Abstract
Abundant literature confirms that intravenous (IV) and intra-articular (IA) administration of tranexamic acid (TXA) reduces blood loss in total knee arthroplasty (TKA). Oral formulations of TXA exhibit profound cost-saving benefits. However, comparisons of the clinical efficacy among three different modalities of TXA administration have not been previously investigated in the setting of TKA with no closed suction drain and tourniquet. A total of 180 patients undergoing TKA were randomized to receive 2-g oral TXA 2 hours preoperatively, 20-mg/kg IV TXA 5 minutes prior to incision, or 2-g IA TXA. The primary outcome was 72-hour blood loss. Secondary outcomes were reductions in hemoglobin, the rate of transfusions, and adverse events. No significant differences were identified with regard to the mean 72-hour blood loss among the three groups (1003 mL in oral group, 1108 mL in IV group, and 1059 mL in IA group, respectively). Similarly, hemoglobin reduction was equivalent among the groups. Only one patient in IV group exhibited deep venous thrombosis. No difference was identified regarding transfusion rates. Oral TXA results in similar blood loss in TKA, with a profound cost-saving benefit, compared with the IA and IV formulations.
Similar content being viewed by others
Introduction
Total knee arthroplasty (TKA) is associated with substantial intra- and postoperative blood loss that may carry a substantial risk of anemia and allogeneic transfusions1,2. Allogeneic blood transfusion may lead to adverse outcomes (i.e., infection and myocardial infarction), which increases morbidity, mortality, and cost3,4. Various blood-saving protocols, including blood-salvaging techniques, autologous blood transfusion, and cryotherapy, as well as the perioperative administration of antifibrinolytic agents such as tranexamic acid, were adopted to reduce bleeding and allogeneic blood transfusions with varied success5,6.
Tranexamic acid (TXA) is a synthetic derivative of the amino acid lysine that prevents plasminogen activation via blockade of the lysine binding site of plasminogen, which promotes the coagulation process7. Numerous randomized controlled trials confirmed that perioperative intravenous (IV), intra-articular (IA), and oral routes of TXA administration exhibited beneficial blood-saving effects and reduced transfusion requirements without apparently increasing the risk of postoperative complications in comparison with placebo8,9,10. Most studies focused on the investigation of IA and IV modalities of TXA administration, but only two prospective studies investigated the efficacy of oral TXA administration compared to IV or IA TXA in TKA when this trial was designed11,12. However, the 1st study12 involved two groups (oral vs. IV), and the 2nd study11 involved four groups, including the use of a postoperative drain and a tourniquet, which enhances local fibrinolysis and affects bleeding kinetics13. No tourniquet or postoperative drain was used in our center on the basis of a well-established fast-track setup focusing on multimodal analgesia strategy14, perioperative blood-saving management15,16, and early function recovery17. Compared with an equivalent IV formulation, the oral TXA dosage produces a cost savings of $33 to $94 per dose, leading to a cost difference of about 70.2% to 90.4%18. However, the blood-sparing efficacy of oral TXA remains unclear in TKA without tourniquet and postoperative closed suction drain.
Therefore, our randomized controlled trial was performed with an enhanced-recovery protocol to compare the efficacy of oral administration of TXA with that of IA and IV administration of TXA without the use of closed suction drain and tourniquet. We hypothesized that oral administration of TXA would produce an equivalent reduction in postoperative blood loss compared with IA and IV administration of TXA, with a better cost-effectiveness profile.
Materials and Methods
Study design and Participants
This single-center, prospective, randomized, double-blinded study was performed in the Department of Orthopaedic Surgery at West China Hospital. Following the approval of the Clinical Trials and Biomedical Ethics Committee of Sichuan University (No. 201302008), the study was registered in the Chinese Clinical Trial Registry (ChiCTR-INR-17010965). Written informed consent was obtained from all participants. All the methods were conducted according to the CONSORT statement19.
All adult patients (≥18 years of age) with primary knee osteoarthritis who were scheduled for elective primary unilateral total knee replacement from March 2016 to January 2017 were assessed for eligibility. At our center, the perioperative managements were performed according to a well-established fact-track protocol. The criteria for exclusion included secondary osteoarthritis (e.g., post-septic arthritis and post-traumatic arthritis), simultaneous bilateral or revision TKA, allergic reaction to TXA, history of major comorbidities (severe arterial thromboembolic event, severe renal failure, or severe pulmonary disease), history of hematopoietic disease, history of pulmonary embolism (PE) or deep venous thrombosis (DVT), alcohol or drug abuse, and current anticoagulant therapy (warfarin or heparin) within one week.
Randomization and trial intervention
The recruited patients were randomized to one of three interventions. The oral group received a total dose of 2 g of oral TXA with use of four 500-mg tablets approximately 2 hours before incision. The patients in the oral group were also administered 100 mL of an IV and IA placebo solution (normal saline solution) in a manner identical to administration in IV and IA groups, respectively. Oral TXA dosing and timing were based on pharmacokinetic studies, which demonstrate that 2.0 grams of oral TXA reaches peak levels after about 2 to 3 hours and falls below the therapeutic threshold 6 hours after administration10. The IV group received a 20-mg/kg dose of TXA in 100 mL of normal saline solution administered intravenously 5 minutes prior to incision and 100 mL of a placebo solution administered intra-articularly, based on previous efficacy studies20. The IA group received a 2-g dose of TXA, diluted in 100 mL of saline solution administered intra-articularly according to previous studies that demonstrated the efficacy of TXA in reducing bleeding at doses of 1.5 g to 3 g21,22, and a placebo dose of 100-mL saline solution administered intravenously. IA TXA was administered at two time points: (1) the open joint surface was soaked with 50 mL of a 1-g TXA solution following component implantation and was left in contact with the tissue for five minutes; (2) the remaining 50 mL of a 1-g TXA solution was given using a needle to penetrate the tissue of knee capsule before capsule closure16,23. The oral and IV groups were also subjected to the 2-stage exposure to TXA placebo as described for the IA group. The patients in the IV and IA groups also received a total dose of 2-g small placebo pills that were identical in appearance but contained no active ingredient.
A random allocation sequence was computer-generated online (www.randomization.org), with an allocation ratio of 1:1:1 and a block size of 60. One of two surgeons responsible for all arthroplasties enrolled patients, and all the patients were screened in clinics. Dedicated research personnel rechecked the inclusion and exclusion criteria of the patients. The patients were assigned a unique randomization number, and patient assignments were concealed into consecutively numbered opaque-sealed envelopes. An envelope was opened on the morning of operation, and a research pharmacist (who was not otherwise involved with the collection of patient data or patient care) handled all the study drug preparations to ensure identical appearance and blinding. This information was linked to a confidential database, which was used for collection and analysis by an independent research statistician. The patients, surgeons, anesthesiologists, data collectors, research assistants, and outcome assessors were blinded.
Surgical technique and postoperative management
The operations were performed by two senior surgeons. In all patients, standardized general anesthesia and a medial para-patellar approach were used. Perioperative multimodal analgesia was standardized and consisted of an adductor canal block and periarticular multi-site infiltration14 as well as standard analgesia24. The tourniquet and intra-articular closed suction drain were not used17,25.
Venous thromboembolism prophylaxis was administered as standard practice, with a combination of mechanical and chemical thromboprophylaxis. A portable intermittent inflatable calf pump was used for mechanical prophylaxis, and lower-extremity strength training was conducted on the day after surgery. While hospitalized, chemical prophylaxis consisted of subcutaneous administration of low-molecular-weight heparin (2000 IU) beginning 8 hours postoperatively, which was then administered once daily (4000 IU). Rivaroxaban (10 mg orally, Xarelto; Bayer, Leverkusen, Germany) was administered daily, which continued for 10 days after discharge.
During hospitalization, a standardized blood-transfusion protocol was followed for all patients on the basis of the perioperative transfusion guidelines of the Chinese Ministry of Health. Red blood cells were transfused when hemoglobin (Hb) levels were <70 g/L in asymptomatic patients or when a patient exhibited any organ dysfunction associated with anemia, regardless of Hb concentration.
Outcome measures
The primary outcome measure was 72-hour blood loss. The calculated blood loss was estimated via application of the Gross formula26, and total blood value was calculated using the formula described by Nadler et al.27: Patient blood volume (PBV) = k1 x height (m) + k2 x weight (kg) + k3 (k1 = 0:3669, k2 = 0:03219, and k3 = 0:6041 for men; k1 = 0:3561, k2 = 0:03308, and k3 = 0:1833 for women). Estimated total blood loss = PBV x (Hctpre − Hctpost)/Hctave (Hctpre = the initial preoperative hematocrit level. Hctpost = the postoperative 72-hour hematocrit level during hospitalization. Hctave = the average of the Hctpre and Hctpost). The total blood loss was equivalent to the volume transfused plus the blood loss when an allogenic transfusion was performed28,29.
The secondary outcome measures were Hb and hematocrit (Hct) level, Hb drops, coagulation indicators on postoperative days (PODs) 1 and 3, the amount of IV fluid, and intraoperative blood loss. Other secondary outcome measures were thromboembolic complications, the rate of blood transfusions, the total number of blood units transfused, adverse events, the costs of allogeneic blood transfusion and TXA, 30-day mortality, 90-day readmission, hospital stay, and knee function.
Intraoperative blood loss was evaluated via measurement of suction drain contents and surgical swabs30. All the patients received examination for clinical symptoms of DVT every day during hospitalization. Both lower limbs were examined using a diagnostic Doppler ultrasound on postoperative day 3, or earlier, if a patient exhibited any clinical symptom (e.g., limb swelling or calf pain). All the adverse events that occurred during the first 90 days were recorded postoperatively.
Sample size
Sample size requirement was determined on differences in the calculated 72-hour blood loss. A review of a historical cohort of 114 patients undergoing primary TKA using a similar fast-track setup at our center from September 2014 to June 2015 revealed that the 72-hour total blood loss was 1104 mL, with a standard deviation of 254 mL. We assumed a clinically important blood loss difference of 110 mL, which was equivalent to a reduction in blood loss of 10% in the historical cohort. The sample size was calculated using a fixed-effect one-way analysis of variance, with an assumed alpha level of 5% and a power of 90%. A minimum of 147 patients was required. Sixty patients per arm were needed to compensate for a 20% expected dropout rate.
Statistical analysis
The distributions of all the variables were tested using the Kolmogorov-Smirnov test prior to analyses. Distributions of the demographic and perioperative data were evaluated with summary statistics, including means and standard deviations (normalized data) or medians and interquartile ranges (non-normalized data) for quantitative data as well as numbers and percentages for qualitative data. One-way analysis of variance (ANOVA) with Tukey’s post hoc test was used for normally distributed continuous variables, and Kruskal-Wallis with Nemenyi post hoc test was used for skewed continuous variables. The categorical variables were analyzed with use of Chi-square test or Fisher’s exact test. All data were analyzed using SPSS software (Version 21.0; SPSS Inc., Chicago, IL). A P <0.05 (two-sided) was considered significant. The statistical power of the study was calculated using G-Power software (Version 3.1.9.2, Germany).
Results
Patients
A total of 421 patients who were scheduled for TKA were screened for eligibility from March 2016 to January 2017, and a total of 241 patients were excluded (Fig. 1). Therefore, 180 enrolled study participants were included in the randomization in terms of interventions (oral TXA, IV TXA, or IA TXA; 60 per arm). One patient in the oral group was lost to follow-up for the analysis of thromboembolic events due to lack of interest in this trial. However, this patient was included in the primary outcome analysis. The baseline characteristics were similar between groups (Table 1).
Primary Outcome
The 72-hour calculated blood loss was 1003.99 ± 414.44 mL in the oral group, 1108.31 ± 392.11 mL in the IV group, and 1059.37 ± 422.99 mL in the IA group (p = 0.29) (Table 2).
Secondary Outcomes
No differences among groups were observed with respect to intraoperative blood loss (p = 0.84) or 24-hour calculated blood loss (p = 0.54). The mean reductions in Hb at 24 and 72 hours were 1.89 g/dL and 2.91 g/dL in the oral group, 1.74 g/dL and 2.99 g/dL in the IA group, and 1.90 g/dL and 3.13 g/dL in the IV group, with no significant intergroup differences (Table 2). There were no significant differences in terms of blood coagulation values (Table 3).
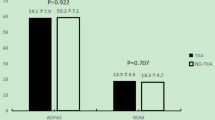
Knee function was similar among the groups 3 months postoperatively. Eight patients (4.4%) were given allogeneic blood transfusion (two patients in oral group, four patients in IV group, and two patients in IA group) (Table 4). The total number of transfused units were similar among the groups.
The cost analysis was based on blood transfusion and TXA costs. The oral, IV, and IA TXA costs were approximately ¥ 8, ¥ 36.3, and ¥ 29 at our institution, respectively (Table 4). The lowest total TXA cost was recorded in the oral group (¥ 480) compared to the IV group (¥ 3341) and the IA group (¥ 3540) (p < 0.001). Oral TXA indicated a net savings of ¥ 2861 and ¥ 3060 per 60 treated patients when compared with the IV and IA TXA groups, respectively. However, no significant differences were identified for transfusion costs per 60 TKA patients (Table 4).
One patient in the IV TXA group exhibited DVT, which was confirmed using Doppler ultrasonography, without any clinical symptoms and was managed using the standard thromboembolism-prophylaxis protocol31. No PE occurred in any of the groups. The 3 groups exhibited similar numbers of other adverse events, which were all successfully resolved (Table 4).
Discussion
Numerous studies have reported that IV and IA TXA administration represented an effective strategy to reduce postoperative blood loss and transfusions with no increased risk of thromboembolic complications in TKA8,9,16,28. Oral TXA formulations largely reduced costs and exhibited blood-sparing efficacy32,33, but only two studies investigated the efficacy of oral versus IV or IA TXA administration in total knee replacement when the trial was designed and developed11,12. Nevertheless, the application of closed suction drain and tourniquet was standard in these previous studies. The present study is novel because it assessed the effect of three different modalities of TXA administration in a fast-track protocol for TKA with no tourniquet or postoperative drain at the same time. The primary finding of our study was that no significant difference was identified in the blood-saving efficacy of the three different modalities, and oral TXA provided greater cost-saving benefits.
Many randomized controlled trials confirmed the safety and efficacy of IA and IV TXA administration compared to placebo during TKA, and various dosages and modalities of TXA administration exist16,20,34. A well-performed randomized controlled trial confirmed significantly decreased blood loss and transfusion requirements in patients treated with a single 2-g dose of IA TXA compared to the placebo35. A meta-analysis by Shin et al. demonstrated that 2-g IA TXA was a safer alternative within the therapeutic range for high-risk patients21. The routine dosing for TXA as an IV bolus is 20 mg/kg at our center, which exhibits therapeutic levels for 3 hours. Its efficacy and safety were confirmed in our randomized controlled trials20,36. Therefore, we compared 2-g oral TXA with 2-g IA and 20-mg/kg IV TXA to investigate the clinical efficacy of reducing blood loss.
Alipour et al.37 demonstrated that 1-g oral TXA administered 2 h prior to surgery exhibited blood-saving effects compared to placebo. Lee et al.32 reported that the total blood loss in patients receiving oral TXA was much smaller than that of placebo. However, only 2 trials investigated the efficacy of oral TXA compared to other routes of TXA administration in TKA11,12 (Table 5). Fillingham et al.37 reported that a single 2-g oral dose of TXA provided an equivalent reduction in blood loss and a lower TXA-dosage cost compared to IV TXA in a small trial. Yuan et al.11 included four study arms (oral, IV, IA, and placebo) and used drain output to roughly measure blood loss. TXA administration via any of the 3 routes significantly reduced blood loss and transfusion rates, and oral TXA similarly reduced blood loss compared to IV and IA TXA. However, a tourniquet and postoperative closed suction drain were used routinely. Moreover, the Hct in drain output gradually decreased, which may affect the accuracy of postoperative blood loss measurements38. Our randomized controlled trail25 and meta-analysis39 reported that the use of a drain could not significantly reduce postoperative blood loss and improve knee function following TKA. Therefore, postoperative drains are not used routinely at our center. Numerous studies indicated that tourniquet application caused muscle damage and delayed recovery17, increased thromboembolic event rates, and stimulated fibrinolysis with increased bleeding40. Moreover, some trials reported that the absence of tourniquet did not appear to affect tibial cementation penetration41 or fixation42. Therefore, the use of a tourniquet is not standard in our institution. Our study estimated changes in blood loss with adequate power. Our findings suggested that no significant differences were observed in calculated blood loss among the groups. This finding is comparable to the findings of a recent meta-analysis that demonstrated that IA TXA provided equivalent reductions in blood loss when compared with IV administration43. Moreover, a similar trial that was conducted later, based on this current study results, has recently been published (during the review process), confirming that oral TXA is effective even against higher IA doses44. However, IA or IV TXA administration should only be applied for patients with swallowing difficulties.
TXA use is routine practice in TKA because of its clinical efficacy and cost-saving benefits. Two retrospective studies analyzed the economic impact of TXA use and reported that TXA administration could produce an estimated cost savings of $879 to $1500 per operation compared with that of placebo45,46. Moreover, Irwin et al.10 reported that a 2-g oral TXA bolus could more effectively reduced transfusion rates and significantly decrease the cumulative hospital cost of around £29,788 in patients undergoing TKA, compared with 15-mg/kg intravenous TXA. In double-blind, randomized, controlled trial comparing oral TXA with IV TXA, Kayupov et al. found a significant decrease in pharmacy cost of approximately $33 to $94 per patient in oral TXA group, resulting in a significant cost difference18. A randomized controlled trial by Luo et al. reported that the application of oral form of TXA could produce a greater pharmacy cost savings, compared with the intravenous and IA formulations in total hip arthroplasty47. In our study, the estimated savings of an appropriate oral TXA dose at our center was $13 to $28 for every 60 patients treated compared with an equivalent dose of IA and IV TXA. More than 200,000 TKAs are performed annually in China, and the numbers of this knee surgery will continue to grow over time48. Therefore, a switch to oral TXA may yield corresponding cost savings of $2.6 million to $5.6 million annually for our health-care system. Moreover, our randomized controlled trials have indicated that multiple postoperative boluses of oral or IV TXA can effectively reduce inflammatory responses and postoperative blood loss in TKA compared with the single preoperative dose of TXA20,49. Therefore, the transition to oral TXA may provide even greater cumulative savings.
This study has several limitations. First, this trial included no placebo group because substantial evidence confirmed the efficacy of TXA at any dose, timing, or route of administration32,34 and favored the use of TXA in this setting10,12. Withholding of TXA would deprive TKA patients the potential beneficial effects of TXA use, including reduced blood loss, Hb drop, and decreased risk of blood transfusion. Second, no significant differences were identified in terms of transfusions, DVT, and PE. The present study may be underpowered for detecting these meaningful comparisons. However, increasing evidence has confirmed the safety of TXA administration. Third, several potential variations, such as hemodilution, may affect estimated blood loss. However, these variations should cancel each other out because of the randomized design.
In conclusion, this randomized controlled trial indicated that 2 g of oral TXA results in similar blood loss compared to 20 mg/kg of IV or 2 g of IA TXA in TKA with no closed suction drain and tourniquet. The equivalent efficacy, larger cost-saving effects and convenient administration of oral TXA support a transition to oral TXA to reduce health-care costs.
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Good, L., Peterson, E. & Lisander, B. Tranexamic acid decreases external blood loss but not hidden blood loss in total knee replacement. Br J Anaesth 90, 596–599 (2003).
Freedman, J. et al. A provincial program of blood conservation: The Ontario Transfusion Coordinators (ONTraC). Transfus Apher Sci 33, 343–349, https://doi.org/10.1016/j.transci.2005.07.011 (2005).
Fuller, A. K., Uglik, K. M., Savage, W. J., Ness, P. M. & King, K. E. Bacterial culture reduces but does not eliminate the risk of septic transfusion reactions to single-donor platelets. Transfusion 49, 2588–2593, https://doi.org/10.1111/j.1537-2995.2009.02348.x (2009).
Kirksey, M. et al. Trends in in-hospital major morbidity and mortality after total joint arthroplasty: United States 1998–2008. Anesth Analg 115, 321–327, https://doi.org/10.1213/ANE.0b013e31825b6824 (2012).
Markert, S. E. The use of cryotherapy after a total knee replacement: a literature review. Orthop Nurs 30, 29–36, https://doi.org/10.1097/NOR.0b013e318205749a (2011).
Leigheb, M. et al. Postoperative blood salvage versus allogeneic blood transfusion in total knee and hip arthroplasty: a literature review. Acta Biomed 87(Suppl 1), 6–14 (2016).
Astedt, B. Clinical pharmacology of tranexamic acid. Scand J Gastroenterol Suppl 137, 22–25 (1987).
Kim, T. K. et al. Clinical value of tranexamic acid in unilateral and simultaneous bilateral TKAs under a contemporary blood-saving protocol: a randomized controlled trial. Knee Surg Sports Traumatol Arthrosc 22, 1870–1878, https://doi.org/10.1007/s00167-013-2492-1 (2014).
Wang, C. G., Sun, Z. H., Liu, J., Cao, J. G. & Li, Z. J. Safety and efficacy of intra-articular tranexamic acid injection without drainage on blood loss in total knee arthroplasty: A randomized clinical trial. Int J Surg 20, 1–7, https://doi.org/10.1016/j.ijsu.2015.05.045 (2015).
Irwin, A. et al. Oral versus intravenous tranexamic acid in enhanced-recovery primary total hip and knee replacement: results of 3000 procedures. Bone Joint J 95-b, 1556–1561, https://doi.org/10.1302/0301-620x.95b11.31055 (2013).
Yuan, X., Li, B., Wang, Q. & Zhang, X. Comparison of 3 Routes of Administration of Tranexamic Acid on Primary Unilateral Total Knee Arthroplasty: A Prospective, Randomized, Controlled Study. J Arthroplasty, https://doi.org/10.1016/j.arth.2017.03.059 (2017).
Fillingham, Y. A. et al. The James A. Rand Young Investigator’s Award: A Randomized Controlled Trial of Oral and Intravenous Tranexamic Acid in Total Knee Arthroplasty: The Same Efficacy at Lower Cost? J Arthroplasty 31, 26–30, https://doi.org/10.1016/j.arth.2016.02.081 (2016).
Roy, S. P., Tanki, U. F., Dutta, A., Jain, S. K. & Nagi, O. N. Efficacy of intra-articular tranexamic acid in blood loss reduction following primary unilateral total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc 20, 2494–2501, https://doi.org/10.1007/s00167-012-1942-5 (2012).
Li, D., Tan, Z., Kang, P., Shen, B. & Pei, F. Effects of multi-site infiltration analgesia on pain management and early rehabilitation compared with femoral nerve or adductor canal block for patients undergoing total knee arthroplasty: a prospective randomized controlled trial. Int Orthop 41, 75–83, https://doi.org/10.1007/s00264-016-3278-0 (2017).
Ma, J., Huang, Z., Shen, B. & Pei, F. Blood management of staged bilateral total knee arthroplasty in a single hospitalization period. J Orthop Surg Res 9, 116, https://doi.org/10.1186/s13018-014-0116-1 (2014).
Huang, Z., Ma, J., Shen, B. & Pei, F. Combination of intravenous and topical application of tranexamic acid in primary total knee arthroplasty: a prospective randomized controlled trial. J Arthroplasty 29, 2342–2346, https://doi.org/10.1016/j.arth.2014.05.026 (2014).
Huang, Z. Y. et al. Comparison of three different tourniquet application strategies for minimally invasive total knee arthroplasty: a prospective non-randomized clinical trial. Arch Orthop Trauma Surg 134, 561–570, https://doi.org/10.1007/s00402-014-1948-1 (2014).
Kayupov, E. et al. Oral and Intravenous Tranexamic Acid Are Equivalent at Reducing Blood Loss Following Total Hip Arthroplasty: A Randomized Controlled Trial. J Bone Joint Surg Am 99, 373–378, https://doi.org/10.2106/jbjs.16.00188 (2017).
Moher, D., Schulz, K. F. & Altman, D. The CONSORT Statement: revised recommendations for improving the quality of reports of parallel-group randomized trials 2001. Explore (NY) 1, 40–45 (2005).
Xie, J., Ma, J., Yao, H., Yue, C. & Pei, F. Multiple Boluses of Intravenous Tranexamic Acid to Reduce Hidden Blood Loss After Primary Total Knee Arthroplasty Without Tourniquet: A Randomized Clinical Trial. J Arthroplasty 31, 2458–2464, https://doi.org/10.1016/j.arth.2016.04.034 (2016).
Shin, Y. S., Yoon, J. R., Lee, H. N., Park, S. H. & Lee, D. H. Intravenous versus topical tranexamic acid administration in primary total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc, https://doi.org/10.1007/s00167-016-4235-6 (2016).
Wong, J. et al. Topical application of tranexamic acid reduces postoperative blood loss in total knee arthroplasty: a randomized, controlled trial. J Bone Joint Surg Am 92, 2503–2513, https://doi.org/10.2106/jbjs.i.01518 (2010).
Gomez-Barrena, E., Ortega-Andreu, M., Padilla-Eguiluz, N. G., Perez-Chrzanowska, H. & Figueredo-Zalve, R. Topical intra-articular compared with intravenous tranexamic acid to reduce blood loss in primary total knee replacement: a double-blind, randomized, controlled, noninferiority clinical trial. J Bone Joint Surg Am 96, 1937–1944, https://doi.org/10.2106/jbjs.n.00060 (2014).
Zhuang, Q. et al. Efficacy and safety of Postoperative Intravenous Parecoxib sodium Followed by ORal CElecoxib (PIPFORCE) post-total knee arthroplasty in patients with osteoarthritis: a study protocol for a multicentre, double-blind, parallel-group trial. BMJ Open 6, e011732, https://doi.org/10.1136/bmjopen-2016-011732 (2016).
Wang, D. et al. Closed Suction Drainage Is Not Associated with Faster Recovery after Total Knee Arthroplasty: A Prospective Randomized Controlled Study of 80 Patients. Orthop Surg 8, 226–233, https://doi.org/10.1111/os.12247 (2016).
Gross, J. B. Estimating allowable blood loss: corrected for dilution. Anesthesiology 58, 277–280 (1983).
Nadler, S. B., Hidalgo, J. H. & Bloch, T. Prediction of blood volume in normal human adults. Surgery 51, 224–232 (1962).
Yue, C., Kang, P., Yang, P., Xie, J. & Pei, F. Topical application of tranexamic acid in primary total hip arthroplasty: a randomized double-blind controlled trial. J Arthroplasty 29, 2452–2456, https://doi.org/10.1016/j.arth.2014.03.032 (2014).
Yi, Z. et al. Tranexamic Acid Administration in Primary Total Hip Arthroplasty: A Randomized Controlled Trial of Intravenous Combined with Topical Versus Single-Dose Intravenous Administration. J Bone Joint Surg Am 98, 983–991, https://doi.org/10.2106/jbjs.15.00638 (2016).
Zhou, K. et al. Non-drainage versus drainage in tourniquet-free knee arthroplasty: a prospective trial. ANZ J Surg, https://doi.org/10.1111/ans.14183 (2017).
Xie, J. et al. Does tranexamic acid alter the risk of thromboembolism following primary total knee arthroplasty with sequential earlier anticoagulation? A large, single center, prospective cohort study of consecutive cases. Thromb Res 136, 234–238, https://doi.org/10.1016/j.thromres.2015.05.014 (2015).
Lee, Q. J., Ching, W. Y. & Wong, Y. C. Blood Sparing Efficacy of Oral Tranexamic Acid in Primary Total Knee Arthroplasty: A Randomized Controlled Trial. Knee Surg Relat Res 29, 57–62, https://doi.org/10.5792/ksrr.16.074 (2017).
Zohar, E. et al. The postoperative blood-sparing efficacy of oral versus intravenous tranexamic acid after total knee replacement. Anesth Analg 99, 1679–1683, table of contents, https://doi.org/10.1213/01.ane.0000136770.75805.19 (2004).
Martin, J. G. et al. Topical administration of tranexamic acid in primary total hip and total knee arthroplasty. J Arthroplasty 29, 889–894, https://doi.org/10.1016/j.arth.2013.10.005 (2014).
Oztas, S. et al. The effect of local and systemic application of tranexamic acid on the amount of blood loss and allogeneic blood transfusion after total knee replacement. Acta Orthop Belg 81, 698–707 (2015).
Lei, Y. et al. The efficacy and safety of multiple-dose intravenous tranexamic acid on blood loss following total knee arthroplasty: a randomized controlled trial. Int Orthop, https://doi.org/10.1007/s00264-017-3519-x (2017).
Alipour, M., Tabari, M., Keramati, M., Zarmehri, A. M. & Makhmalbaf, H. Effectiveness of oral Tranexamic acid administration on blood loss after knee artroplasty: a randomized clinical trial. Transfus Apher Sci 49, 574–577, https://doi.org/10.1016/j.transci.2013.09.005 (2013).
Quinn, M., Bowe, A., Galvin, R., Dawson, P. & O’Byrne, J. The use of postoperative suction drainage in total knee arthroplasty: a systematic review. Int Orthop 39, 653–658, https://doi.org/10.1007/s00264-014-2455-2 (2015).
Si, H. B., Yang, T. M., Zeng, Y. & Shen, B. No clear benefit or drawback to the use of closed drainage after primary total knee arthroplasty: a systematic review and meta-analysis. BMC Musculoskelet Disord 17, 183, https://doi.org/10.1186/s12891-016-1039-2 (2016).
Zhang, W. et al. The effects of a tourniquet used in total knee arthroplasty: a meta-analysis. J Orthop Surg Res 9, 13, https://doi.org/10.1186/1749-799x-9-13 (2014).
Pfitzner, T. et al. Influence of the tourniquet on tibial cement mantle thickness in primary total knee arthroplasty. Knee Surg Sports Traumatol Arthrosc, https://doi.org/10.1007/s00167-014-3341-6 (2014).
Ledin, H., Aspenberg, P. & Good, L. Tourniquet use in total knee replacement does not improve fixation, but appears to reduce final range of motion: A randomized RSA study involving 50 patients. Acta orthopaedica 83, 499–503 (2012).
Li, J., Zhang, Z. & Chen, J. Comparison of efficacy and safety of topical versus intravenous tranexamic acid in total hip arthroplasty: A meta-analysis. Medicine (Baltimore) 95, e4689, https://doi.org/10.1097/md.0000000000004689 (2016).
Wang, D. et al. Comparison of oral versus intra-articular tranexamic acid in enhanced-recovery primary total knee arthroplasty without tourniquet application: a randomized controlled trial. BMC Musculoskelet Disord 19, 85, https://doi.org/10.1186/s12891-018-1996-8 (2018).
Chimento, G. F. et al. An evaluation of the use of topical tranexamic acid in total knee arthroplasty. J Arthroplasty 28, 74–77, https://doi.org/10.1016/j.arth.2013.06.037 (2013).
Gillette, B. P. et al. Economic impact of tranexamic acid in healthy patients undergoing primary total hip and knee arthroplasty. J Arthroplasty 28, 137–139, https://doi.org/10.1016/j.arth.2013.04.054 (2013).
Luo, Z. Y. et al. Oral vs Intravenous vs Topical Tranexamic Acid in Primary Hip Arthroplasty: A Prospective, Randomized, Double-Blind, Controlled Study. J Arthroplasty, https://doi.org/10.1016/j.arth.2017.09.062 (2017).
Dai, K.-R., Li, H.-W. & Yan, M.-N. Twenty-year accelerated development of artificial joints in China. Chin J Joint Surg 9, 691–694 (2015).
Wang, D. et al. Blood-conserving efficacy of multiple doses of oral tranexamic acid associated with an enhanced-recovery programme in primary total knee arthroplasty: a randomized controlled trial. Bone Joint J 100-b, 1025–1032, https://doi.org/10.1302/0301-620x.100b8.bjj-2017-1598.r1 (2018).
Acknowledgements
The trial was registered in the Chinese Clinical Trial Registry on March 23, 2017 (ChiCTR-INR-17010965).
Author information
Authors and Affiliations
Contributions
Z.K.Z., F.X.P., D.H.L. and W.N.Z. conceived and designed this study; D.W., C.C., H.Y.W., L.L.L. and W.K.M. collected the data; D.W., L.L.L., C.C. and H.Y.W. performed the statistical analysis; D.W., H.Y.W. and W.K.M. prepared Tables 1–5; D.W. prepared Fig. 1; D.W. and H.Y.W. wrote the manuscript. All authors reviewed the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, D., Wang, HY., Cao, C. et al. Tranexamic acid in primary total knee arthroplasty without tourniquet: a randomized, controlled trial of oral versus intravenous versus topical administration. Sci Rep 8, 13579 (2018). https://doi.org/10.1038/s41598-018-31791-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31791-x
Keywords
This article is cited by
-
The effect of perioperative tranexamic acid (TXA) in patients with calcaneal fractures: a meta-analysis and systematic review of randomized controlled trials
Journal of Orthopaedic Surgery and Research (2023)
-
Are cementation quality and clinical outcomes affected by the use of tourniquet in primary total knee arthroplasty?
Archives of Orthopaedic and Trauma Surgery (2022)
-
Intra-articular versus intravenous administration of tranexamic acid in lower limb total arthroplasty: a systematic review and meta-analysis of randomised clinical trials
European Journal of Orthopaedic Surgery & Traumatology (2022)
-
Comparison of efficacy and safety between oral and intravenous administration of tranexamic acid for primary total knee/hip replacement: a meta-analysis of randomized controlled trial
Journal of Orthopaedic Surgery and Research (2020)
-
Finding the optimal control level of intraoperative blood pressure in no tourniquet primary total knee arthroplasty combine with tranexamic acid: a retrospective cohort study which supports the enhanced recovery strategy
Journal of Orthopaedic Surgery and Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.