Abstract
Acute respiratory distress syndrome (ARDS) has a high mortality rate in intensive care units (ICU). The elderly patients remain to be increased of ICU patients. The aim is to investigate the survival predictors of elderly patients with ARDS. We reported a prospective observational cohort research, including the patients with ARDS between October 2012 and May 2015. Demographic, comorbidities, severity, lung mechanics, laboratory data and survival outcomes were analyzed. A total of 463 patients with ARDS were ≥65 years old were enrolled and analyzed. Multivariate logistic regression analysis identified Charlson comorbidity index (CCI) [odds ratio (OR) 1.111, 95% CI 1.010–1.222, p = 0.031], Sequential Organ Failure Assessment (SOFA) score (OR 1.127, 95% CI 1.054–1.206, p < 0.001) and peak inspiratory pressure (PIP) (OR 1.061, 95% CI 1.024–1.099, p = 0.001) which were independently associated with hospital mortality. Regarding the subgroups patients as 65–74 years old, 75–84 years old and ≥85 years old, the baseline characteristics were not significant difference and the hospital mortality rates were also not significant difference. In conclusion, CCI, SOFA score and PIP were identified as survival predictors in elderly patient with ARDS. Assessing comorbidities with CCI is essential in predicting the survival for elderly patients with ARDS.
Similar content being viewed by others
Introduction
Some epidemiological studies have reported that acute respiratory distress syndrome (ARDS) accounts for 4% of all hospital admissions1,2, 10.4% of intensive care unit (ICU) admissions, and 23.4% of patients needed mechanical ventilation for more than 4 weeks3. Wang et al.4 reported that 15–20% of the patients with ARDS who survive will die by 1 year, mainly because of underlying comorbidities rather than pulmonary sequelae of ARDS. Furthermore, previous studies have reported that the mortality rate of ARDS among elderly patients may be as high as 69–80%5,6.
The number of elderly patients in the ICU continues to rise with the increasing age of the general population7. It has been estimated that 7% to 25% of patients in the ICU are 85 years old and older in developed countries8,9. Several studies have concluded that age is not a predictor of a poor prognosis for elderly patients admitted to an ICU, and that severity of chronic illness and premorbid functional status mainly decided the patients’ outcomes9,10,11. In addition, few studies have investigated the role of advanced age on the survival outcomes of patients with ARDS.
ARDS is a significant cause of morbidity and mortality in patients admitted to an ICU. Clinical trials on the management of ARDS usually exclude very old patients, however, these elderly patients will be admitted to an ICU more frequently and their management will be challenging. Therefore, the object of this study was to explore the survival predictors of elderly patients with ARDS. Understanding these factors may help intensivists when making decisions regarding the appropriate use of life support in this particular patient population.
Material and Methods
Study design and population
This prospective observational cohort research was conducted from October 2012 to May 2015 at Chang Gung Memorial Hospital, Linkou branch, a tertiary referral medical center in northern Taiwan. The hospital consists of 3,700 general ward beds and 278 adult ICU beds. All of the patients admitted to ICU needed invasive mechanical ventilation with available data on both PaO2/FiO2 ratio and chest X-ray were screened for eligibility via the Hospital Information System. This study was approved by the Institutional Review Board Ethics Committee of Chang Gung Memorial Hospital (CGMH IRB No. 102–1729B) and was carried out in accordance with relevant guidelines and regulations. All clinical investigations were conducted according to the principles expressed in the Declaration of Helsinki. The IRB approval exempted the study from informed consent due to the non-intervention and observational data collection nature.
Data collection
We enrolled patients into this study if they met the criteria of the Berlin definition of ARDS1. Patients were excluded if they were younger than 18 years old, were referred from other hospitals, died within 48 hours, and had incomplete data. Demographics, baseline clinical characteristics and laboratory data were collected on enrollment. The following data were recorded on ICU admission: date of hospital and ICU admission, age, gender, predicted body weight, past underlying diseases history, risk factors and severity of ARDS on the day of diagnosis. The mechanical ventilator settings such as tidal volume, lowest PaO2/FiO2 ratio with the highest PEEP and peak inspiratory pressure (PIP) were recorded during mechanical ventilation when ARDS was recognized within the first 24 hours of ARDS diagnosis. The severity index were recorded within the first 24 hours of ARDS diagnosis including Charlson comorbidity index (CCI)12, Acute Physiology and Chronic Health Evaluation (APACHE) II score13, Sequential Organ Failure Assessment (SOFA) score14, and lung injury score15.
Managements of ARDS
The general mechanical ventilation settings of the patients included a lung protective ventilation strategy using a low tidal volume of 4–8 mL/kg of the predicted body weight, and the PEEP setting guided by low PEEP - FiO2 table for volume-controlled or pressure-controlled ventilation. Oxygenation was monitored by SpO2 through pulse oximetry, and the FiO2 level was adjusted to maintain SpO2 at more than 90%. Hemodynamics and lung water were monitored if the clinical condition of the patient indicated the need using a PiCCO plus monitor (version 5.2.2; Pulsion Medical System AG, Muenchen, Germany).
Statistical analysis
Data analysis was carried out by SPSS software version 22 (SPSS for Windows, SPSS Inc., Chicago, IL, USA). Student’s t test and ANOVA were used to compare the continuous variables. Categorical data were compared using the chi square test. The risk factor for hospital mortality was analyzed using univariate analysis, and the variables statistically significant (p < 0.05) were included for multivariate analysis by applying multiple logistic regressions based on backward elimination of data. Cumulative survival curves as a function of time were generated using the Kaplan-Meier approach and compared using the log-rank test. P value < 0.05 is considered to be statistically significant.
Results
During the research period, 22,470 admitted adult patients with invasive mechanical ventilation were screened, of whom 1,034 (4.6%) met the criteria of ARDS (Fig. 1). The sources of patients included 9 medical ICUs, 5 post-surgical ICUs, 2 trauma ICUs, 1 burn ICU and emergency department. Eighty-nine patients were excluded, and the remaining 945 patients with ARDS were included for analysis.
The demographic and clinical characteristics of the included population are shown in Table 1. There were no significantly different in gender, CCI, lung injury score and mechanical ventilator settings between the younger (<65 years old) and older (≥65 years old) patients. The older patients had a lower body mass index and higher APACHE II and SOFA scores than the younger population. For the initial oxygenation, the older patients had a higher PaO2/FiO2 ratio (147.9 ± 77.3 vs. 134.3 ± 70.5 mmHg, p = 0.005) and less severe ARDS (34.1% vs. 40.2%, p = 0.041) than the younger patients. The hospital mortality rate was significantly higher in older patients than in younger patients (63.9% vs. 50.2%, p < 0.001). For the ARDS patients without co-morbidities (n = 186), the younger patients (n = 108) had lower hospital mortality rate than older patients (n = 78) (34.3% vs. 57.5%, p = 0.001).
The Table 2 compared the baseline characteristics of the older patients (≥65 years old) with ARDS between survivors and nonsurvivors. Regarding the risk factors of ARDS in these 463 older patients (≥65 years old), pneumonia was the most common (n = 354, 76.5%), followed by sepsis (n = 118, 25.5%), aspiration (n = 37, 8%), and others (n = 18, 4.2%). Of the 463 older patients with ARDS, the hospital survival rate was 36.1% (167/463). Univariate analysis showed that the CCI, APACHE II score, SOFA score, lung injury score and PIP were predictors of hospital mortality (Table 3). Multivariate logistic regression analysis revealed that CCI [odds ratio (OR) 1.111, 95% confidence interval (CI) 1.010–1.222, p = 0.031], SOFA score (OR 1.127, 95% CI 1.054–1.206, p < 0.001) and PIP (OR 1.061, 95% CI 1.024–1.099, p = 0.001) were significantly and independently associated with hospital mortality. Regression coefficients of these variables were used to calculate a natural logarithm of the odds (logit) of the probability of death (p), as follows: logit (p) = −2.5 + (0.11 × CCI) + (0.12 × SOFA score) + (0.06 × PIP).
Of these 463 older patients, 194 (41.9%) were 65–74 years old, 189 (40.8%) were 75–84 years old, and 80 (17.3%) were ≥85 years old. Demographic and clinical characteristics of these three age groups are compared in Table 4. There was no significant difference in gender, CCI, APACHE II, SOFA, lung injury score, mechanical ventilator settings and severity of ARDS among these three groups. For these older ARDS patients (≥65 years old), the ICU and hospital mortality rates were not significantly different in mild (n = 119), moderate (n = 186) and severe (n = 158) ARDS (43.7% vs. 47.8% vs 57%, respectively, p = 0.07; and 63.9% vs. 61.3% vs. 67.1%, respectively, p = 0.536).
The ≥85 years old group had a significantly lower body mass index than the 65–74 years group (22.3 ± 3.4 vs. 23.8 ± 4.1, p = 0.025). There was no significant difference in ICU or hospital mortality rates among the three groups (45% vs. 48.7% vs. 53.1%, respectively, p = 0.433; and 60% vs. 65.6% vs. 63.9%, respectively, p = 0.682), and no significantly different in days of mechanical ventilation among the three groups (19.1 ± 14.6 days, 20.5 ± 15.7 days, and 21.0 ± 15.4 days, respectively, p = 0.583). The lengths of stay in the ICU and hospital were not significantly different among the three groups (24.0 ± 18.8 days vs. 25.9 ± 21.0 days vs. 26.4 ± 20.6 days, respectively, p = 0.565; and 34.7 ± 29.2 days vs. 38.2 ± 31.8 days vs. 35.7 ± 23.4 days, respectively, p = 0.459). The leading causes of death of the older patients with ARDS were multiple organ failure (n = 203), followed by septic shock (n = 46) and refractory hypoxemia (n = 20). Between the three groups, these three leading causes of death were not significant difference (68% vs. 72% vs. 69%, p = 0.81; 17% vs. 15% vs. 16%, p = 0.846; 7% vs. 6% vs. 8%, p = 0.947). For the older patients without co-morbidities (n = 78), the hospital mortality rates were not significantly different between 65–74 years old (n = 32), 75–84 years old (n = 32), and ≥85 years old (n = 14). (56.2% vs. 56.2 vs. 64.3%, respectively, p = 0.859).
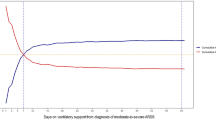
Kaplan-Meier survival curves for hospital survival in the different age groups are shown in Fig. 2. The younger patients (<65 years old) had a significantly higher survival rate than the older patients (≥65 years old) (p = 0.0049). However, the survival rate was not significantly different among the 65–74, 75–84 and ≥85 years old groups (p = 0.774).
Kaplan-Meier survival curves of patients with acute respiratory distress syndrome in different age groups. (A) The patients <65 years old had a significantly higher survival rate than those ≥65 years old (p = 0.0049). (B) The survival rate was not significantly different among those aged 65–74 years, 75–84 years old and ≥85 years old groups (p = 0.774).
Discussion
The main results of this prospective observational cohort study revealed that the older (≥65 years old) patients with ARDS had a lower survival rate than the younger (<65 years old) patients with ARDS. For the elder ARDS patients, the CCI, SOFA score and PIP were significantly and independently associated with hospital mortality. However there was no significant difference in ICU or hospital survival rates among the 65–74 years old, 75–84 years old and ≥85 years old groups.
The Berlin definition classifies the severity of ARDS by the PaO2/FiO2 ratio, and mild, moderate, and severe ARDS are associated with increased mortality (27%, 32% and 45%, respectively)1. Recent reports have shown that stratification of severity of ARDS based on baseline value of PaO2/FiO2 did not completely correlate with mortality16,17,18. Often as much as 50% of patients classified as having moderate or severe ARDS respond quickly to routine ventilator and oxygenation measures that they do not meet the criteria for moderate/severe ARDS at 24 hours after diagnosis17. A 9 - point score based on age, PaO2/FiO2, and plateau pressure was proposed to predict mortality in patients with ARDS19. Compared to patients with ARDS who were younger than 47 years old, those 47–66 years and >66 years old had significantly higher hospital mortality rates (27.5% vs. 44.4% vs. 66.0%, respectively; p < 0.001)19. Increasing age is a known risk factor for death in patients with ARDS, and older patients have a higher risk of mortality than younger patients20,21. However, little is known about the risk of mortality for patients with ARDS who are older than 65 years. In this study, we found that the major determinants of mortality were underlying disease (e.g. CCI), organ function (e.g. SOFA score) and pulmonary condition (e.g. PIP), but not age. Therefore, the impact of age on mortality in patients with ARDS seems to be limited, especially in elderly patients.
A study on patients with ARDS found that patients with serious comorbidities had a mortality rate three times higher than patients without serious comorbidities22. The CCI is an index of multiple comorbidities including 22 items which was initially developed in a cohort of 559 internal medicine patients to predict 1 year mortality16. In lung cancer patients, the CCI has been shown to be a prognostic predictor23, and several studies have reported that the CCI can predict survival and physiological outcomes in patients with ARDS24,25,26. In this study on elderly patients with ARDS, we found that the CCI was significantly positively correlated with survival outcomes (OR 1.11, p = 0.031). Therefore, we suggest assessing comorbidities using the CCI to predict survival in elderly patients with ARDS in addition to age.
The SOFA score involves organ dysfunction across six vital organs and it has been shown to be associated with more severe disease and a higher risk of death14. Only about 20% of patients with ARDS die from refractory hypoxemia, and approximately 80% of all deaths are caused by multiple organ dysfunction syndromes27,28. The SOFA score has been used for patients with ARDS to evaluate organ dysfunction as a surrogate marker of mortality29. In terms of liver failure, patients with ARDS and cirrhosis have been reported to have a significantly higher mortality rate (62%) than patients without cirrhosis (43%) (p = 0.02)30. An observational study of patients with indirect ARDS found that age, lung injury score, and number of non-pulmonary organ failures (OR 1.67, p = 0.01) were independent risk factors for hospital mortality31. The LUNG SAFE study of patients with ARDS found that a higher non-pulmonary SOFA score was associated with poorer outcomes (OR 1.12, p < 0.001)32. In our study on elderly patients with ARDS, SOFA score was significantly correlated with hospital mortality (OR 1.18, p < 0.001). The prognosis for elderly patients with ARDS therefore appears to be related to extra-pulmonary organ dysfunction rather than pulmonary dysfunction alone.
A study including 3562 patients with ARDS in nine randomized controlled trials concluded that driving pressure as an index of pulmonary mechanics of the respiratory system was the strongest predictor of mortality33. Another study on 56 patients with ARDS reported that treatment strategies leading to decreased transpulmonary driving pressure at 24 hours may be associated with an improved 28 - day mortality rate34. In addition to driving pressure, plateau pressure has also been reported to be a predictor of mortality in patients with ARDS19,32. A prospective, descriptive, and validation study reported that the hospital mortality rates of patients with ARDS with a plateau pressure >30 cm H2O and <27 cm H2O were 64.0% and 28.7%, respectively (relative risk 2.2, p < 0.001)19. Peak inspiratory pressure, which is an easily measurable parameter of lung mechanics was also associated with hospital mortality in the LUNG SAFE study (OR 1.02, p = 0.002)32. For our elderly patients with ARDS, we found that PIP was significantly correlated with hospital mortality (OR 1.068, p = 0.001). In theory, the PIP is different from plateau pressure. However, the peak airway has a good collinearity with plateau pressure especially when patients were to be deep sedated or paralyzed in early stage of ARDS. Due to its convenience and feasibility, PIP may be useful as a prognostic index in real world patient care.
There are several limitations to this study. First, this study was conducted at one referral medical center, and our results may not be generalizable to patients in community hospitals or other models of intensive care. Nevertheless, the number of enrolled elderly patients with ARDS was reasonably high, and thus we believe our findings are of value. Second, few studies have investigated elderly patients with ARDS, and these studies have mostly focused on critically ill patients as a whole. We chose 65 years of age as a cutoff value mainly because previous studies on critically ill patients have used this cutoff to define “elderly” patients. We further classified the elderly patients into three arbitrary age groups of 65–74, 75–84 and ≥85 years old without considering morbidities, functional status and other disabilities, and this may have affected the outcomes. Third, different health care systems in different countries will have different policies for intensive care for critically ill elderly patients. Finally, it is possible to have a selection bias from the patients’ collection. Some patients were possibly rejected to be admitted in ICU because of underlying comorbidity by the physician in charge.
In conclusion, CCI, SOFA score and PIP were predictors of hospital survival in elderly patients with ARDS. The risk of mortality in the elderly patients ARDS was associated with the degree of lung injury and also with the underlying disease and presence of other organ dysfunction. When making decisions regarding life-sustaining therapy for elderly patients with ARDS, both comorbidities and advanced age should be taken into consideration.
References
Ranieri, V. M. et al. Acute respiratory distress syndrome: the Berlin Definition. JAMA 307, 2526–33 (2012).
Riviello, E. D. et al. Hospital incidence and outcomes of ARDS using the Kigali modification of the Berlin definition. Am J Respir Crit Care Med 193, 52–9 (2016).
Bellani, G. et al. Epidemiology, recognition, management and outcome of acute respiratory distress syndrome in the 21st century: the LUNG SAFE Study. JAMA 315, 788–800 (2016).
Wang, C. Y. et al. One-year mortality and predictors of death among hospital survivors of acute respiratory distress syndrome. Intensive Care Med 40, 388–96 (2014).
Gee, M. H. et al. Physiology of aging related to outcome in the adult respiratory distress syndrome. J Appl Physiol 69, 822–9 (1990).
Milberg, J. A., Davis, D. R., Steinberg, K. P. & Hudson, L. D. Improved survival of patients with acute respiratory distress syndrome (ARDS): 1983–1993. JAMA 273, 306–9 (1995).
Nguyen, Y. L., Angus, D. C., Boumendil, A. & Guidet, B. The challenge of admitting the very elderly to intensive care. Ann Intensive Care 1, 29 (2011).
de Rooij, S. E. et al. Short-term and long-term mortality in very elderly patients admitted to an intensive care unit. Intensive Care Med 32, 1039–44 (2006).
Bagshaw, S. M. et al. Very old patients admitted to intensive care in Australia and New Zealand: a multi-centre cohort analysis. Crit Care 13, R45 (2009).
Sacanella, E. et al. Mortality in healthy elderly patients after ICU admission. Intensive Care Med 35, 550–5 (2009).
Nathanson, B. H. et al. Do elderly patients fare well in the ICU? Chest 139, 825–31 (2011).
Charlson, M. E., Pompei, P., Ales, K. L. & MacKenzie, C. R. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40, 373–83 (1987).
Knaus, W. A., Draper, E. A., Wagner, D. P. & Zimmerman, J. E. APACHE II: a severity of disease classification system. Crit Care Med 130, 818–29 (1985).
Vincent, J. L. et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med 22, 707–10 (1996).
Murray, J. F., Matthay, M. A., Luce, J. M. & Flick, M. R. An expanded definition of adult respiratory distress syndrome. Am Rev Respir Dis 138, 720–3 (1988).
Hernu, R. et al. An attempt to validate the modification of the American-European consensus definition of acute lung injury/acute respiratory distress syndrome by the Berlin definition in a university hospital. Intensive Care Med 39, 2161–70 (2013).
Villar, J. et al. Spanish Initiative for Epidemiology, Stratification, and Therapies for ARDS (SIESTA) Network: A universal definition of ARDS: the PaO2/FiO2 ratio under a standard ventilator setting – prospective, multicenter validation study. Intensive Care Med 39, 583–92 (2013).
Caser, E. B., Zandonade, E., Pereira, E., Gama, A. M. & Barbas, C. S. Impact of distinct definitions of acute lung injury on its incidence and outcomes in Brazilian ICUs: prospective evaluation of 7,133 patients. Crit Care Med 42, 574–82 (2014).
Villar, J. et al. Age, PaO2/FIO2, and Plateau Pressure Score: A Proposal for a Simple Outcome Score in Patients With the Acute Respiratory Distress Syndrome. Crit Care Med 44, 1361–9 (2016).
Ely, E. W. et al. Recovery rate and prognosis in older persons who develop acute lung injury and the acute respiratory distress syndrome. Ann Intern Med 136, 25–36 (2002).
Eachempati, S. R., Hydo, L. J., Shou, J. & Barie, P. S. Outcomes of Acute Respiratory Distress Syndrome (ARDS) in Elderly Patients. J Trauma 63, 344–50 (2007).
Davidson, T. A., Rubenfeld, G. D., Caldwell, E. S., Hudson, L. D. & Steinberg, K. P. The effect of acute respiratory distress syndrome on long-term survival. Am J Respir Crit Care Med 160, 1838–42 (1999).
Asmis, T. R. et al. Age and comorbidity as independent prognostic factors in the treatment of non small-cell lung cancer: a review of National Cancer Institute of Canada Clinical Trials Group Trials. J Clin Oncol 26, 54–9 (2008).
Groll, D. L., Heyland, D. K., Caeser, M. & Wright, J. G. Assessment of long-term physical function in acute respiratory distress syndrome (ARDS) patients: comparison of the Charlson Comorbidity Index and the Functional Comorbidity Index. Am J Phys Med Rehabil 85, 574–81 (2006).
Fan, E. et al. The functional comorbidity index had high inter-rater reliability in patients with acute lung injury. BMC Anesthesiology 13(12), 21 (2012).
Ando, K. et al. The Effect of Comorbidity on the Prognosis of Acute Lung Injury and Acute Respiratory Distress Syndrome. Intern Med 51, 1835–40 (2012).
Stapleton, R. D. et al. Causes and timing of death in patients with ARDS. Chest 128, 525–32 (2005).
Villar, J. et al. The ALIEN Study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive Care Med 37, 1932–41 (2011).
Diaz, J. V., Brower, R., Calfee, C. S. & Matthay, M. A. Therapeutic strategies for severe acute lung injury. Crit Care Med 38, 1644–50 (2010).
Gacouin, A. et al. Liver Cirrhosis is Independently Associated With 90-Day Mortality in ARDS Patients. Shock 45, 16–21 (2016).
Luo, L. et al. Clinical predictors of hospital mortality differ between direct and indirect acute respiratory distress syndrome. Chest 151, 755–63 (2016).
Laffey, J. G. et al. Potentially modifiable factors contributing to outcome from acute respiratory distress syndrome: the LUNG SAFE study. Intensive Care Med 42, 1865–76 (2016).
Amato, M. B. et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 372, 747–55 (2015).
Baedorf Kassis, E., Loring, S. H. & Talmor, D. Mortality and pulmonary mechanics in relation to respiratory system and transpulmonary driving pressures in ARDS. Intensive Care Med 42, 1206–13 (2016).
Acknowledgements
This study was supported by grant CMRPG3F0791 from Chang Gung Memorial Hospital.
Author information
Authors and Affiliations
Contributions
K.C.K. conceived the study, M.J.H., S.W.L., L.P.C. and C.H.C. conducted the study, H.C.H., C.H.W., L.F.L. and C.C.H. analyzed the results. K.C.K. and H.P.W. writed the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kao, KC., Hsieh, MJ., Lin, SW. et al. Survival predictors in elderly patients with acute respiratory distress syndrome: a prospective observational cohort study. Sci Rep 8, 13459 (2018). https://doi.org/10.1038/s41598-018-31811-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31811-w
This article is cited by
-
Added value of chest CT images to a personalized prognostic model in acute respiratory distress syndrome: a retrospective study
Chinese Journal of Academic Radiology (2023)
-
Using real-time visualization system for data-driven decision support to achieve lung protective strategy: a retrospective observational study
Critical Care (2022)
-
Characteristics, management, and prognosis of elderly patients with COVID-19 admitted in the ICU during the first wave: insights from the COVID-ICU study
Annals of Intensive Care (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.