Abstract
This study aims to investigate how the frequency settings of deep brain stimulation (DBS) targeting the subthalamic nucleus (STN) influence the motor symptoms of Parkinson’s disease (PD). Stimulation with frequencies less than 100 Hz (mostly 60 or 80 Hz) is considered low-frequency stimulation (LFS) and with frequencies greater than 100 Hz (mostly 130 or 150 Hz) is considered high-frequency stimulation (HFS). We conducted a comprehensive literature review and meta-analysis with a random-effect model. Ten studies with 132 patients were included in our analysis. The pooled results showed no significant difference in the total Unified Parkinson Disease Rating Scale part III (UPDRS-III) scores (mean effect, −1.50; p = 0.19) or the rigidity subscore between HFS and LFS. Compared to LFS, HFS induced greater reduction in the tremor subscore within the medication-off condition (mean effect, 1.01; p = 0.002), while no significance was shown within the medication-on condition (mean effect, 0.01; p = 0.92). LFS induced greater reduction in akinesia subscore (mean effect, −1.68, p = 0.003), the time to complete the stand-walk-sit (SWS) test (mean effect, −4.84; p < 0.00001), and the number of freezing of gait (FOG) (mean effect, −1.71; p = 0.03). These results suggest that two types of frequency settings may have different effects, that is, HFS induces better responses for tremor and LFS induces greater response for akinesia, gait, and FOG, respectively, which are worthwhile to be confirmed in future study, and will ultimately inform the clinical practice in the management of PD using STN-DBS.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a neurodegenerative disorder, characterized by pathological motor symptoms including tremors, rigidity, bradykinesia and postural instability1. After an initial honeymoon period, pharmacological treatments often fail to alleviate the burden from those symptoms, severely compromising the quality of life for PD patients. The pathological symptoms in PD may arise at least in part due to the dysfunction in thalamic region in the brain. Deep brain stimulation (DBS) targeting the subthalamic nucleus (STN) has been evidenced as an effective intervention to improve the functional performance in those suffering from advanced PD. The STN-DBS modulates the activity of certain nucleus via implanted electrodes and thus disrupts the pathologic oscillations of alpha- (8–12 Hz), beta- (12–30 Hz), and gamma-(30–100 Hz) bands within the cortico-basal ganglia loop2,3,4,5. Previous studies showed that STN-DBS can improve the functional performance of PD patients as evidenced by a 25% decrease in the Unified Parkinson Disease Rating Scale part III (UPDRS-III) scores, a 25% decrease in the average levodopa-equivalent daily dose (LEDD) and an 80% decrease in drug-induced dyskinesia6.
The effectiveness of STN-DBS on PD depends mainly upon the parameters used in the intervention, including the intensity, frequency, pulse width (PW) and contact configuration7. No guidelines are currently available to determine the optimal parameters of the DBS intervention. Only the impact of the intensity (i.e., amplitude of the applied current) and pulse width on the intervention effects are fairly well-understood. For example, the high voltage and/or narrow PW (e.g., 30 µs) of stimulation can induce greater effects (e.g., longer therapeutic window) compared to low voltage and/or wide PW8,9. However, the influence of the frequency settings on the therapeutic effects of STN-DBS on motor symptoms are still not fully understood.
The frequency of DBS is often categorized as high frequency (i.e., HFS, >100 Hz, mostly 130 or 150 Hz) or low frequency (i.e., LFS, <100 Hz, mostly 60 or 80 Hz)1. These two categories have varied therapeutic effects on motor function in those with PD. For example, Khoo et al. reported that LFS induced greater improvements in motor control performance (i.e., lower UPDRS scores, particularly in akinesia and axial subscores) compared to HFS. Xie et al. showed using LFS significantly improved aspiration, swallowing, FOG and axial symptoms10,11. However, Vallabhajosula et al. observed no significant improvements in total UPDRS-III scores, step length and velocity during gait initiation, and gait speed after LFS12. As such, no consensus on the effects of STN-DBS frequency settings has been reached, and the potential roles of frequency in ameliorating motor symptoms in PD may be underestimated.
Therefore, we here completed a meta-analysis to quantitatively analyze the acute effects (i.e., several minutes to hours) of LFS and HFS of STN-DBS on motor symptoms in PD patients based upon previously published studies. This work may provide us a better understanding on the influences of the frequency settings on motor symptoms in PD, which will ultimately optimize the frequency-programming protocols of STN-DBS for different motor symptoms in clinical practice.
Methods
Search criteria
We conducted a comprehensive review of the published literature reporting the acute effects of LFS and HFS in STN-DBS on motor symptoms in PD. The Pubmed, Embase, the Cochrane Library and the Web of Science were used for the literature research. We reviewed publications up to February 2018 by searching citing and cited articles. We also checked cross-references for certain crucial articles. We used a combination of MeSH and text word searching for the following terms: “(deep brain stimulation OR electrical stimulation OR neuromodulation or DBS) AND frequency AND (subthalamic nucleus OR STN) AND (Parkinson’s disease OR PD)”.
Eligibility criteria
The inclusion criteria for the searched studies were: written in English; focusing on human participants; participants with PD; unilateral or bilateral procedures; STN-DBS; prospective studies; comparison between LFS (<100 Hz, mostly 60 or 80 Hz) and HFS (>100 Hz, mostly 130 or 150 Hz); UPDRS-III, stand-walk-sit (SWS) test and FOG questionnaire as measurements. The exclusion criteria were: DBS procedure as a treatment for diseases other than PD; targeting other than STN or combined targets; less than 5 participants; retrospective analyses; case reports; review articles; editorials; letters; conference articles. The Prospero registration number of this study is 42017060545.
Quality evaluation and data collection
Quality evaluation was conducted according to the Cochrane Collaboration’s tool for assessing the risk of bias by two physicians separately. Subsequently, study details were extracted from the retrieved studies, including the number of patients, mean age, duration of disease, study designs, post-surgery duration, medication status (on and off), time for adapting to the changed stimulation conditions. The means and standard deviations (SDs) of UPDRS-III scores and subscores, as well as the time to complete SWS tests were collected. The means and SDs were calculated for those data presented as medians and range values as previously described13. These procedures were also conducted by two physicians independently to ensure accuracy of analyses.
Statistical analysis
The acute effects of LFS and HFS on motor performance, tremors, rigidity, gait and akinesia were assessed by the changes in the corresponding subscores in the UPDRS III. The results of the SWS test were also summarized. Using Review Manager Version 5.3, a random-effect meta-analysis of continuous variables was performed to pool the mean effect and 95% confidence intervals (CI) for a more conservative estimate of pooled effects. The Cochran’s Q-test and I2-values were adapted for assessment of statistical heterogeneity between studies. Heterogeneity was regarded as mild, moderate and high separately when the I2 was above 25%, 50% and 75%, respectively14. The extent of DBS-induced improvement in motor symptoms may possibly influenced by the medication states (i.e., on and off)15,16. But due to the limited number of studies within the medication-on state, we only summarized and analyzed the data within the medication-off condition. No publication bias analysis was performed due to the low number of included studies.
Results
Study identification and characteristics of included studies
We identified a total of 876 records from databases following our search strategy. After reviewing titles and abstracts, we excluded 241 unqualified records. We then retrieved full-text articles and found 24 studies that compared the acute effects of low- and high- frequency STN-DBS on motor symptoms in PD. Ten of them met the inclusion criteria (Fig. 1). We then evaluated the allocation, blinding, incomplete outcome data, selective reporting, and other potential sources of bias of the 10 eligible studies (Fig. 2).
Four out of the ten studies were completed under the medication-on condition10,11,17, five were completed under the medication-off condition12,18,19,20, and the other one did not separate different medication conditions. The age of participants ranged from 31 to 76 years old, and their history of PD ranged from 8 to 29 years. Time intervals between DBS parameter adjustment and motor performance evaluation ranged from 10 to 180 minutes. Among these studies, UPDRS-III scores were reported in 9 studies, and the results of SWS tests were reported in 4 studies. Characteristics of the included studies are presented in Table 1.
Overall motor performance
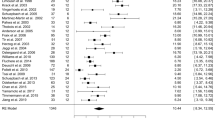
No significant difference was found between LFS and HFS on total UPDRS-III scores across all studies (Fig. 3a). Within the medication-off state, UPDRS-III scores improved more after using HFS DBS compared to LFS (mean effect, 1.58; 95% CI, 0.25–2.91; p = 0.02) (Fig. 3b). However, under the medication-on condition, LFS was more effective than HFS (mean effect, −10.17; 95% CI, −17.2–3.15; p = 0.005) (Fig. 3c). The inconsistency among studies was moderate to high (Q = 8.51, I2 = 53% and Q = 6.9, I2 = 71%).
Tremor
Eight studies, consisting of 123 patients, used Item 20 and/or Item 21 in UPDRS-III to measure tremors. HFS had better effects on tremors than LFS across all eight studies when combining studies in both medication-on and -off states (mean effect, 0.75; 95% CI, 0.23–1.28; p = 0.005) (Fig. 4a). Particularly, within the medication-on condition (four studies included), significant effects of HFS on tremor were observed compared to LFS (mean effect, 1.01; 95% CI, 0.38–1.65; p = 0.002) (Fig. 4b), while, within the medication-off condition, no significant differences were found between LFS and HFS (mean effect, 0.01; 95% CI, −0.22–0.25; p = 0.92) (Fig. 4c). The inconsistency among the studies was low for the studies in medication-on condition (Q = 0.43, I2 = 0%) and it was relatively high for those in medication-off condition (Q = 29.38, I2 = 90%).
Gait
Based upon the gait subscores of UPDRS-III in the medication-off condition and the time to complete the SWS test in the medication-on condition, LFS was more effective on gait than HFS (mean effect, −0.07; 95% CI, −0.13 to 0; p = 0.04 and mean effect, −4.84; 95% CI, −6.89- to −2.80; p < 0.00001) (Fig. 6b,d). The inconsistency was low across these studies (Q = 2.22, I2 = 10% and Q = 0.08, I2 = 0%).
Freezing of gait
LFS induced greater reduction in FOG of PD compared to HFS. Specifically, the number of FOG during SWS tests (mean effect, −1.71; 95% CI, −3.26 to −0.16; p = 0.03, Fig. 6e) and the scores of FOG questionnaires (mean effect, −6.52; 95% CI, −9.39 to −3.66; p < 0.00001, Fig. 6f) were lower after the LFS intervention than HFS. The inconsistency between the studies using SWS test was moderate (Q = 1.96, I2 = 49%) and the inconsistency was low across studies using FOG questionnaires (Q = 0.08, I2 = 0%).
Akinesia
Four studies including 49 participants showed that the akinesia subscore was significantly lower after LFS compared to HFS (mean effect, −1.68; 95% CI, −2.78 to −0.57; p = 0.003) (Fig. 5a). The inconsistency among studies was moderate (Q = 7.19, I2 = 72%). However, no significance was observed within medication-on or -off condition separately, except a trend of greater effect in LFS towards significance (Fig. 5b,c).
Rigidity
Four studies including 49 patients measured the UPDRS-III Item 22 rigidity subscore. No significant difference between the HFS and LFS was observed regardless of the medication condition (p > 0.05) (Fig. 5d–f). High inconsistency was found between the studies.
Discussion
In this meta-analysis, we provide first-of-its kind evidence of frequency-dependent effects of STN-DBS on different motor symptoms. By analyzing the acute effects of LFS and HFS in STN-DBS on the motor symptoms of patients with PD, our results demonstrated that HFS alleviates tremors better than LFS in the medication-off state, but not in the medication-on state, which is probably due to ceiling-effects on the improvement of tremor in the medication-on state. LFS had greater alleviating effects on akinesia and FOG, and improvement in gait speed compared to HFS. These findings indicates that frequency is an essential parameter for the therapeutic effects of STN-DBS on motor symptoms in PD, and the determination of the frequency setting is critical for the use of DBS in clinical practice.
Pathological oscillations at different power bands (e.g., alpha- (8–12 Hz), beta- (12–30 Hz)/gamma-(30–100 Hz) bands) may be the neural substrate of the frequency-dependent response of DBS2,3,4, contributing to the varied effects between HFS and LFS. For instance, Blumenfeld et al. recorded intra-operative local field potentials (LFPs) in the STN, and found that HFS decreased the baseline STN alpha- and beta-band oscillations compared to LFS with equivalent power4. Further, 60-Hz DBS amplified alpha/low-beta power (11–15 Hz) and attenuated high-beta power (19–27 Hz), whereas 140 Hz DBS broadly attenuated beta power (15–30 Hz)2. Such effects of LFS and HFS on beta-band oscillation attenuation may result in various extent of improvement in akinesia as observed in our analysis.
Similarly, greater reduction in tremors induced by HFS in the medication-off state may arise from the frequency-specific interference effect of STN-DBS on pathological oscillations, that is, HFS attenuates low-beta power, while LFS cannot. Blumenfeld et al. proposed that the superior effect of HFS on tremors might be due to reduced coupling in the cortico-striato-STN circuitry with low-beta bands2. In addition, other pathological oscillations may be involved in the regulation of tremors. Anzak et al. found that low gamma oscillations in the STN are associated with tremor severity in PD. In another study, Beudel et al. reported that suppression of the gamma-band in the STN by STN-DBS is inversely related to tremor amplitude. However, as the influence of LFS on gamma-band oscillation is unclear, the frequency-specific effects within gamma power band requires further exploration.
The underlying mechanism through which LFS and HFS influence gait and FOG events may both relate to the pedunculopontine nucleus (PPN), which is located in the caudal pontomesencephalic tegmentum and five mm away from the STN21,22, projecting to the cortex and the spinal cord23. LFS in the STN may thus affect neural activity in the PPN24 with diffused current delivered by the implanted electrode or reciprocal connections between the STN and the PPN. In animal studies, Sitti et al. explored the effects of STN-DBS on PPN neural activity and observed that compared to stimulation with 10 Hz or 130 Hz, only 60 Hz STN-DBS produced entrainment of the neural firing pattern in the PPN25. Moreover, several studies have comfimed that stimulation of PPN with lower frequency such as 10–25 Hz, sometimes even 60 Hz, had better efficacy of improving gait and locomotion26,27,28,29,30. Therefore, LFS targeting the STN may yield a potential propagation to the PPN and induces the changes in the neural activities in PPN, which is needed to be confirmed in future studies by comparing the effects of stimulation targeting STN and PPN.
Limitations
Two out of four studies on the effects on FOG were excluded. Specifically, in study reported by Xie et al.11, after the LFS, people perform no any FOG symptom, and thus the scores of FOG were zero, which cannot be used in the meta-analysis11. But the findings of Xie et al.11 was consistent with the results of our analysis, that is, LFS had better efficacy on reducing numbers of FOG11. In the other study, no participants presented FOG though the FOG score was measured17. The number and the sample sizes of the literatures are thus relatively small and the study designs are of high heterogeneity. Future randomized and double-blinded studies with larger sample sizes are needed to explore explicitly the effects of different frequency bands of on the pathological motor symptoms in population with PD.
Conclusion
In conclusion, our meta-analysis reveal that HFS of STN-DBS is an effective strategy to alleviate tremors within medication-off condition, and LFS is helpful for severe akinesia and gait disturbances in patients with PD. Although these results are needed to be further confirmed in future, the observed frequency-specific effects can ultimately inform the frequency programming of STN-DBS in the clinic use.
References
di Biase, L. & Fasano, A. Low-frequency deep brain stimulation for Parkinson’s disease: Great expectation or false hope? Mov Disord 31, 962–967, https://doi.org/10.1002/mds.26658 (2016).
Blumenfeld, Z. et al. Sixty-hertz stimulation improves bradykinesia and amplifies subthalamic low-frequency oscillations. Mov Disord 32, 80–88, https://doi.org/10.1002/mds.26837 (2017).
Aziz, M. B. et al. Tremor Reduction by Deep Brain Stimulation Is Associated With Gamma Power Suppression in Parkinson’s Disease. Neuromodulation: Technology at the Neural Interface, https://doi.org/10.1111/ner.12297 (2014).
Blumenfeld, Z. et al. Sixty hertz neurostimulation amplifies subthalamic neural synchrony in Parkinson’s disease. Plos One 10, e0121067, https://doi.org/10.1371/journal.pone.0121067 (2015).
Fogelson, N. et al. Frequency dependent effects of subthalamic nucleus stimulation in Parkinson’s disease. Neurosci Lett 382, 5–9, https://doi.org/10.1016/j.neulet.2005.02.050 (2005).
Chiou, S. M., Lin, Y. C., Lu, M. K. & Tsai, C. H. Bilateral subthalamic stimulation for advanced Parkinson disease: early experience at an Eastern center. Neurol Sci 36, 515–520, https://doi.org/10.1007/s10072-014-2008-x (2015).
Picillo, M., Lozano, A. M., Kou, N., Puppi Munhoz, R. & Fasano, A. Programming Deep Brain Stimulation for Parkinson’s Disease: The Toronto Western Hospital Algorithms. Brain Stimul 9, 425–437, https://doi.org/10.1016/j.brs.2016.02.004 (2016).
Moro, E. et al. The impact on Parkinson’s disease of electrical parameter settings in STN stimulation. Neurology (2002).
Reich, M. M. et al. Short pulse width widens the therapeutic window of subthalamic neurostimulation. Ann Clin Transl Neurol 2, 427–432, https://doi.org/10.1002/acn3.168 (2015).
Khoo, H. M. et al. Low-frequency subthalamic nucleus stimulation in Parkinson’s disease: a randomized clinical trial. Mov Disord 29, 270–274, https://doi.org/10.1002/mds.25810 (2014).
Tao Xie, M. E. Low-frequency stimulation of STN-DBS reduces aspiration and freezing of gait in patients with PD. Neurology (2015).
Vallabhajosula, S. et al. Low-frequency versus high-frequency subthalamic nucleus deep brain stimulation on postural control and gait in Parkinson’s disease: a quantitative study. Brain Stimul 8, 64–75, https://doi.org/10.1016/j.brs.2014.10.011 (2015).
Hozo, S. P., Djulbegovic, B. & Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 5, 13, https://doi.org/10.1186/1471-2288-5-13 (2005).
Higgins, J. P. T., Jonathan, S. G. T., Douglas, J. D. & Altman, G. Measuring inconsistency in meta-analyses. BMJ (2003).
St George, R. J., Nutt, J. G., Burchiel, K. J, Horak, F. B. A meta-regression of the long-term effects of deep brain stimulation on balance and gait in PD. Neurology (2010).
Hamani, C., Richter, E., Schwalb, J. M. & Lozano, A. M. Bilateral Subthalamic Nucleus Stimulation for Parkinson’s Disease: A Systematic Review of the Clinical Literature. Neurosurgery 56, 1313–1324, https://doi.org/10.1227/01.neu.0000159714.28232.c4 (2005).
Ricchi, V. et al. Transient effects of 80 Hz stimulation on gait in STN DBS treated PD patients: a 15 months follow-up study. Brain Stimul 5, 388–392, https://doi.org/10.1016/j.brs.2011.07.001 (2012).
Annic, A. et al. Predictive factors for improvement of gait by low-frequency stimulation in Parkinson’s disease. J Parkinsons Dis 4, 413–420, https://doi.org/10.3233/JPD-130337 (2014).
Stegemo llera, E. L. et al. Selective use of low frequency stimulation in Parkinson’s disease based on absence of tremor. NeuroRehabilitation, https://doi.org/10.3233/NRE-130960 (2013).
C. Moreau, M. et al. STN-DBS frequency effects on freezing of gait in advanced Parkinson disease. Neurology (2008).
Xie, T. et al. Effect of low versus high frequency stimulation on freezing of gait and other axial symptoms in Parkinson patients with bilateral STN DBS: a mini-review. Transl Neurodegener 6, 13, https://doi.org/10.1186/s40035-017-0083-7 (2017).
Peter H. Weiss, M. et al. Subthalamic Nucleus Stimulation Improves Parkinsonian Gait via Brainstem Locomotor Centers. Movement Disorders, https://doi.org/10.1002/mds.26229 (2015).
Baizabal-Carvallo, J. F. & Alonso-Juarez, M. Low-frequency deep brain stimulation for movement disorders. Parkinsonism Relat Disord 31, 14–22, https://doi.org/10.1016/j.parkreldis.2016.07.018 (2016).
Herrington, T. M., Cheng, J. J. & Eskandar, E. N. Mechanisms of deep brain stimulation. J Neurophysiol 115, 19–38, https://doi.org/10.1152/jn.00281.2015 (2016).
Sitti, I. et al. Effect of Subthalamic Nucleus Stimulation on Pedunculopontine Nucleus Neural Activity. Stereotactic and Functional Neurosurgery 94, 54–59, https://doi.org/10.1159/000442892 (2016).
Xie, T. et al. Long-term effect of low frequency stimulation of STN on dysphagia, freezing of gait and other motor symptoms in PD. Journal of Neurology, Neurosurgery & Psychiatry, jnnp-2018-318060, https://doi.org/10.1136/jnnp-2018-318060 (2018).
Stefani, A. et al. Bilateral deep brain stimulation of the pedunculopontine and subthalamic nuclei in severe Parkinson’s disease. Brain 130, 1596–1607, https://doi.org/10.1093/brain/awl346 (2007).
Nosko, D. et al. Low-frequency versus high-frequency stimulation of the pedunculopontine nucleus area in Parkinson’s disease: a randomised controlled trial. J Neurol Neurosurg Psychiatry 86, 674–679, https://doi.org/10.1136/jnnp-2013-307511 (2015).
Paolo Mazzonea, A. L. et al. Implantation of human pedunculopontine nucleus: a safe and clinically relevant target in Parkinson’s disease. Neuroreport (2005).
Gill, S. & Plaha, P. Bilateral deep brain stimulation of the pedunculopontine nucleus for Parkinson’s disease. Neuroreport (2005).
Acknowledgements
This research was supported by the National Key Technology Research and Development Program of the Ministry of Science and Technology of The People’s Republic of China, code: 2016YFC1306501., National Natural Science Foundation of China (No. 81571226, 81771367), Beijing Municipal Science and Technology Commission (No. Z17110700100000, Z151100003915117 and No. Z151100003915150), Beijing Natural Science Foundation (No. 7164254), and Irma and Paul Milstein Program for Senior Health Fellowship Award from Milstein Medical Asian American Partnership (MMAAP) Foundation.
Author information
Authors and Affiliations
Contributions
Tao Feng and Dongning Su had the idea and designed the study. Dongning Su and Yuye Liu finished the literature review. Dongning Su and Wanli Hu conducted the studies’ quality assessment. The statistical analysis and article draft was finished by Dongning Su. Huimin Chen, Junhong Zhou and Zhan Wang critically revised the article. Xuemei Wang, Genliang Liu, and Huizi Ma subsequently revised the article and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Su, D., Chen, H., Hu, W. et al. Frequency-dependent effects of subthalamic deep brain stimulation on motor symptoms in Parkinson’s disease: a meta-analysis of controlled trials. Sci Rep 8, 14456 (2018). https://doi.org/10.1038/s41598-018-32161-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-32161-3
Keywords
This article is cited by
-
Closed-loop modulation of model parkinsonian beta oscillations based on CAR-fuzzy control algorithm
Cognitive Neurodynamics (2023)
-
Causal mapping of human brain function
Nature Reviews Neuroscience (2022)
-
Electroceutically induced subthalamic high-frequency oscillations and evoked compound activity may explain the mechanism of therapeutic stimulation in Parkinson’s disease
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.