Abstract
Extracellular vesicles (EVs), originating from multivesicular bodies by invagination of the endosomal membrane, are communication channels between distant cells. They are natural carriers of exogeneous cellular materials and have been exploited as drug delivery carriers in various diseases. Here, we found that tumor cell-derived EVs can be used as efficient targets in tumors by monitoring with an optical reporter system. Anaplastic thyroid cancer (CAL62) cell-derived EVs with Renilla luciferase (Rluc) were used to target CAL62 tumors in a mouse model. Optical imaging revealed that cancer cell-derived EVs (EV-CAL62/Rluc) targeted the original tumor (CAL62) in mice within 30 min after systemic injection. Furthermore, fluorescence imaging revealed that EV-CAL62/Rluc were internalized into CAL62 tumors in the mice. Ex vivo Optical imaging further confirmed the in vivo finding. Here, we successfully monitored the tumor targeting ability of tumor cell-derived EVs by optical imaging. Based on these results, tumor cell-derived EVs are highly effective natural carriers for drug delivery for cancer therapies.
Similar content being viewed by others
Introduction
Naturally produced biological nanoparticles are known as extracellular vesicles (EVs). EVs are released from cells into the extracellular space and found in various body fluids such as the blood, urine, and central nervous system fluids1,2,3. EVs are classified into exosomes and microvesicles. Exosomes (50–200 nm) are membrane vesicles released by multi-vesicular bodies. Microvesicles (50–1000 nm) are released from the cell membrane via a budding process in the cell and are larger than exosomes4,5. EVs are capable of carrying vary biological materials such as lipids, mRNA, miRNA, proteins, and extrachromosomal DNA3,6,7,8,9. Cancer cells produce and secrete larger numbers of EVs compared to normal cells10. Tumor-derived EVs are information carriers that convey molecular and genetic messages from tumor cells to normal or other abnormal cells residing at close or distant sites11.
EVs are a novel class of intercellular signal mediators that are involved in a number of different physiological and pathological processes11,12. Previous studies suggested that the contact between EVs and recipient cells occurs through receptor-ligand binding13,14,15. Another study showed that primary melanoma exosomes can be preferentially delivered to metastatic melanoma tumor cells16. A recent study used cancer-derived exosomes as a useful delivery vehicle with low immunogenicity for efficient CRISPR/Cas9-mediated genome editing in cancer cells. Particularly, cancer-derived exosomes showed preferential uptake to into cancer cells compared to epithelial cell-derived exosomes17. Tumor targeting and selective drug delivery using cancer-derived EVs has been proposed because of their specific expression of tetraspanins, which preferentially interact with certain ligands18. The precise mechanisms of these interactions are not clearly understood. Few studies have demonstrated that tumor-derived EVs can target a parental tumor in vitro and in vivo17, and further studies are necessary to assess this phenomenon in vivo. These characteristics of tumor-derived EVs can be used to target the tumor and in drug delivery vehicles. EVs are excellent endogenous nanocarriers for exogeneous drug delivery systems. In recent years, several studies showed that drug-loaded EVs improved disease conditions19, including studies performed using different cancer models20,21,22,23. One way of reducing side effects is to target the delivery of an anti-cancer drug to the tumor.
Recent studies showed that EVs can be visualized in in vivo animal models by using a lipophilic dye24, radionuclides25,26, magnetic particles27,28 and bioluminescence reporter system29. The labeling process with lipophilic dyes is simple and the labeling is suitable for real-time monitoring of EVs, but lipophilic dyes promotes clumping of EVs30; significant EV damage29; non-specific signals from dye remains in tissues5,29. Nuclear imaging could be a good candidate for tracking EVs in both preclinical and clinical studies, a limitation of this technology is the possibility of altering EV characteristics by the labeling procedure5. In recent studies, EVs were loaded with MRI contrasts and visualized using MRI27,28,31. A large amount of iron oxide-loaded EVs are needed as low sensitivity of MRI technology5,28. In preclinical studies bioluminescent imaging (BLI) has an extremely high signal-to-noise ratio, low auto-luminescence in mammalian tissue; low background emission compared to fluorescent-based imaging32,33.
Noninvasive or in vivo bioluminescent imaging is particularly advantageous for studying various live cells in small animals32,34,35. We recently developed a highly sensitive in vivo visualization method for EVs by employing a new BLI reporter (Renilla luciferase) system33. Studies involving real-time visualization of tumor-derived EVs as tumor-targeting agents are urgently needed. The present study was performed to determine whether EVs can preferentially target their parent cell, which may be useful for tumor targeting and drug delivery. Here, we tested a newly developed bioluminescent EV reporter system in an in vivo animal model to monitor the targeting ability of thyroid cancer-derived EVs to original tumors.
Results
Generation of Stable Cell Lines Expressing Luciferase Reporter Genes
Anaplastic thyroid cancer cells (CAL62) transfected with retrovirus containing the Rluc gene or effluc gene were used to generate cells expressing a reporter gene. CAL62 cells transduced with the Rluc gene were named as CAL62/Rluc and those transduced with the Effluc gene were named as CAL62/Effluc. Successful insertion of the Rluc or effluc gene into CAL62 cells was confirmed by BLI, as shown in Fig. 1A–D. As the BLI signal in CAL62/Rluc and CAL62/Effluc cells increased, there was increase in BLI signal in dose-dependent manner. (Cal62/Rluc: R2 = 0.985; Cal62/Effluc: R2 = 0.976) and no signals were observed in the parental CAL62 cells. In addition, successful transduction of the Rluc or Effluc gene into cells was confirmed by RT-PCR and western blotting (Figs 1E,F and S1A,B). Taken together, these results indicate that Rluc and Effluc were stably expressed in CAL62 cells. CAL62/Rluc cells were used for the isolation of EVs, and CAL62/Effluc cells were used to prepare a subcutaneous tumor mouse model.
Generation of stable reporter gene expression in a cancer cell line. (A) Representative bioluminescent imaging of the in vitro luciferase assay in CAL62 and CAL-62/Rluc cells. (B) In vitro luciferase assay in CAL62 and CAL62/Rluc cells. Data are expressed as the mean ± standard deviation (SD). (C) Representative bioluminescent imaging of the in vitro luciferase assay in CAL62 and CAL62/Effluc cells. (D) In vitro luciferase assay in CAL62 and CAL62/Effluc cells. Data are expressed as the mean ± standard deviation (SD). (E) Western blot analysis of the Rluc and Effluc proteins in CAL62, CAL62/Rluc, and CAL62/Effluc cells; β-actin was used as an internal control. (F) RT-PCR analysis to determine the expression of the Rluc and Effluc genes in CAL62, CAL62/Rluc, and CAL62/Effluc cells; GAPDH served as an internal control.
Characterization of EV-CAL62/Rluc
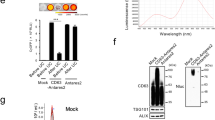
We isolated EVs (EV-CAL62/Rluc) from conditioned medium obtained from CAL62/Rluc cells by ultracentrifugation as described previously33. EV-CAL62/Rluc was examined by TEM, which revealed that the EVs were 100–300-nm round shape vesicles with an intact membrane structure, which are recognized characteristics of EVs (Fig. 2A). Further, western blotting analysis confirmed the enrichment of EVs-biomarkers such as tetraspanins protein cluster of differentiation 63 (CD63) and tumor susceptibility gene 101 (TSG101) in the EVs. Furthermore, absence of Golgin subfamily A member 2 (GM130), the endoplasmic reticulum protein (Calnexin) and proliferating cell nuclear antigen (PCNA) was confirmed and these proteins were present in cells (Figs 2B and S2A). In addition, the average size and range concentration of EVs were analyzed by ELS, which revealed that average size of EVs was 93.1 ± 21.3 nm and approximately 75% of EVs were 50–300 nm (Fig. 2C,D). Together, these results confirm the characterization and purification of isolated EVs.
Characterization of EVs. (A) Examination of EVs from CAL62/Rluc cells using electron microscopy Scale bar: 100, 200 nm. (B) Western blotting analysis of EVs. CD63 and TSG101 (EV marker proteins) GM130, CALNEXIN and PCNA (cell marker proteins) were detected by specific antibodies. (C,D) EV size and concentrations were determined by DLS (Avg. 93.1 ± 21.3 nm). (D) Distribution of EVs population in the isolated EVs.
Renilla Luciferase Activity of EV-CAL62/Rluc
To confirm the presence of Rluc protein in CAL62 cell-derived EVs, BLI was performed, which revealed that Rluc levels and BLI signals increased in a dose-dependent manner (Fig. 3A,B; P < 0.001). The western blotting analysis further confirmed the presence of Rluc protein in EV-CAL62/Rluc and not in EV-CAL62 (Figs 3C and S2B).
EV-CAL-62/Rluc showed EV-specific Rluc activity and serum stability in vitro. (A) Representative bioluminescent imaging of an in vitro luciferase assay in EVs from CAL-62 and CAL-62/Rluc cells. (B) Quantitative in vitro luciferase data in EVs are expressed as the mean ± SD. (C) Western blot analysis of the Rluc protein in EVs from CAL-62, CAL-62/Rluc cells, detected by means of Rluc-specific antibodies. TSG served as loading control. (D) Stability of Rluc in EV-CAL62/Rluc and recombinant Rluc protein in serum. Time course of stability of Rluc at 37 °C in 20% FBS/PBS buffer. (E) Representative confocal images of Rluc (green) and DiD (red) in EV-CAL62/Rluc/DiD or EV-CAL62/DiD or EV-CAL62 in CAL62 cells. Scale bars: 10 μm.
Rluc in EV-CAL62/Rluc Showed High Stability in the Serum
The Rluc stability of EV-CAL62/Rluc and recombinant Rluc (r-Rluc) in serum was tested. Rluc activity was decreased marginally EV-CAL62/Rluc, the percentage of serum stability of Rluc in EV-CAL62/Rluc was approximately 98% (1 h), 98% (2 h) and 97% (3 h) but r-Rluc serum stability was significantly decreased (P < 0.001) compare to EV-CAL62/Rluc and it was approximately 77% (1 h), 58% (2 h) and 47% (3 h) (Fig. 3D).
Immunofluorescent (IF) Imaging Confirms the Presence of Rluc in EV-CAL62/Rluc
EV-CAL62/Rluc or EV-CAL62 were labeled with NIR dye-DiD (a fluorescent lipophilic tracer) and then incubated with CAL62 cells. After 2-hour, IF was performed with Rluc antibody. IF results revealed the presence of Rluc signals in the EV-CAL62/Rluc/DiD incubated CAL62 cells and no Rluc signals present in EV-CAL62 /DiD incubated CAL62 cells. Further, Rluc signals were co-localized with DiD signals in EV-CAL62/Rluc/DiD incubated CAL62 cells. Whereas only DiD signals were observed in EV-CAL62/DiD incubated CAL62 cells (Fig. 3E).
EV-CAL62 are internalized by CAL62 and CAL62/Effluc Cells
EV-based drug delivery to cells is possible by internalization of EVs into targeted cells. Therefore, we measured the internalization of EV-CAL62/Rluc into recipient parent CAL62 and CAL62/Effluc cancer cells. EVs were purified and labeled with the NIR dye-DiD. Confocal laser microscopy, performed 1 h after the incubation of EVs, revealed the effective internalization of EV-CAL62/Rluc into CAL62 and CAL62/Effluc cells (Supplementary Fig. 3A). Total number of DiD (EV-CAL62/Rluc/DiD) positive cells were not significantly (P = 0.349) changed between CAL62 (58.7) and CAL62/Effluc (60.5) cells (Supplementary Fig. 3B). Further, we also confirmed the internalization of EVs using flow cytometry. Results revealed that EV-CAL62/Rluc were effectively internalized into CAL62 and CAL62/Effluc cells (Fig. 4A,B). Total number of DiD (EV-CAL62/Rluc/DiD) positive cells were not significantly (P = 0.112) different between CAL62 (89.5%) and CAL62/Effluc (86.6%) cells (Fig. 4C). These results indicated that EV-CAL62/Rluc actively internalized irrespective of effluc gene expression in CAL62 cells.
Internalization of EVs into cancer cells. (A,B) Representative flowcytometry images of CAL62 or CAL62/Effluc cells incubated with unlabeled EV-CAL62/Rluc or DiD-labeled EV-CAL62/Rluc. (B) Quantification of DiD positive cells from flowcytometry of CAL62 or CAL62/Effluc cells treated with unlabeled EV-CAL62/Rluc or DiD-labeled EV-CAL62/Rluc was represented in bar graph (p = 0.112).
In vivo Tumor Targeting Monitored by Bioluminescent Imaging
CAL62/Effluc cells were subcutaneously injected into mice and the mice were randomly divided into two groups (EV-CAL62/Rluc and PBS) at the 6th week. The tumor was assessed by BLI after intraperitoneal D-luciferin injection 5 days prior to EV administration (Fig. 5A,B). We isolated EVs (EV-CAL62/Rluc) from conditioned medium obtained from CAL62/Rluc cells by ultracentrifugation as described previously33. We intravenously administered 50 µg of EV-CAL62/Rluc to mice with or without tumor xenograft, while control mice were administered PBS. Coelenterazine was intravenously injected at 5, 30, 60, and 120 min after EV-Cal62/Rluc (50 µg) or PBS injection and BLI was obtained using an IVIS Lumina III.
BLI showed that no signals were visualized at 5 min post-injection all the three groups and EV-CAL-62/Rluc was visualized in the region of lungs, liver and spleen of mice and in the tumor of CAL62/Effluc tumor bearing mice within 30 min after injection (Fig. 5C,D); quantitative analysis revealed that significant (P < 0.001) BLI signals were observed in the tumor (CAL62/Effluc) region at 30 min and No signals were visualized at the tumor site (CAL62/Effluc) in PBS-injected mice at 30 min by imaging (Fig. 5C,D). Furthermore, BLI imaging at 60 and 120 min revealed that EV-CAL62/Rluc remained in the region of lung in mice without tumor xenograft and in the tumor of CAL62/Effluc tumor bearing mice (Fig. 5C,D). Quantitative analysis revealed significantly (P < 0.001) higher BLI signals in the tumor region (CAL62/Effluc) at 60 and 120 min in EV-CAL62/Rluc-injected CAL62/Effluc tumor bearing mice compared to those in control PBS-injected CAL62/Effluc tumor bearing mice (Fig. 5D). Taken together, these results revealed that EV-CAL62/Rluc are predominantly accumulated in lung of mice without tumor xenograft and accumulated in the tumor of tumor bearing mice.
In vivo visualizing and monitoring tumor-derived EVs targeting the same tumor. (A) Image of Effluc activity of CAL62/Effluc nude mice at six weeks after subcutaneous injection of CAL62/Effluc cells. (B) Quantification of tumor BLI signals from derived EV-CAL62/Rluc (n = 3) or PBS (n = 3) mouse groups.(C) In vivo imaging of EV-CAL62/Rluc intravenously administered to naïve or CAL62/Effluc tumor-bearing mice. (D) Quantification of Rluc (EV-CAL62/Rluc) signals in the tumor region of mice mentioned in 3C.
Ex Vivo Bioluminescent Imaging of EV-CAL62/Rluc Targeting Tumors and Subcellular Visualization EV-CAL62/Rluc by Fluorescent Imaging
Ex vivo bioluminescent imaging of excised tumor showed bioluminescent signals after incubated with Coelenterazine in EV-CAL62/Rluc injected mice and no signals were observed in PBS control (Fig. 6A). These results further confirmed the in vivo imaging of EV-CAL62/Rluc targeting CAL62/Effluc Tumor. The quantification of bioluminescent imaging showed significant signals (P < 0.001) in EV-CAL62/Rluc injected mice compare to PBS control (Fig. 6B). Tumors were sectioned and subjected to immunofluorescent staining with Rluc- and Effluc-specific antibodies, which revealed that EV-CAL-62/Rluc localized to the CAL62/Effluc tumor. No Rluc signals were observed in PBS control tumors (CAL62/Effluc) (Fig. 6C).
Ex vivo and Subcellular visualization of EV-CAL62/Rluc in tumors. (A) Representative ex vivo bioluminescent imaging of EV-CAL62/Rluc excised tumor from EV-CAL62/Rluc or PBS intravenously administered to CAL62/Effluc tumor-bearing mice (n = 3). (B) Quantification of EV-CAL62/Rluc signal from the tumor above mentioned in (A); the values are expressed as mean ± SD. (C) Representative confocal images of Rluc in harvested tumors (CAL62/Effluc) from mice described in Fig. 3C after 120 min. Scale bars: 50 μm.
Discussion
EVs are naturally occurring carriers that can target tumors and deliver a wide range of endogenous and exogeneous and small molecules, anti-cancer drugs, and nucleic acids20,36,37,38. Thus, using EVs as drug delivery vehicles shows more potential compared to other nanoparticles that induce high immunogenicity in vivo and are easily eliminated by the immune system17,39. Tumor-derived EVs affect tumors in various manners, including effects on the epithelial to mesenchymal transition in nasopharyngeal carcinoma40, converting mesenchymal stem cells into cancer-associated fibroblasts41, transporting paracrine signaling factors and mRNA to induce angiogenesis in the metastatic microenvironment42, and facilitating organotropic metastatic growth43,44, among others. EV proteins and miRNA can be used as biomarkers for the early detection of tumors45,46. Tumor-derived EVs can be used for cell-free anti-tumor vaccination to induce T-cell activation and T-cell dependent immune responses against tumor cells in vivo47. They also have antitumor immunogenicity even without expressing MHC-I molecules, which can provide antigens against various cancer types48.
In vivo visualization and monitoring the EVs is an animal model can provide a foundation for developing EV-based therapies. Here, we tested the targeting ability of tumor-derived EVs to its parental tumor in vivo in a mouse model by using a newly developed reporter gene-based optical imaging system for in vivo EV monitoring, which may be useful as a monitoring platform for EV-based delivery vehicles. We used anaplastic thyroid cancer cells (CAL62) to test our hypotheses that CAL62 cancer cell-derived EVs can target CAL62 tumors in an in vivo animal model. We successfully inserted an optical reporter gene (Rluc) in CAL62 cells and isolated EVs with the optical reporter protein (Rluc). We also separately transduced CAL62 cells with the Effluc gene to prepare a subcutaneous tumor mouse model.
First, we isolated EVs from the CAL62/Rluc cell culture media using a conventional ultracentrifugation method. The round morphology, size, positive or negative and Rluc Protein in EV-CAL62/Rluc which is consistent with previous study33. Rluc in the EV-CAL62/Rluc showed a strong stability compare to r-Rluc under serum condition and consistent with other study33. Furthermore, Immunofluorescent assay confirmed the Rluc present in the EVs compartment. Immunofluorescent and flowcytometry analysis confirmed the EV-CAL62/Rluc are able to internalize into the same cancer cells more effectively regardless of Effluc gene expression in the cancer cells, This results support the hypothesis that cancer cell-derived EVs harbor a specific tropism, based on their cellular origin17, which can be exploited to deliver the drugs into cancer cells.
Furthermore, we used a newly developed optical reporter system to track EVs in vivo; the results suggested that most EVs accumulated in the tumor, while previous studies showed that a portion or more than half of tumor-derived EVs accumulated in the same tumor in in vivo14,17. Our previous work showed that labeling EVs with lipophilic dye affected their biodistribution in vivo33, as EV targeting occurs through integrins present in the EV membrane49, which can be blocked by dye labeling on the EV surface membrane and can lead to less accumulation at the tumor in vivo. Further, we visualized the subcellular distribution of EV-CAL-62/Rluc in excised tumors by immunofluorescence. Our ex vivo results further confirmed the in vivo finding and further results showed that most EVs were co-localized with CAL-62/Effluc cells, and previous studies found that cancer cells readily internalize EVs to a greater extent than normal cells50.
We confirmed that EVs derived from the tumor targeted the same tumor when injected intravenously into an in vivo mouse model. BLI imaging clearly showed that intravenously injected EV-CAL62/Rluc accumulated at the tumor region of mice at 30 min after injection but EV-CAL62/Rluc injected into nude mice without tumor xenograft by the intravenous route accumulated predominantly in the lung and liver, which is consistent with previous study33. Similar to our results (EV-CAL62 targeting CAL62 cells in vivo), a recent study evaluated exosomes derived from tumor cells (SKOV3) injected intravenously into the tail veins of SKOV3 xenograft mice; optical imaging revealed that Cy5.5-labeled SKOV3-Exo was significantly accumulated in tumor sites in vivo17. Other studies suggested that tumor-derived EVs are more highly associated with cancer cells than with normal cells, and an acidic pH may be one factor affecting tumor exosome trafficking to the tumor16. Another study suggested that tumor-derived EVs have unique protein and lipids compartments resembling those of cells from which they are derived and may interact very uniquely with the same tumor cells50. In addition, more SKOV3-derived exosomes were internalized than HEK293-derived exosomes in SKOV3 tumors17. Based on these data, tumor-derived EVs are an attractive therapeutic cargo carrier for targeted drug delivery systems. Furthermore, tumor derived EVs can be administered via intracardiac or intraperitoneal routes to enhance tumor targeting ability in the future.
Recently, EVs were considered as new biological drug delivery vehicles. EVs are small and have a negative zeta potential to ensure long circulation51 and they can escape degradation52 and can evade the immune system53. EVs can carry drugs, DNA, mRNA, miRNA, and proteins54. In this study, Rluc proteins inside the EVs were successfully delivered to the target tissue. A recent study of cancer-derived EVs suggested that they can promote cancer progression and form a pre-metastatic niche54; advancements in the engineering of EVs may reduce these effects of cancer-derived EVs. Furthermore, drugs can be effectively delivered to the tumor area using cancer-derived EVs to destroy cancer cells. Cancer-derived EVs are produced in greater numbers than EVs in normal cells, reducing the effort needed to collect EVs.
Conclusion
We successfully visualized EVs derived from CAL-62 targets CAL-62 tumors in vivo using an optical imaging system. These cancer-derived EVs show potential as candidates for drug delivery systems.
Materials and Methods
Cell Culture and Transduction
An anaplastic thyroid cancer cell line (ATC), CAL-62, was cultured in Dulbecco’s modified Eagle medium (DMEM, Gibco, Grand Island, NY, USA) supplemented with 10% fetal calf serum (Hyclone, Logan, UT, USA) and 1% antibiotics (Gibco) and incubated at 37 °C in a 5% CO2 atmosphere. CAL-62 cells were transduced with a lentivirus expressing Rluc with puromycin genes under control of the CMV promoter (Genecopoeia, Rockville, MD, USA)33. CAL-62 cells were retrovirally transduced to express both effluc and thy1.1. Thy1.1-positive cells were sorted using CD90.1 micro-beads (Miltenyi Biotec, Bergisch Gladbach, Germany)55.
Luciferase Activity Assays
CAL-62 cells, CAL-62/Rluc and CAL-62/Effluc (1.25 × 104, 2.5 × 104, 5 × 104, and 105 cells/well) were plated into white and clear-bottom 96-well plates containing serum-free DMEM. Twenty-four hours later, 5 µl of coelenterazine/100 µl media (15 µg/ml final concentration) for Rluc or 2 µl D-luciferin/100 µl media (15 µg/ml final concentration) for Effluc substrate (Caliper, PerkinElmer, Waltham, MA, USA) was added to each well. Fluc emits photons in a reaction that requires ATP, Mg2+, oxygen, and D-luciferin56. In the presence of oxygen, Rluc catalyzes the non-ATP-dependent oxidation of coelenterazine to generate a luminescent signal57. Rluc or Effluc activity was determined by BLI using the IVIS Lumina III instrument (In Vivo Imaging System, IVIS Lumina III, PerkinElmer).
RT-PCR Analysis
CAL-62, CAL-62/Rluc and CAL-62/Effluc cells were lysed using Trizol solution (Invitrogen, Carlsbad, CA, USA), and total RNA was extracted according to the manufacturer’s instructions. Reverse transcription was performed as previously described33 using a High-Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific, Inc., Waltham, MA, USA). After denaturing the samples for 2 min at 94 °C, 40 cycles for 20 s at 94 °C, 10 s at 57 °C, and 30 s at 72 °C were conducted with an additional 5 min at 72 °C. DNA polymerase (Takara, Shiga, Japan) and a Takara PCR system were used. Primers sequences were as follows: Rluc; forward: 5′-TATGATTCCGAGAAGCACGC-3′; reverse: 5′-TGATCCAGGAGGCGATATGA-3′; Fluc; forward: 5′-GCACAAGGCCATGAAGAGAT-3′; reverse: 5′-CTTCTTGCTCACGAACACCA-3′; GAPDH; forward: 5′-AGTGATGGCATGGACTGTGG-3′; reverse: 5′-GTCAAGGCTGAGAACGGGAA-3′. Samples were separated by electrophoresis on an ethidium bromide-stained agarose gel. Gels were imaged on a UV trans-illuminator using a UVP Gel-Doc-IT imaging system.
Western Blot Analysis
Western blotting was performed as described previously33. Whole-cell or EV lysates were prepared in RIPA buffer (Thermo Fisher Scientific) with protease inhibitor cocktail (Thermo Fisher Scientific). An equal amount of protein (20 µg per well) was loaded and separated by 10% SDS–PAGE. The proteins were transferred from the gel to a polyvinylidene fluoride membrane (Millipore, Billerica, MA, USA) and probed with the primary antibody and then with a secondary antibody conjugated to horseradish peroxidase (HRP). The following primary antibodies were used: CD63, TSG101, GM130, Calnexin, Rluc (Abcam, Cambridge, UK; Dilution: 1:4000), PCNA (Cell Signaling Technology, Danvers, MA, USA; Dilution: 1:6000), Effluc (Promega, Madison, WI, USA; Dilution: 1:5000). The following secondary antibodies were used: HRP-conjugated anti-mouse (Cell Signaling Technology, Danvers, MA, USA; Dilution: 1:8000), HRP-conjugated anti-rabbit (Cell Signaling Technology; Dilution: 1:8000), HRP-conjugated anti-goat (Cell Signaling Technology; Dilution: 1:8000). The signals were detected using an electrochemiluminescence detection system (Thermo Fisher Scientific) according to the manufacturer’s protocol.
Isolation of EVs
Cal62 and Cal62/Rluc cells were cultured with DMEM supplemented with 10% EV-depleted fetal bovine serum (filtered through a 0.22-µm syringe filter and then centrifuged for 18 h at 100,000 × g at 4 °C) for isolation of EVs. EVs were isolated as described previously33. Briefly, the supernatant was centrifuged at 300 × g for 10 min, 1500 × g for 20 min, and finally 2500 × g for 20 min (to remove cells and debris). The supernatant was filtered through a 0.45-µm syringe filter and centrifuged at 100,000 × g for 60 min. The pellet was resuspended and centrifuged at 100,000 × g for 60 min. The final pellet was resuspended in 50–100 µL of PBS and stored at −80 °C. All EVs were used within one week. All ultracentrifugation steps were performed (SW-28 rotor; Ultra-Clear tube) using the OptimaTM L-100 XP ultracentrifuge (Beckman Coulter, Brea, CA, USA). All centrifugation steps were conducted at 4 °C.
Transmission Electron Microscopy (TEM)
EVs were imaged by TEM. Briefly, after isolation, EVs were fixed at 4 °C overnight. The fixative contained 2.5% glutaraldehyde in 0.01 M phosphate buffer at pH 7.4 (filtered through 0.22-µm filters) and was washed with PBS. EVs were post-fixed in 1% OsO4 (Taab Laboratories Equipment Ltd., Reading, UK) for 30 min. EV pellets were washed with distilled water and dehydrated with graded ethanol. EV pellets were negative-stained with 1% uranyl-acetate in 50% ethanol for 30 min and then embedded in Taab 812 (Taab), followed by polymerization at 60 °C overnight and ultra-sectioning for TEM. Ultra-thin sections were examined, and images captured with a HT-7700 transmission electron microscope (Hitachi, Tokyo, Japan) operated at 100 kV.
Electrophoretic Light Scattering (ELS) Analysis
EV-Cal62/Rluc resuspended in PBS was further diluted by 200–400-fold in distilled water to determine the size and distribution with an ELS-Z (Otsuka Electronics, Osaka, Japan).
Rluc Activity Assay for EV-Cal62/Rluc
EV samples were plated in white and clear-bottom 96-well plates with increasing concentrations of EV-Cal62 and EV-Cal62/Rluc (0, 25, and 50 μg) at the same volume. The substrate coelenterazine was added to each well. Rluc activity was determined by bioluminescence imaging as described above.
Rluc Stability Assay
EV-CAL-62/Rluc or Free Rluc protein was incubated in 20% FBS in a PBS solution at 37 °C33. Stability of Rluc was evaluated by measuring Rluc activity at 0, 6, 12, 18, 24 hours for EV-CAL-62/Rluc. Stability of Free Rluc protein (Creative BioMart) was evaluated by measuring Rluc activity at 0, 1, 2, 3, 4 and 5 hours. The appropriate substrate coelenterazine was added to each well. Rluc activity was determined by BLI as described above.
Rluc Stability Assay Recombinant Rluc Protein (r-Rluc)
Stability of Free Rluc protein (Creative BioMart) was evaluated by measuring Rluc activity at 0, 1, 2 and 3 hours. The appropriate substrate coelenterazine was added to each well. Rluc activity was determined by BLI as described above.
Labeling EVs with DiD
To analyze the internalization of EVs, 20 µg of EVs were labeled with 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt (DiD; Invitrogen), incubated for 20 min at room temperature, and washed with PBS. Next, a two-step OptiPrep density gradient ultracentrifugation was performed as described9.
Immunostaining and Confocal Analysis of EV-CAL62 and EV-CAL62/Rluc
Immunofluorescence on EV-CAL62 and EV-CAL62/Rluc were performed as described58. DiD labeled and purified EV-CAL62/DiD and EV-CAL62/Rluc/DiD were added CAL62 cells for 2-hours, Fixed in 4% paraformaldehyde and permeabilized by 0.5% Triton-X-100. After a blocking step in 3% BSA in PBS, slides were stained overnight at 4 °C with anti-rabbit Rluc (Abcam; Dilution: 1:400) antibodies. After PBS washes and goat anti-rabbit FITC incubation (Abcam; Dilution: 1:500) were mounted with Vectastain anti-fade mounting medium with DAPI (Vector Laboratories, Burlingame, CA, USA). Images were acquired with a Zeiss super-resolution confocal microscope (LSM 5 Exciter, Zeiss, Oberkochen, Germany).
Internalization Assay
The DiD-labeled EVs were incubated together with CAL62 parental cell lines for 1 h at 37 °C under 5% CO2 and were then fixed in methanol. Coverslips were then mounted using the VECTASHIELD anti-fade mounting medium (Vector Laboratories, Burlingame, CA, USA) and sealed. The cellular internalization of EVs was observed using an LSM 780 laser scanning microscope (Carl Zeiss). Total number of DiD (EV-CAL62/Rluc/DiD) positive cells were counted by two independent observers from CAL62 and CAL62/Effluc cells.
Flow cytometry
Flow Cytometry Analysis was performed as described59, Briefly, The DiD-labeled EVs were incubated together with CAL62 or CAL62/Effluc cells for 1-hour at 37 °C under 5% CO2. Cells were washed with PBS (trice) and centrifuged for 3 min at 3500 g to remove free EVs. The supernatant was discarded, and the cells were resuspended in 1 mL of PBS for flow cytometry analysis on a BD Biosciences Aria III (BD Biosciences, San Jose, CA, USA).
Animals
Five-week-old female BALB/c nude mice were purchased from Japan SLC, Inc. (Shizuoka, Japan). All procedures were reviewed and approved by Kyungpook National University (KNU-2012-43) Animal Care and Use Committee and performed in accordance with the Guiding Principles for the Care and Use of Laboratory Animals.
Establishment of a CAL62/effluc Subcutaneous Animal Model
Nude mice were subcutaneously implanted with CAL62/effluc (5 × 106 cells) on the right lower flank regions and allowed to grow for 6 weeks. The mice were anesthetized with 2.5% isoflurane (Merial, Lyon, France) and bioluminescent images were captured using the IVIS Lumina III imaging system after intraperitoneal injection of 100 µL of D-luciferin (3 mg/mouse; Caliper). BLI was subsequently assessed up to the 6th week and mice were dived into two groups (EV-Cal62/Rluc or PBS).
In vivo Imaging of EV-Cal62/Rluc Targeting Tumors
The mice were anesthetized with 2.5% isoflurane and bioluminescent images were captured using the IVIS III imaging system with an appropriate amount of coelenterazine administered intravenously after 5, 30, 60 and 120 min after EV-Cal62/Rluc (50 µg) (n = 3) injection to naïve mice, EV-Cal62/Rluc (50 µg) (n = 3) or PBS (n = 3) injection to tumor bearing mice through same route. Animals were sacrificed after 120 min and tumors were immediately excised and incubated with coelenterazine. Bioluminescent signals were captured and quantified as described above.
Immunofluorescence (IF)
Tumor tissues were cryo-sectioned and processed for immunofluorescence assay as described previously58. CAL-62/Rluc- or PBS-injected mouse tumor sections were stained with Rluc (Abcam; Dilution: 1:400), followed by goat anti-rabbit FITC (Abcam; Dilution: 1:500); Effluc (Promega; Dilution: 1:300), and donkey anti-goat Alexa Fluor® 647 (Abcam; Dilution: 1:500). Tissue sections were mounted using VECTASHIELD mounting medium (Vector Laboratories, Burlingame, CA, USA). IF-stained sections were imaged under a confocal microscope (LSM 5 Exciter, Zeiss, Oberkochen, Germany).
Statistical analysis
All data are expressed as the means ± standard deviation (SD). Two groups of data were statistically analyzed by t-test using GraphPad Prism7 software version 7.04 (GraphPad Software, Inc., La Jolla, CA, USA). A P value less than 0.05 was considered statistically significant.
Data Availability
The authors declare that all the relevant data supporting the findings of this study are available within the article or from the corresponding author upon request.
References
Yáñez-Mó, M. et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 4 (2015).
Zhu, L. et al. Targeting and therapy of glioblastoma in a mouse model using exosomes derived from natural killer cells. Front. Immunol. 9 (2018).
Zhu, L. et al. Exosomes Derived From Natural Killer Cells Exert Therapeutic Effect in Melanoma. Theranostics 7, 2732–2745 (2017).
Gangadaran, P., Hong, C. M. & Ahn, B.-C. Current Perspectives on In Vivo Noninvasive Tracking of Extracellular Vesicles with Molecular Imaging. BioMed Res. Int. 2017, e9158319 (2017).
Gangadaran, P., Hong, C. M. & Ahn, B.-C. An Update on In Vivo Imaging of Extracellular Vesicles as Drug Delivery Vehicles. Front. Pharmacol. 9 (2018).
Yamamoto, H. et al. BARHL2 Methylation Using Gastric Wash DNA or Gastric Juice Exosomal DNA is a Useful Marker For Early Detection of Gastric Cancer in an H. pylori-Independent Manner. Clin. Transl. Gastroenterol. 7, e184 (2016).
Mathivanan, S., Fahner, C. J., Reid, G. E. & Simpson, R. J. ExoCarta 2012: database of exosomal proteins, RNA and lipids. Nucleic Acids Res. 40, D1241–1244 (2012).
Rajendran, R. L. et al. Extracellular vesicles derived from MSCs activates dermal papilla cell in vitro and promotes hair follicle conversion from telogen to anagen in mice. Sci. Rep. 7, 15560 (2017).
Gangadaran, P. et al. Extracellular vesicles from mesenchymal stem cells activates VEGF receptors and accelerates recovery of hindlimb ischemia. J. Control. Release Off. J. Control. Release Soc., https://doi.org/10.1016/j.jconrel.2017.08.022 (2017).
Whiteside, T. L. Tumor-derived exosomes and their role in cancer progression. Adv. Clin. Chem. 74, 103–141 (2016).
Ratajczak, J., Wysoczynski, M., Hayek, F., Janowska-Wieczorek, A. & Ratajczak, M. Z. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia 20, 1487–1495 (2006).
Keller, S., Sanderson, M. P., Stoeck, A. & Altevogt, P. Exosomes: from biogenesis and secretion to biological function. Immunol. Lett. 107, 102–108 (2006).
Raposo, G. et al. B lymphocytes secrete antigen-presenting vesicles. J. Exp. Med. 183, 1161–1172 (1996).
Smyth, T. et al. Biodistribution and delivery efficiency of unmodified tumor-derived exosomes. J. Control. Release Off. J. Control. Release Soc. 199, 145–155 (2015).
Klibi, J. et al. Blood diffusion and Th1-suppressive effects of galectin-9-containing exosomes released by Epstein-Barr virus-infected nasopharyngeal carcinoma cells. Blood 113, 1957–1966 (2009).
Parolini, I. et al. Microenvironmental pH is a key factor for exosome traffic in tumor cells. J. Biol. Chem. 284, 34211–34222 (2009).
Kim, S. M. et al. Cancer-derived exosomes as a delivery platform of CRISPR/Cas9 confer cancer cell tropism-dependent targeting. J. Control. Release Off. J. Control. Release Soc. 266, 8–16 (2017).
Rana, S., Yue, S., Stadel, D. & Zöller, M. Toward tailored exosomes: the exosomal tetraspanin web contributes to target cell selection. Int. J. Biochem. Cell Biol. 44, 1574–1584 (2012).
Johnsen, K. B. et al. A comprehensive overview of exosomes as drug delivery vehicles - endogenous nanocarriers for targeted cancer therapy. Biochim. Biophys. Acta 1846, 75–87 (2014).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat. Biotechnol. 29, 341–345 (2011).
Jang, S. C. et al. Bioinspired Exosome-Mimetic Nanovesicles for Targeted Delivery of Chemotherapeutics to Malignant Tumors. ACS Nano 7, 7698–7710 (2013).
Kim, M. S. et al. Development of exosome-encapsulated paclitaxel to overcome MDR in cancer cells. Nanomedicine Nanotechnol. Biol. Med. 12, 655–664 (2016).
Yang, T. et al. Exosome delivered anticancer drugs across the blood-brain barrier for brain cancer therapy in Danio rerio. Pharm. Res. 32, 2003–2014 (2015).
Wiklander, O. P. B. et al. Extracellular vesicle in vivo biodistribution is determined by cell source, route of administration and targeting. J. Extracell. Vesicles 4, 26316 (2015).
Gangadaran, P. et al. In vivo Non-invasive Imaging of Radio-Labeled Exosome-Mimetics Derived From Red Blood Cells in Mice. Front. Pharmacol. 9 (2018).
Hwang, D. W. et al. Noninvasive imaging of radiolabeled exosome-mimetic nanovesicle using (99m)Tc-HMPAO. Sci. Rep. 5, 15636 (2015).
Hu, L., Wickline, S. A. & Hood, J. L. Magnetic resonance imaging of melanoma exosomes in lymph nodes. Magn. Reson. Med. 74, 266–271 (2015).
Busato, A. et al. Magnetic resonance imaging of ultrasmall superparamagnetic iron oxide-labeled exosomes from stem cells: a new method to obtain labeled exosomes. Int. J. Nanomedicine 11, 2481–2490 (2016).
Lai, C. P. et al. Dynamic Biodistribution of Extracellular Vesicles in Vivo Using a Multimodal Imaging Reporter. ACS Nano 8, 483–494 (2014).
Grange, C. et al. Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging. Int. J. Mol. Med. 33, 1055–1063 (2014).
Hood, J. L., Scott, M. J. & Wickline, S. A. Maximizing exosome colloidal stability following electroporation. Anal. Biochem. 448, 41–49 (2014).
Kim, J. E., Kalimuthu, S. & Ahn, B.-C. In vivo cell tracking with bioluminescence imaging. Nucl. Med. Mol. Imaging 49, 3–10 (2015).
Gangadaran, P. et al. A new bioluminescent reporter system to study the biodistribution of systematically injected tumor-derived bioluminescent extracellular vesicles in mice. Oncotarget 5 (2017).
Ahn, B.-C. Requisites for successful theranostics with radionuclide-based reporter gene imaging. J. Drug Target. 22, 295–303 (2014).
Gangadaran, P. & Ahn, B.-C. Molecular Imaging: A Useful Tool for the Development of Natural Killer Cell-Based Immunotherapies. Front. Immunol. 8, 1090 (2017).
Tian, Y. et al. A doxorubicin delivery platform using engineered natural membrane vesicle exosomes for targeted tumor therapy. Biomaterials 35, 2383–2390 (2014).
Sun, D. et al. A novel nanoparticle drug delivery system: the anti-inflammatory activity of curcumin is enhanced when encapsulated in exosomes. Mol. Ther. J. Am. Soc. Gene Ther. 18, 1606–1614 (2010).
Momen-Heravi, F., Bala, S., Bukong, T. & Szabo, G. Exosome-mediated delivery of functionally active miRNA-155 inhibitor to macrophages. Nanomedicine Nanotechnol. Biol. Med. 10, 1517–1527 (2014).
Sercombe, L. et al. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 6 (2015).
Hood, J. L. Melanoma exosome induction of endothelial cell GM-CSF in pre-metastatic lymph nodes may result in different M1 and M2 macrophage mediated angiogenic processes. Med. Hypotheses 94, 118–122 (2016).
Webber, J., Steadman, R., Mason, M. D., Tabi, Z. & Clayton, A. Cancer exosomes trigger fibroblast to myofibroblast differentiation. Cancer Res. 70, 9621–9630 (2010).
Skog, J. et al. Glioblastoma microvesicles transport RNA and protein that promote tumor growth and provide diagnostic biomarkers. Nat. Cell Biol. 10, 1470–1476 (2008).
Fong, M. Y. et al. Breast-cancer-secreted miR-122 reprograms glucose metabolism in premetastatic niche to promote metastasis. Nat. Cell Biol. 17, 183–194 (2015).
Lu, X. & Kang, Y. Organotropism of breast cancer metastasis. J. Mammary Gland Biol. Neoplasia 12, 153–162 (2007).
Melo, S. A. et al. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature 523, 177–182 (2015).
Mitchell, P. S. et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA 105, 10513–10518 (2008).
Zitvogel, L. et al. Eradication of established murine tumors using a novel cell-free vaccine: dendritic cell derived exosomes. Nat. Med. 4, 594–600 (1998).
Wolfers, J. et al. Tumor-derived exosomes are a source of shared tumor rejection antigens for CTL cross-priming. Nat. Med. 7, 297–303 (2001).
Hoshino, A. et al. Tumour exosome integrins determine organotropic metastasis. Nature 527, 329–335 (2015).
Smyth, T. J., Redzic, J. S., Graner, M. W. & Anchordoquy, T. J. Examination of the specificity of tumor cell derived exosomes with tumor cells in vitro. Biochim. Biophys. Acta BBA - Biomembr. 1838, 2954–2965 (2014).
Malhotra, H. et al. Exosomes: Tunable Nano Vehicles for Macromolecular Delivery of Transferrin and Lactoferrin to Specific Intracellular Compartment. J. Biomed. Nanotechnol. 12, 1101–1114 (2016).
Luan, X. et al. Engineering exosomes as refined biological nanoplatforms for drug delivery. Acta Pharmacol. Sin. 38, 754–763 (2017).
Kamerkar, S. et al. Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 (2017).
Blackwell, R. H., Foreman, K. E. & Gupta, G. N. The Role of Cancer-Derived Exosomes in Tumorigenicity & Epithelial-to-Mesenchymal Transition. Cancers 9 (2017).
Li, X. J. et al. Role of pulmonary macrophages in initiation of lung metastasis in anaplastic thyroid cancer. Int. J. Cancer 139, 2583–2592 (2016).
Wilson, T. & Hastings, J. W. Bioluminescence. Annu. Rev. Cell Dev. Biol. 14, 197–230 (1998).
Lorenz, W. W., McCann, R. O., Longiaru, M. & Cormier, M. J. Isolation and expression of a cDNA encoding Renilla reniformis luciferase. Proc. Natl. Acad. Sci. USA 88, 4438–4442 (1991).
Alam, H. et al. Fascin overexpression promotes neoplastic progression in oral squamous cell carcinoma. BMC Cancer 12, 32 (2012).
Tharmalingam, J. et al. Host Th1/Th2 immune response to Taenia solium cyst antigens in relation to cyst burden of neurocysticercosis. Parasite Immunol. 38, 628–634 (2016).
Acknowledgements
This work was also supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2016R1D1A1A02936968); This work supported by the Korean Thyroid Association Outstanding Investigator Award 2017 and by Natural Science Foundation of Shandong Providence Of China (ZR2017MH063).
Author information
Authors and Affiliations
Contributions
P.G., X.J.L. and B.C.A. initiated the project. P.G. and X.J.L. conducted most of the experiments and data analysis. S.K., O.J.M., C.M.H., R.L.R., H.W.L., L.Z. and S.H.B. conducted some of the experiments. S.Y.J., S.W.L., J.L. and B.C.A. provided the tool/materials/methodology. P.G. prepared the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gangadaran, P., Li, X.J., Kalimuthu, S.k. et al. New Optical Imaging Reporter-labeled Anaplastic Thyroid Cancer-Derived Extracellular Vesicles as a Platform for In Vivo Tumor Targeting in a Mouse Model. Sci Rep 8, 13509 (2018). https://doi.org/10.1038/s41598-018-31998-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31998-y
This article is cited by
-
Radioiodine labeling and in vivo trafficking of extracellular vesicles
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.