Abstract
We here employed a model animal of Caenorhabditis elegans to perform toxicity assessment of original surface water samples collected from Three Gorges Reservoir (TGR) in the quiet season in Wanzhou, Chongqing. Using some sublethal endpoints, including lifespan, body length, locomotion behavior, brood size, and intestinal reactive oxygen species (ROS) induction, we found that the examined five original surface water samples could not cause toxicity on wild-type nematodes. Nevertheless, the surface water sample collected from backwater area induced the significant increase in expressions of genes (sod-2 and sod-3) encoding Mn-SODs in wild-type nematodes. Among the examined five original surface water samples, exposure to the original surface water sample collected from backwater area could further cause the toxicity in decreasing locomotion behavior and in inducing intestinal ROS production in sod-3 mutant nematodes. Moreover, the solid phase of surface water sample collected from backwater area might mainly contribute to the observed toxicity in sod-3 mutant nematodes. Our results are helpful for understanding the potential effects of surface water in the TGR region in the quiet season on environmental organisms.
Similar content being viewed by others
Introduction
Three Gorges Reservoir (TGR), the world’s largest reservoir, has a novel ecosystem in the upper stream of Yangtze River in China due to the formation of the largest and the longest yearly water-level drop1,2. In the recent years, with the development of industrialization and urbanization, both organic and inorganic pollutants may be released from the industrial and the residential wastewater and accumulated in the water or in the soils/sediments in the TGR region along Yangtze River3,4,5,6,7,8,9,10,11,12. Besides this, the spatial-temporal dynamics of bacterioplankton community and the suspended particulate matter have been detected in the TGR region13,14,15. Based on these possibilities, the ecological safety for the Yangtze River in the TGR region has received the great attention16,17,18.
Nematodes Caenorhabditis elegans is a classic model animal with the sensitivity to various environmental toxicants or stresses19,20,21,22,23,24. Based on the use of some sublethal endpoints including lifespan, development, reproduction, locomotion behavior, and oxidative stress, C. elegans has been proven to be valuable for in vivo toxicological assessment of environmental samples or toxicants25,26,27,28. Moreover, due to the property of model animal, C. elegans is helpful for elucidating the underlying cellular and molecular mechanisms for the observed toxicity of environmental toxicants29,30,31. Recently, the safety assessment of original surface water samples collected from TGR region in the flood season in Wanzhou, Chongqing has been performed using C. elegans as an assay animal model32. It has been shown that, after acute exposure, the original surface water sample collected from backwater area in the flood season could induce the obvious toxicity on wild-type nematodes32.
Besides the flood season, the quiet season is another important duration for the water in the TGR region, and most of the time (October-May) in TGR region in Wanzhou is in the quiet season. We hypothesize that some of the original surface water samples collected from TGR region in the quiet season may also have ecological risk under certain conditions. In this study, we further employed the animal model of C. elegans to perform the toxicity assessment on the original surface water samples collected from TGR region in the quiet season in Wanzhou, Chongqing. Our data suggest that original surface water sample collected from backwater area might have adverse effects on sod-3 mutant nematodes. Additionally, the solid phase may contribute greatly to the observed toxicity of surface water sample collected from backwater area on sod-3 mutant nematodes.
Results
Toxicity assessment of surface water samples in the TGR region in the quiet season in wild-type nematodes
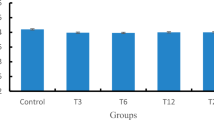
We first employed the endpoints of lifespan, body length, locomotion behavior, and brood size to perform the toxicity assessment of five surface water samples (W1, W2, W3, W4, and W5) in the TGR region in the quiet season in wild-type nematodes. After acute exposure from L4-larvae for 24-h, surface water samples of W1, W2, W3, W4, and W5 all did not significantly affect the lifespan, the body length, the locomotion behavior as reflected by the head thrash and the body bend, and the brood size in wild-type nematodes (Fig. 1).
Effects of different surface water samples in the TGR region in the quiet season on lifespan (a), body length (b), locomotion behavior (c), and brood size (d) in wild-type nematodes. Exposures were performed from L4-larvae for 24-h. The differences between groups were analyzed using analysis of variance (ANOVA). The survival curve data were statistically analyzed using the log-rank test. Bars represent means ± SD.
Effect of surface water samples in the TGR region in the quiet season on induction of intestinal reactive oxygen species (ROS) production in wild-type nematodes
Considering the crucial role of oxidative stress in the toxicity induction of environmental toxicants or stresses33,34,35, we further determined the possible effect of surface water samples in the TGR region in the quiet season on wild-type nematodes. After acute exposure from L4-larvae for 24-h, the surface water samples of W1, W2, W3, W4, and W5 all could not induce the obvious intestinal ROS production in wild-type nematodes (Fig. 2).
Effect of different surface water samples in the TGR region in the quiet season on induction intestinal ROS production in wild-type nematodes. Paraquat (2 mM), positive control. Exposures were performed from L4-larvae for 24-h. The differences between groups were analyzed using analysis of variance (ANOVA). Bars represent means ± SD. **P < 0.01 vs control.
Effect of surface water samples in the TGR region in the quiet season on molecular basis for oxidative stress in wild-type nematodes
In nematodes, the superoxide dismutases (SODs) provide the antioxidative systems, and the proteins of MEV-1, GAS-1, ISP-1, and CLK-1 are components in mitochondrial complex or electron transport chain. The functions of these proteins act as the core molecular mechanisms of induction of oxidative stress in nematodes36,37,38,39,40,41,42. Although we did not observe the abnormal phenotypes, we still wonder whether certain molecular response associated with the oxidative stress could be activated in wild-type nematodes exposed to the examined surface water samples. Acute exposure to the surface water sample of W1, W2, W3, or W4 did not significantly alter the transcriptional expressions of all these examined genes in wild-type nematodes (data not shown). Acute exposure to the surface water sample of W5 also could not significantly affect the transcriptional expressions of sod-1, sod-4, sod-5, mev-1, gas-1, isp-1, and clk-1 in wild-type nematodes (Fig. 3). Different from these, acute exposure to the surface water sample of W5 significantly increased the transcriptional expressions of sod-2 and sod-3 in wild-type nematodes (Fig. 3).
Effect of surface water sample of W5 on transcriptional expressions of sod-1-5, mev-1, gas-1, isp-1, and clk-1 in wild-type nematodes. Relative quantification of the examined genes in comparison to reference tba-1. Exposures were performed from L4-larvae for 24-h. The differences between groups were analyzed using analysis of variance (ANOVA). Bars represent means ± SD. **P < 0.01 vs control.
Effect of sod-3 mutation on potential toxicity of surface water samples in nematodes
In nematodes, sod-2 and sod-3 encode mitochondiral Mn-SODs. Mutation of any of these genes can induce a susceptibility to toxicity of environmental toxicants33,43. We further examined the possible toxicity of different surface water samples in sod-3 mutant nematodes. We used locomotion behavior and induction of intestinal ROS production as the toxicity assessment endpoints. Even in the sod-3 mutant nematodes, acute exposure to the surface water sample of W1, W2, W3, or W4 still could not cause the significant decrease in locomotion behavior and induction of intestinal ROS production (Fig. 4). In contrast, acute exposure to the surface water sample of W5 resulted in the significant decrease in locomotion behavior and induction of intestinal ROS production in sod-3 mutant nematodes (Fig. 4).
Effect of sod-3 mutation on potential toxicity of surface water samples in the TGR region in the quiet season in decreasing locomotion behavior (a) and in inducing intestinal ROS production (b). Exposures were performed from L4-larvae for 24-h. The differences between groups were analyzed using analysis of variance (ANOVA). Bars represent means ± SD. **P < 0.01 vs control.
Analysis on the chemical pollutants from the sample W5
To determine the possible contributors from the surface water sample of W5 in inducing toxicity on sod-3 mutant nematodes, both liquid phase and solid phase from the surface water sample of W5 were isolated by centrifugation at 10000 g for 10-min. After the centrifugation, the pellet was re-suspended with the equal volume of K medium to obtain the solution for solid phase, and the liquid phase existed in the supernatant. After acute exposure, the solid phase for surface water sample of W5 could lead to the obvious toxicity in decreasing locomotion behavior and in inducing intestinal ROS production in sod-3 mutant nematodes (Fig. 5). In contrast, the liquid phase for surface water sample of W5 could only induce the moderate toxicity in decreasing locomotion behavior and in inducing intestinal ROS production in sod-3 mutant nematodes (Fig. 5).
Effect of liquid phase or solid phase of the sample of W5 on locomotion behavior (a) and induction of intestinal ROS production (b) in sod-3 mutant nematodes. Exposures were performed from L4-larvae for 24-h. The differences between groups were analyzed using analysis of variance (ANOVA). Bars represent means ± SD. *P < 0.05 vs control, **P < 0.01 vs control.
Discussion
In this study, we first investigated the possible effects of five original surface water samples (W1, W2, W3, W4, and W5) in the TGR region in the quiet season on wild-type nematodes. With the aid of some sublethal endpoints (lifespan, body length, locomotion behavior, brood size, and intestinal ROS production), acute exposure to all these examined surface water samples in the TGR region in the quiet season did not cause the obvious toxicity on wild-type nematodes (Figs 1 and 2). This observation is very different from the effects of surface water samples in the TGR region in the flood season on wild-type nematodes. In wild-type nematodes, it has been shown that acute exposure to the surface water sample collected from the backwater area (W5) in the flood season could cause the significant decrease in locomotion behavior and induction of intestinal ROS production, although exposure to other four surface water samples in the flood season could not cause obvious toxicity32. These observations imply that, at least for the site of backwater area, the noticeable difference for the potential effect between surface water in the flood season and surface water in the quiet season may exist on environmental organisms. Additionally, our data also implies that, in the flood season, more chemical pollutants might be washed off into the TGR region than that in quiet season.
Although the surface water sample of W5 collected in the quiet season could not result in the obvious toxicity in wild-type nematodes, we found that exposure to this surface water sample could alter the molecular basis for oxidative stress to a certain degree. Exposure to surface water sample of W5 significantly increased the transcriptional expressions of genes (sod-2 and sod-3) encoding mitochondrial Mn-SODs (Fig. 3). This observation implies one important possibility. That is, in the nematodes exposed to the surface water sample of W5, the potential toxicity of surface water sample of W5 could already induce a protection response mediated by the increased Mn-SODs, although the toxicity of surface water sample of W5 was still not enough to induce the obvious alterations in various phenotypes.
In this study, we further employed the sod-3 mutant with the susceptible property to environmental toxicants to assess the potential toxicity of five surface water samples collected in the TGR region in the quiet season. We found that exposure to the surface water sample of W5 caused the significant decrease in locomotion behavior and induction of intestinal ROS production in sod-3 mutant nematodes (Fig. 4). That is, mutation of sod-3 may amplify the potential toxicity of the surface water sample of W5, which allows us to detect the obvious alteration in toxicity assessment endpoints, such as locomotion behavior and intestinal ROS production.
For the surface water sample of W5, our results imply that its solid phase may contribute greatly to the observed toxicity in sod-3 mutant nematodes (Fig. 5). In contrast, the contribution of liquid phase for the surface water sample of W5 to its toxicity formation might be limited. The elemental analysis did not show obvious differences for the metals among the examined five surface water samples (Table S1), which implies that the observed moderate toxicity in the liquid phase for the surface water sample of W5 may be largely due to the organic pollutants. For the components existed in the solid phase in water in the TGR region, previous studies have implied the important roles of bacterioplankton community and suspended particulate matter13,14,15. Especially, previous study has detected the high prevalence and concentrations of pathogens and already identified some pathogens in the corresponding backwater area44. The detailed biological effects of identified pathogens on environmental organisms still need to be further examined. In the area for surface water sample of W5, we also observed a large amount of microplastics. For the technical limitations, it still unclear for the potential effects of these observed microplastics in the solid phase on environmental organisms. The assessment on the contribution of bacterioplankton community and suspended particulate matter to the toxicity formation of surface water sample of W5 in the TGR region in the quiet season is suggested to be further performed.
In conclusion, we examined the effects of five original surface water samples in the TGR region in the quiet season on nematodes. Using some sublethal endpoints, we found that all the examined original surface water samples in the TGR region in the quiet season could not cause the toxicity on wild-type nematodes. Nevertheless, acute exposure to the surface water sample of W5 induced the significant increase in transcriptional expressions of genes encoding Mn-SODs in wild-type nematodes. Moreover, mutation of sod-3 resulted in the susceptibility to potential toxicity of the surface water sample of W5. Our data further imply that the solid phase of the surface water sample of W5 might mainly contribute to the observed toxicity in sod-3 mutant nematodes. Our data will be helpful for our understanding the potential effects of surface water in the TGR region in the quiet season on environmental organisms.
Methods
Sample collection
The surface water samples were from 5 sampling sites (W1, shore water; W2, upstream; W3, shore water; W4, downstream; and W5, backwater area) in Wanzhou, Chongqing (Fig. 6), which are the same as those analyzed in our previous study32. The sampling season was selected in the quiet season (December 15, 2017). Water samples were collected according to the description of a standard method45. Surface water samples were collected at a depth of 0.5 m using a TN-S water sampler (Jintan Taina Instrument Factory, Jiangshu, China). Samples collected were stored in a car refrigerator (0 °C), and analyzed after being transported back to the laboratory in a cooler.
Water temperature, pH value, total dissolved solids, and turbidity were analyzed as described by Xiao et al.32. Information for daily flow and water level of the sampling sites was from Yangtze River Hydrological Network (http://www.cjh.com.cn). Major elements of surface water samples were determined by inductively coupled plasma atomic emission spectroscopy (ICP, GE Co., USA), and provided in Table S1. The related information for the collected surface water samples in TGR region is provided in Table S2.
Animal maintenance and exposure
Wild-type N2 and mutant of sod-3(gk235) were used in this study. The animals were maintained on nematode growth medium (NGM) plates with Escherichia coli OP50 as a food source as described by Brenner46. Age synchronous L4-larvae were prepared by lysis of the hermaphrodite adults with bleaching mixture (0.45 M NaOH, 2% HOCl) in order to separate the eggs and the animals. Exposures were performed from L4-larvae for 24-h (acute exposure) in liquid solutions in the presence of food at 20 °C. Approximately 4 × 106 colony-forming units (CFUs) of OP50 were added into the exposure solutions. The examined L4-larvae nematodes were directed transferred into the exposure solutions using the picker. Control animals were treated with liquid K medium.
Toxicity assessment
After the exposure, the toxicity assessment was performed using lifespan, body length, brood size, locomotion behavior, and intestinal ROS production as the toxicity assessment endpoints.
Lifespan, an endpoint to reflect long-term effect of certain environmental toxicants47, was performed as described by Zhi et al.48. To examine the lifespan, the examined nematodes were transferred daily for the first 7 days of adulthood using a picker, and checked the survival every two-day. Nematodes will be scored as dead if they can not move even after repeated taps with a picker. Fifty nematodes were examined per treatment. Graphs are representative of three trials. The survival curve data were statistically analyzed using the log-rank test.
Body length was used to assess the growth of nematodes32. Body length was determined by measuring the flat surface length of nematodes using Image-Pro® Express software. Thirty nematodes were examined per treatment.
Head thrash and body bend, endpoints to reflect the locomotion behavior49,50, were performed as described by Chen et al.51. After the exposure, the nematodes were first allowed to crawl freely for 1-min. Head thrashes were scored as the number of wavelengths the nematodes moved in a 1-min interval. Body bends were scored by eye for the number generated in a 20 s time interval. A change in the direction of propagation of the part corresponding to the posterior bulb of pharynx is defined as a body bend. Fifty nematodes were examined per treatment.
Brood size, an endpoint to reflect the reproductive capacity52, was performed as described by Zhao et al.53. The number of offspring at all stages beyond the egg was counted. Thirty nematodes were examined per treatment.
Intestinal ROS production, the endpoint to reflect the activation of oxidative stress54, was performed as described by Yang et al.55. The 5′,6′-chloromethyl-2′,7′dichlorodihydro-fluorescein diacetate (CM-H2DCFDA) can detect the presence of intracellular produced ROS species. The examined nematodes were labeled with CM-H2DCFDA (1 μM) for 3 h at 20 °C in the dark. After the labeling, the nematodes were analyzed at 488 nm of excitation wavelength and 510 nm of emission filter under a laser scanning confocal microscope. The semiquantified ROS was expressed as relative fluorescence units (RFU) and normalized to the autofluorescence. Thirty nematodes were examined per treatment. For intestinal ROS production assay, exposure to paraquat (2 mM), a ROS generator, was used as a positive control.
Reverse-transcription and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA of nematodes was isolated using Trizol (Invitrogen, UK) according to the manufacturer’s protocols. After cDNA synthesis, relative transcriptional expressions of certain genes were determined by real-time PCR in an ABI 7500 real-time PCR system with Evagreen (Biotium, USA). All reactions were performed in triplicate with the same cDNA samples. Relative quantification of the examined genes in comparison to reference tba-1 gene encoding a tubulin protein was determined. The primer information was shown in Table S3.
Statistical analysis
Data in this study were expressed as means ± standard deviation (SD). Statistical analysis using SPSS 12.0 software (SPSS Inc., Chicago, USA) and differences between groups using analysis of variance (ANOVA) were analyzed. Probability levels of 0.05 and 0.01 were considered to be statistically significant.
Change history
17 October 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Li, Z. et al. Soil–air greenhouse gas fluxes influenced by farming practices in reservoir drawdown area: A case at the Three Gorges Reservoir in China. J. Environ. Manag. 181, 64–73 (2016).
Ye, L., Han, X., Xu, Y. & Cai, Q. Spatial analysis for spring bloom and nutrient limitation in Xiangxi Bay of Three Gorges Reservoir. Environ. Monitor. Assess. 127, 1351145 (2017).
Zhang, J. & Deng, W. Industrial structure change and its eco-environmental influence since the establishment of municipality in Chongqing, China. Proc. Environ. Sci. 2, 517–526 (2010).
Xiao, G. et al. Occurrence and potential health risk of Cryptosporidium and Giardia in the Three Gorges Reservior, China. Water Res. 47, 2431–2445 (2013).
Zhao, P., Tang, X., Tang, J. & Wang, C. Assessing water quality of Three Gorges Reservoir, China, over a five-year period from 2006 to 2011. Water. Resour. Manag. 27, 4545–4558 (2013).
Ye, C., Cheng, X., Liu, W. & Zhang, Q. Revegetation impacts soil nitrogen dynamics in the water level fluctuation zone of the Three Gorges Reservoir, China. Sci. Total. Environ. 517, 76–85 (2015).
Floehr, T. et al. Yangtze Three Gorges Reservoir, China: A holistic assessment of organic pollution, mutagenic effects of sediments and genotoxic impacts on fish. J. Environ. Sci. 38, 63–82 (2015).
Wang, W., Ndungu, A. W. & Wang, J. Monitoring of endocrine-disrupting compounds in surface water and sediments of the Three Gorges Reservior region, China. Arch. Environ. Contam. Toxicol. 71, 509–517 (2016).
Wang, T., Pan, J. & Liu, X. Characterization of heavy metal contamination in the soil and sediment of the Three Gorges Reservior, China. J. Environ. Sci. Health Part A 52, 201–209 (2017).
Du, H. et al. Mercury-methylating genes dsrB and hgcA in soils/sediments of the Three Gorges Reservior. Environ. Sci. Pollut. Res. 24, 5001–5011 (2017).
Zhang, K. et al. Occurrence and characteristics of microplastic pollution in Xiangxi Bay of Three Gorges Reservior, China. Environ. Sci. Technol. 51, 3794–3801 (2017).
Zhao, X., Li, T., Zhang, T., Luo, W. & Li, J. Distribution and health risk assessment of dissolved heavy metals in the Three Reservior, China (section in the main urban area of Chongqing). Environ. Sci. Pollut. Res. 24, 2697–2710 (2017).
Yan, Q. et al. Impact of the Three Gorges Dam on microbial structure and potential function. Sci. Rep. 5, 8605 (2015).
Gao, M., Zhu, K., Bi, Y. & Hu, Z. Spatiotemporal patterns of surface-suspended particulate matter in the Three Gorges Reservior. Environ. Sci. Pollut. Res. 23, 3569–3577 (2016).
Li, Z. et al. Responses of spatial-temporal dynamics of bacterioplankton community to large-scale reservoir operation: a case study in the Three Gorges reservoir, China. Sci. Rep. 7, 42469 (2017).
Schramm, K. et al. Chemical- and effect-oriented exposomics: Three Gorges Reservior (TGR). Environ. Sci. Pollut. Res. 20, 7075–7062 (2013).
Tang, J. et al. Tempo-spatial analysis of water quality in tributary bays of the Three Gorges Reservior region (China). Environ. Sci. Pollut. Res. 22, 16709–16720 (2015).
Huang, Y. et al. Air-water CO2 and CH4 fluxes along a river-reservior continuum: case study in the Pengxi River, a tributary of the Yangtze River in the Three Gorges Reservior, China. Environ. Monit. Assess. 189, 223 (2017).
Dong, S.-S., Qu, M., Rui, Q. & Wang, D.-Y. Combinational effect of titanium dioxide nanoparticles and nanopolystyrene particles at environmentally relevant concentrations on nematodes Caenorhabditis elegans. Ecotoxicol. Environ. Safety 161, 444–450 (2018).
Qu, M., Xu, K.-N., Li, Y.-Y., Wong, G. & Wang, D.-Y. Using acs-22 mutant Caenorhabditis elegans to detect the toxicity of nanopolystyrene particles. Sci. Total Environ. 643, 119–126 (2018).
Zhu, C. et al. Hormetic effect and mechanism of imidazolium-based ionic liquids on the nematode Caenorhabditis elegans. Chemosphere 157, 65–70 (2016).
Yang, R.-L. et al. Metallothioneins act downstream of insulin signaling to regulate toxicity of outdoor fine particulate matter (PM2.5) during Spring Festival in Beijing in nematode Caenorhabditis elegans. Toxicol. Res. 5, 1097–1105 (2016).
Huang, C. W., Li, S. W. & Hsiu-Chuan Liao, V. Chronic ZnO-NPs exposure at environmentally relevant concentrations results in metabolic and locomotive toxicities in Caenorhabditis elegans. Environ. Pollut. 220, 1456–1464 (2017).
Zhao, L., Wan, H.-X., Liu, Q.-Z. & Wang, D.-Y. Multi-walled carbon nanotubes-induced alterations in microRNA let-7 and its targets activate a protection mechanism by conferring a developmental timing control. Part. Fibre Toxicol. 14, 27 (2017).
Li, J. et al. Acrylamide induces locomotor defects and degeneration of dopamine neurons in Caenorhabditis elegans. J. Appl. Toxicol. 36, 60–67 (2016).
Yu, Z., Sun, G., Liu, Y., Yin, D. & Zhang, J. Trans-generational influences of sulfamethoxazole on lifespan, reproduction and population growth of Caenorhabditis elegans. Ecotoxicol Environ Safety 135, 312–318 (2017).
Qu, M., Li, Y.-H., Wu, Q.-L., Xia, Y.-K. & Wang, D.-Y. Neuronal ERK signaling in response to graphene oxide in nematode Caenorhabditis elegans. Nanotoxicology 11, 520–533 (2017).
Ding, X.-C., Wang, J., Rui, Q. & Wang, D.-Y. Long-term exposure to thiolated graphene oxide in the range of μg/L induces toxicity in nematode Caenorhabditis elegans. Sci. Total Environ. 616–617, 29–37 (2018).
Zhuang, Z.-H. et al. Function of RSKS-1-AAK-2-DAF-16 signaling cascade in enhancing toxicity of multi-walled carbon nanotubes can be suppressed by mir-259 activation in Caenorhabditis elegans. Sci. Rep. 6, 32409 (2016).
Gonzalez-Moragas, L. et al. Toxicogenomics of iron oxide nanoparticles in the nematode C. elegans. Nanotoxicology 11, 647–657 (2017).
Ren, M.-X. et al. Developmental basis for intestinal barrier against the toxicity of graphene oxide. Part. Fibre Toxicol. 15, 26 (2018).
Xiao, G.-S. et al. Toxicity evaluation of Wanzhou watershed of Yangtze Three Gorges Reservior in the flood season in Caenorhabditis elegans. Sci. Rep. 8, 6734 (2018).
Ren, M.-X., Zhao, L., Lv, X. & Wang, D.-Y. Antimicrobial proteins in the response to graphene oxide in Caenorhabditis elegans. Nanotoxicology 11, 578–590 (2017).
Xiao, G.-S., Chen, H., Krasteva, N., Liu, Q.-Z. & Wang, D.-Y. Identification of interneurons required for the aversive response of Caenorhabditis elegans to graphene oxide. J. Nanobiotechnol. 16, 45 (2018).
Li, W.-J., Wang, D.-Y. & Wang, D.-Y. Regulation of the response of Caenorhabditis elegans to simulated microgravity by p38 mitogen-activated protein kinase signaling. Sci. Rep. 8, 857 (2018).
Hunter, T., Bannister, W. H. & Hunter, G. J. Cloning, expression, and characterization of two manganese superoxide dismutases from Caenorhabditis elegans. J. Biol. Chem. 272, 28652–28659 (1997).
Ishii, N. et al. A mutation in succinate dehydrogenase cytochrome b causes oxidative stress and ageing in nematodes. Nature 394, 694–697 (1998).
Miyadera, H. et al. Altered quinone biosynthesis in the long-lived clk-1 mutants of Caenorhabditis elegans. J. Biol. Chem. 276, 7713–7716 (2001).
Feng, J., Bussiere, F. & Hekimi, S. Mitochondrial electron transport is a key determinant of life span in Caenorhabditis elegans. Dev. Cell 1, 633–644 (2001).
Kayser, E. B., Morgan, P. G., Hoppel, C. L. & Sedensky, M. M. Mitochondrial expression and function of GAS-1 in Caenorhabditis elegans. J. Biol. Chem. 276, 20551–20558 (2001).
Wu, Q.-L., Han, X.-X., Wang, D., Zhao, F. & Wang, D.-Y. Coal combustion related fine particulate matter (PM2.5) induces toxicity in Caenorhabditis elegans by dysregulating microRNA expression. Toxicol. Res. 6, 432–441 (2017).
Zhao, L., Rui, Q. & Wang, D.-Y. Molecular basis for oxidative stress induced by simulated microgravity in nematode Caenorhabditis elegans. Sci. Total Environ. 607–608, 1381–1390 (2017).
Wang, D. -Y. Nanotoxicology in Caenorhabditis elegans. Springer Nature Singapore Pte Ltd (2018).
Xiao, G.-S. et al. Occurrence and infection risk of waterborne pathogens in Wanzhou watershed of the Three Gorges Reservoir, China. J. Environ. Sci. 25, 1913–1924 (2013).
Chen, D. Guidelines for the investigation of aquatic organisms in rivers. China Science Publishing & Media Ltd (2014).
Brenner, S. The genetics of Caenorhabditis elegans. Genetics 77, 71–94 (1974).
Shakoor, S., Sun, L.-M. & Wang, D.-Y. Multi-walled carbon nanotubes enhanced fungal colonization and suppressed innate immune response to fungal infection in nematodes. Toxicol. Res. 5, 492–499 (2016).
Zhi, L.-T. et al. Molecular control of innate immune response to Pseudomonas aeruginosa infection by intestinal let-7 in Caenorhabditis elegans. PLoS Pathog. 13, e1006152 (2017).
Yu, Y.-L., Zhi, L.-T., Wu, Q.-L., Jing, L.-N. & Wang, D.-Y. NPR-9 regulates innate immune response in Caenorhabditis elegans by antagonizing activity of AIB interneurons. Cell. Mol. Immunol. 15, 27–37 (2018).
Zhi, L.-T., Ren, M.-X., Qu, M., Zhang, H.-Y. & Wang, D.-Y. Wnt ligands differentially regulate toxicity and translocation of graphene oxide through different mechanisms in Caenorhabditis elegans. Sci. Rep. 6, 39261 (2016).
Chen, H., Li, H.-R. & Wang, D.-Y. Graphene oxide dysregulates Neuroligin/NLG-1-mediated molecular signaling in interneurons in Caenorhabditis elegans. Sci. Rep. 7, 41655 (2017).
Zhi, L.-T., Fu, W., Wang, X. & Wang, D.-Y. ACS-22, a protein homologous to mammalian fatty acid transport protein 4, is essential for the control of toxicity and translocation of multi-walled carbon nanotubes in Caenorhabditis elegans. RSC Adv. 6, 4151–4159 (2016).
Zhao, L., Qu, M., Wong, G. & Wang, D.-Y. Transgenerational toxicity of nanopolystyrene particles in the range of μg/L in nematode Caenorhabditis elegans. Environ. Sci.: Nano 4, 2356–2366 (2017).
Xiao, G.-S., Zhi, L.-T., Ding, X.-C., Rui, Q. & Wang, D.-Y. Value of mir-247 in warning graphene oxide toxicity in nematode Caenorhabditis elegans. RSC Adv. 7, 52694–52701 (2017).
Yang, R.-L., Ren, M.-X., Rui, Q. & Wang, D.-Y. A. mir-231-regulated protection mechanism against the toxicity of graphene oxide in nematode Caenorhabditis elegans. Sci. Rep. 6, 32214 (2016).
Acknowledgements
This work was supported by the grants from Key Project of Chinese Ministry of Education (No. 211150), Basic and Advanced Research Project of Chongqing CSTC (No. cstc2013jcyjA20011 and cstc2018jcyjA1714), Major Cultivation Projects of Chongqing Three Gorges University (No. 201502), Special Foundation for Scientific and Technological Talents of Chongqing Three Gorges University (No. 17RC11) and Scientific Research Foundation for High-Level Talents in Chongqing Three Gorges University (No.17RC11).
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: D.W. Performed the experiments and analyzed the data: G.X., L.Z., Q.H., J.Y., H.D., D.G., M.X., G.L. and Z.C. Wrote the paper: D.W.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xiao, G., Zhao, L., Huang, Q. et al. Biosafety assessment of water samples from Wanzhou watershed of Yangtze Three Gorges Reservoir in the quiet season in Caenorhabditis elegans. Sci Rep 8, 14102 (2018). https://doi.org/10.1038/s41598-018-32296-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32296-3
Keywords
This article is cited by
-
Seasonal pollution and surface characteristics of microplastics in surface water in the Wanzhou section of the Three Gorges Reservoir, China
Environmental Science and Pollution Research (2023)
-
Biosafety assessment of Acinetobacter strains isolated from the Three Gorges Reservoir region in nematode Caenorhabditis elegans
Scientific Reports (2021)
-
Dysregulation of Neuronal Gαo Signaling by Graphene Oxide in Nematode Caenorhabditis elegans
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.