vertical Transmission of West Nile Virus, Diapause Potential, Autogeny, and Host Selection. Am J Trop Med Hyg 89, 1168–1178 (2013).
Tran, A. et al. An Integrative Eco-Epidemiological Analysis of West Nile Virus Transmission. Ecohealth 14, 474–489 (2017).
Autorino, G. L. et al. West Nile virus epidemic in horses, Tuscany region, Italy. Emerging Infect. Dis. 8, 1372–1378 (2002).
Zehender, G. et al. Reconstructing the recent West Nile virus lineage 2 epidemic in Europe and Italy using discrete and continuous phylogeography. PLOS ONE 12, e0179679 (2017).
Bagnarelli, P. et al. Human case of autochthonous West Nile virus lineage 2 infection in Italy, September 2011. Eurosurveillance 16, 20002 (2011).
Rizzo, C. et al. West Nile virus transmission: results from the integrated surveillance system in Italy, 2008 to 2015. Eurosurveillance 21, 30340 (2016).
Savini, G. et al. Evidence of West Nile virus lineage 2 circulation in Northern Italy. Vet. Microbiol. 158, 267–273 (2012).
Istituto Superiore di Sanita (ISS). West Nile News. Aggiornamento epidemiologico settimanale. http://www.epicentro.iss.it/problemi/westNile/bollettino/WN_News_2013_13.pdf (2013).
Istituto Superiore di Sanita (ISS). West Nile News. Aggiornamento epidemiologico settimanale. http://www.epicentro.iss.it/problemi/westNile/bollettino/WN_News_2016_11.pdf (2016).
Mancini, G. et al. Mosquito species involved in the circulation of West Nile and Usutu viruses in Italy. Vet. Ital. 53, 97–110 (2017).
Cruz-Pacheco, G., Esteva, L., Montaño-Hirose, J. A. & Vargas, C. Modelling the dynamics of West Nile Virus. Bull. Math. Biol. 67, 1157–1172 (2005).
Wonham, M. J., de-Camino-Beck, T. & Lewis, M. A. An epidemiological model for West Nile virus: invasion analysis and control applications. Proc. Biol. Sci. 271, 501–507 (2004).
Hartigan, J. A. & Wong, M. A. Algorithm AS 136: A K-Means Clustering Algorithm. Journal of the Royal Statistical Society. Series C (Applied Statistics) 28, 100–108 (1979).
Arpa Veneto Dati Ambientali. Available: http://www.arpa.veneto.it/dati-ambientali (2018).
Rizzoli, A. et al. Understanding West Nile virus ecology in Europe: Culex pipiens host feeding preference in a hotspot of virus emergence. Parasites & Vectors 8 213 (2015).
Duggal, N. K. et al. Evidence for Co-evolution of West Nile Virus and House Sparrows in North America. PLOS Neglected Tropical Diseases 8, e3262 (2014).
Anderson, T. R. Biology of the Ubiquitous House Sparrow: From Genes to Populations. (Oxford University Press, 2006).
Dohm, D. J., Sardelis, M. R. & Turell, M. J. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera: Culicidae). J. Med. Entomol. 39, 640–644 (2002).
Nelms, B. M. et al. Experimental and natural vertical transmission of West Nile virus by California Culex (Diptera: Culicidae) mosquitoes. J. Med. Entomol. 50, 371–378 (2013).
Hartemink, N. A., Davis, S. A., Reiter, P., Hubálek, Z. & Heesterbeek, J. A. P. Importance of bird-to-bird transmission for the establishment of West Nile virus. Vector Borne Zoonotic Dis. 7, 575–584 (2007).
Moschini, P., Bisanzio, D. & Pugliese, A. A Seasonal Model for West Nile Virus. Math. Model. Nat. Phenom. 12, 58–83 (2017).
Spiegelhalter, D. J., Best, N. G., Carlin, B. R. & van der Linde, A. Bayesian measures of model complexity and fit. J. R. Stat. Soc. Ser. B-Stat. Methodol. 64, 583–616 (2002).
Kilpatrick, A. M., Kramer, L. D., Jones, M. J., Marra, P. P. & Daszak, P. West Nile Virus Epidemics in North America Are Driven by Shifts in Mosquito Feeding Behavior. PLOS Biology 4, e82 (2006).
Faraj, C., Elkohli, M. & Lyagoubi, M. Cycle gonotrophique de Culex pipiens (Diptera: Culicidae), vecteur potentiel du virus West Nile, au Maroc: estimation de la durée en laboratoire. Bulletin de la Société de pathologie exotique 99, 119–121 (2006).
Simpson, J. E. et al. Vector hoWest Nile virus as a model st-feeding preferences drive transmission of multi-host pathogens: system. Proceedings of the Royal Society of London B: Biological Sciences 279, 925–933 (2012).
Bellini, R. et al. The experience of West Nile virus integrated surveillance system in the Emilia-Romagna region: five years of implementation, Italy, 2009 to 2013. Eurosurveillance 19, 20953 (2014).
Nemeth, N. et al. Persistent West Nile virus infection in the house sparrow (Passer domesticus). Arch. Virol. 154, 783–789 (2009).
Durand, B., Balança, G., Baldet, T. & Chevalier, V. A metapopulation model to simulate West Nile virus circulation in Western Africa, Southern Europe and the Mediterranean basin. Vet Res 41 (2010).
Rizzoli, A., Rosà, R., Rosso, F., Buckley, A. & Gould, E. West Nile virus circulation detected in northern Italy in sentinel chickens. Vector Borne Zoonotic Dis. 7, 411–417 (2007).
Monaco, F. et al. 2009 West Nile disease epidemic in Italy: first evidence of overwintering in Western Europe? Res. Vet. Sci. 91, 321–326 (2011).
Sotelo, E. et al. Phylogenetic relationships of Western Mediterranean West Nile virus strains (1996–2010) using full-length genome sequences: single or multiple introductions? J. Gen. Virol. 92, 2512–2522 (2011).
Hernández-Triana, L. M. et al. Emergence of West Nile Virus Lineage 2 in Europe: A Review on the Introduction and Spread of a Mosquito-Borne Disease. Front. Public Health 2 (2014).
Rudolf, I. et al. West Nile virus in overwintering mosquitoes, central Europe. Parasit Vectors 10 (2017).
O’Brien, V. A., Meteyer, C. U., Reisen, W. K., Ip, H. S. & Brown, C. R. Prevalence and Pathology of West Nile Virus in Naturally Infected House Sparrows, Western Nebraska, 2008. Am J Trop Med Hyg 82, 937–944 (2010).
Hamer, G. L. et al. Rapid amplification of West Nile virus: the role of hatch-year birds. Vector Borne Zoonotic Dis. 8, 57–67 (2008).
López, G., Jiménez-Clavero, M. A., Tejedor, C. G., Soriguer, R. & Figuerola, J. Prevalence of West Nile virus neutralizing antibodies in Spain is related to the behavior of migratory birds. Vector Borne Zoonotic Dis. 8, 615–621 (2008).
Martínez-de la Puente, J. et al. Mosquito community influences West Nile virus seroprevalence in wild birds: implications for the risk of spillover into human populations. Sci Rep 8, 2599 (2018).
Kwan, J. L., Kluh, S. & Reisen, W. K. Antecedent Avian Immunity Limits Tangential Transmission of West Nile Virus to Humans. PLOS ONE 7, e34127 (2012).
Neghina, A. M. & Neghina, R. Reemergence of human infections with West Nile virus in Romania, 2010: an epidemiological study and brief review of the past situation. Vector Borne Zoonotic Dis. 11, 1289–1292 (2011).
Paştiu, A. I. et al. Wild Birds in Romania Are More Exposed to West Nile Virus Than to Newcastle Disease Virus. Vector Borne Zoonotic Dis. 16, 176–180 (2016).
Lim, S. M. et al. Susceptibility of European jackdaws (Corvus monedula) to experimental infection with lineage 1 and 2 West Nile viruses. J. Gen. Virol. 95, 1320–1329 (2014).
Lim, S. M. et al. Susceptibility of Carrion Crows to Experimental Infection with Lineage 1 and 2 West Nile Viruses. Emerging Infect. Dis. 21, 1357–1365 (2015).
Jiménez de Oya, N. et al. High susceptibility of magpie (Pica pica) to experimental infection with lineage 1 and 2 West Nile virus. PLoS Negl Trop Dis 12, e0006394 (2018).
Ezenwa, V. O., Godsey, M. S., King, R. J. & Guptill, S. C. Avian diversity and West Nile virus: testing associations between biodiversity and infectious disease risk. Proceedings of the Royal Society of London B: Biological Sciences 273, 109–117 (2006).
Swaddle, J. P. & Calos, S. E. Increased Avian Diversity Is Associated with Lower Incidence of Human West Nile Infection: Observation of the Dilution Effect. PLOS ONE 3, e2488 (2008).
Ciota, A. T., Matacchiero, A. C., Kilpatrick, A. M. & Kramer, L. D. The Effect of Temperature on Life History Traits of Culex Mosquitoes. J. Med. Entomol. 51, 55–62 (2014).
Marini, G. et al. The Role of Climatic and Density Dependent Factors in Shaping Mosquito Population Dynamics: The Case of Culex pipiens in Northwestern Italy. PLOS ONE 11, e0154018 (2016).
Li, Y. et al. Comparative evaluation of the efficiency of the BG-Sentinel trap, CDC light trap and Mosquito-oviposition trap for the surveillance of vector mosquitoes. Parasit Vectors 9, 446 (2016).
Ciota, A. T. et al. Dispersal of Culex mosquitoes (Diptera: Culicidae) from a wastewater treatment facility. J. Med. Entomol. 49, 35–42 (2012).
Tsuda, Y. et al. A mark-release-recapture study on dispersal and flight distance of Culex pipiens pallens in an urban area of Japan. J. Am. Mosq. Control Assoc. 24, 339–343 (2008).
Vogels, C. B. F., Fros, J. J., Göertz, G. P., Pijlman, G. P. & Koenraadt, C. J. M. Vector competence of northern European Culex pipiens biotypes and hybrids for West Nile virus is differentially affected by temperature. Parasites & Vectors 9, 393 (2016).
Reisen, W. K., Fang, Y. & Martinez, V. M. Effects of temperature on the transmission of west nile virus by Culex tarsalis (Diptera: Culicidae). J. Med. Entomol. 43, 309–317 (2006).
Summers-Smith, J. D. The Sparrows: a study of the genus Passer. (T & AD Poyser, 1988).
Acknowledgements
This work was partially funded by the Veneto region and the Ministry of Health.
Author information
Authors and Affiliations
Contributions
G.M., R.R. and A.P. conceived the study and participated in its design and coordination. G.M. performed model simulations. G.M., R.R. and A.P. drafted the manuscript. G.C., F.M. and F.R. coordinated the mosquito collections and built the original entomological dataset. C.R. coordinated the human surveillance for W.N.V. infections at national level and provided the epidemiological data. C.R., A.R., F.R., G.C. and F.M. contributed to interpretation and critical review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Marini, G., Rosà, R., Pugliese, A. et al. West Nile virus transmission and human infection risk in Veneto (Italy): a modelling analysis. Sci Rep 8, 14005 (2018). https://doi.org/10.1038/s41598-018-32401-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32401-6
Keywords
This article is cited by
-
Empirical dynamic modelling and enhanced causal analysis of short-length Culex abundance timeseries with vector correlation metrics
Scientific Reports (2024)
-
A dynamically structured matrix population model for insect life histories observed under variable environmental conditions
Scientific Reports (2022)
-
An epidemiological model for mosquito host selection and temperature-dependent transmission of West Nile virus
Scientific Reports (2022)
-
Predicting West Nile virus transmission in North American bird communities using phylogenetic mixed effects models and eBird citizen science data
Parasites & Vectors (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.