Abstract
Plant potyviruses require eukaryotic translation initiation factors (eIFs) such as eIF4E and eIF(iso)4E to replicate and spread. When Turnip mosaic virus (TuMV) infects a host plant, its viral protein linked to the genome (VPg) needs to interact with eIF4E or eIF(iso)4E to initiate translation. TuMV utilizes BraA.eIF4E.a, BraA.eIF4E.c, BraA.eIF(iso)4E.a, and BraA.eIF(iso)4E.c of Brassica rapa to initiate translation in Arabidopsis thaliana. In this study, the BraA.eIF4E.a, BraA.eIF4E.c, BraA.eIF(iso)4E.a, and BraA.eIF(iso)4E.c genes were cloned and sequenced from eight B. rapa lines, namely, two BraA.eIF4E.a alleles, four BraA.eIF4E.c alleles, four BraA.eIF(iso)4E.a alleles, and two BraA.eIF(iso)4E.c alleles. Yeast two-hybrid (Y2H) and bimolecular fluorescence complementation (BiFC) analyses indicated that TuMV VPg could not interact with eIF4E, but only with eIF(iso)4E of B. rapa. In addition, the VPgs of the different TuMV isolates interacted with various eIF(iso)4E copies in B. rapa. In particular, TuMV-UK1/CDN1 VPg only interacted with BraA.eIF(iso)4E.c, not with BraA.eIF(iso)4E.a. Some single nucleotide polymorphisms (SNPs) were identified that may have affected the interaction between eIF(iso)4E and VPg such as the SNP T106C in BraA.eIF(iso)4E.c and the SNP A154C in VPg. Furthermore, a three-dimensional structural model of the BraA.eIF(iso)4E.c-1 protein was constructed to identify the specific conformation of the variable amino acids from BraA.eIF(iso)4E.c. The 36th amino acid in BraA.eIF(iso)4E.c is highly conserved and may play an important role in establishing protein structural stability. The findings of the present study may lay the foundation for future investigations on the co-evolution of TuMV and eIF(iso)4E.
Similar content being viewed by others
Introduction
Potyvirus is the largest genus of plant viruses (comprising approximately 36% of the total number of plant viruses) and causes severe economic losses in agriculture1,2. The shared characteristics of the Potyviridae family include a positive single-stranded RNA molecule with a covalently-bound viral protein linked to the genome (VPg) that is linked to the 5′-end and a 3′ poly (A) tail at the 3′-end2,3. Turnip mosaic virus (TuMV) is a member of Potyvirus (family Potyviridae) and is one of the most significant potyviruses known to infect brassicas4,5. The genome of TuMV is approximately 10 kb in size and has a single open reading frame (ORF) that is flanked by two untranslated regions (UTRs)5. The 5′ UTR includes an internal ribosome entry site5,6. The ORF is translated as a large polyprotein that is subsequently cleaved into at least 10 smaller functional polypeptides (P1, HC-Pro, P3, 6K1, C1, 6K2, VPg, NIa, Nib, and CP) by viral-encoded proteases3,5,7,8. The 6K2 and VPg proteins are involved in replication complexes on cytoplasmic membranes5.
Approximately 51% of resistance traits to plant viruses are dominant, 35% are recessive, and the remaining are more complex (incomplete dominance or dose-dependent)9. Plants have both active and passive resistance mechanisms against viruses. The active resistance mechanisms are mediated by R genes and/or gene silencing9. R genes are always dominant and have characteristic domains such as NBS-LRR or (CC)-NBS-LRR10. Generally, potyviruses are only able to encode a limited number of proteins, and therefore they depend on host factors for replication and translation and to infect the host and spread systemically11,12. The passive mechanisms of plant virus resistance indicate that loss, deletion, or mutation of a required host factor may cause recessive resistance to the virus2,12,13. Several of these resistance genes (such as pvr1 in Capsicum and retr01 and retr02 in Brassica rapa) have been successfully used for decades in breeding programs as effective and stable sources of resistance14,15,16.
The majority, but not all, of the recessive resistance genes that have been characterized to date encode eukaryotic translation initiation factors (eIFs) [i.e., eIF4E, eIF(iso)4E, eIF4G, and eIF(iso)4 G], which play critical roles in potyviral infection2,12,17. eIFs associated with plant virus resistance are encoded by genes such as lsp118, pvr1/214,19, pvr619, nsv20,21, cum222, cum122,23, sbm124, rymv-125,26, rym4/5/627,28, mol1/229, pot130, cyv-131, cyv-232, tsv133, wlv34, bc-335, retr0115, retr0216, and retr0336. In plants, eIF4E and eIF4G form the eIF4F complex, and eIF(iso)4E and eIF(iso)4 G form the eIF(iso)4F complex37,38. These complexes are involved in the binding of the mRNA cap and ribosome recruitment in the initial steps of translation16,18,26,39. Some studies have indicated that the potyviral protein, VPg, is crucial for virus replication and cell-to-cell communication, as well as long-distance movement in relation to recessive resistance genes in different host species2,40,41,42,43,44,45. A key observation in yeast two-hybrid (Y2H) assays relating to recessive resistance is that the VPg (or its precursor, NIa) protein of some potyviruses has high affinity to eIF4E or eIF(iso)4E46,47. For example, Y2H and enzyme-linked immunosorbent assays revealed interactions between Arabidopsis eIF(iso)4E and TuMV/Tobacco etch virus (TEV) VPg2,46.
Arabidopsis thaliana possesses three eIF4E genes, one eIF(iso)4E gene, three eIF4G genes, and two eIF(iso)4G genes that act as host factors and play an important role in viral infection37. Mutations in eIF(iso)4E in A. thaliana result in broad-spectrum potyvirus resistance to TEV and TuMV18,48. In addition, TuMV VPg solely interacts with the eIF(iso)4E gene (AT5G35620), and not with any other eIF4E genes in A. thaliana49. Y2H assays and co-immunoprecipitation analysis suggest that the W95L, K150L, and W95L/K150E amino acid mutations in B. rapa eIF(iso)4E interrupt its interaction with TuMV VPg and its overexpression in a susceptible Chinese cabbage cultivar confers resistance to multiple TuMV strains50.
Three copies of eIF4E [BraA.eIF4E.a, BraA.eIF4E.b, and BraA.eIF4E.c] and three copies of eIF(iso)4E [BraA.eIF(iso)4E.a, BraA.eIF(iso)4E.b, and BraA.eIF(iso)4E.c] were identified in the TuMV-susceptible, inbred, diploid B. rapa line R-o-1851. In addition, some recessive resistance genes to TuMV have been identified in B. rapa, such as retr0115 and retr0216, which encode eIF(iso)4E proteins. The retr02 gene contains a polymorphism (A/G) that results in an amino acid substitution (Gly/Asp) in the eIF(iso)4E protein that contributes to resistance/susceptibility16. In a further study, splice variants within the retr02 gene produced a stop codon within exon 1 that is predicted to generate a truncated, non-functional protein, and TuMV could use copies of eIF(iso)4E at two loci, which confers a spectrum of resistance and durability52. Subsequently, the Brassica juncea retr03 gene was mapped and determined to be an allele of eIF2Bβ, which acts as a guanine nucleotide exchange factor (GEF) for its GTP-binding protein partner eIF2 by interacting with eIF2.GTP at an early step during translation initiation, thereby conferring resistance to the TuMV isolate (ZJ) from Zhejiang Province in China36. Based on our previous study, TuMV-C4 VPg exclusively interacted with BraA.eIF(iso)4E.a, and TuMV-UK1 VPg solely interacted with BraA.eIF(iso)4E.c53. In the present study, the eIF4E (BraA.eIF4E.a and BraA.eIF4E.c) and eIF(iso)4E (BraA.eIF(iso)4E.a and BraA.eIF(iso)4E.c) alleles from eight B. rapa lines were investigated. The TuMV isolates C4 [HQ46217] from China, CDN1 [D83184] from Canada, and UK1 [NC_002509] from the UK, constituted the three representative pathotypes. The interactions of BraA.eIF4E/BraA.eIF(iso)4E and TuMV-CDN1 VPgs were analyzed by Y2H and bimolecular fluorescence complementation (BiFC) assays. Furthermore, key amino acids influencing the interaction between BraA.eIF(iso)4E and TuMV-C4/UK1/CDN1 VPgs were identified. This is the first report on the systemic relationships between eIF4E families and different TuMV VPgs. This may provide a foundation for the screening and cloning of eIF(iso)4E resistance loci, as well as systematic research into the resistance mechanisms of eIF(iso)4E to TuMV.
Results
Variability in eIF4E and its isoform eIF(iso)4E in B. rapa
Two copies of eIF4E (BraA.eIF4E.a and BraA.eIF4E.c) and two copies of eIF(iso)4E [BraA.eIF(iso)4E.a and BraA.eIF(iso)4E.c] could potentially complement Col-0::dSpm [possessing a transposon knock-out of eIF(iso)4E]. Thus, primers were designed to amplify the cDNAs of these copies in the eight B. rapa lines, followed by sequencing. A sequence analysis identified several variants within the BraA.eIF4E.a of the eight lines (the lines 80122, 80186, 80124, Chiifu, 2079, and BP058 are resistant to TuMV C4, whereas 80425 and R-o-18 are susceptible). Most of the variations were non-synonymous, although some were synonymous (nts 300, 444, 525, and 564) (Table S1). There were no differences in nucleotide/amino acid sequences among lines 80124, BP058, and 2079, which included only three differing bases/amino acids (nts 98, 164, and 638; aas 33, 55, and 213) compared to line 80186 (Tables S1 and 1). The amino acid sequences of 80122, 80425, Chiifu, and R-o-18 were the same, although there were also some synonymous nucleotide variations. Numerous variations were also identified in BraA.eIF4E.c, although most were synonymous (Tables S2 and 2). Notably, the nucleotide/amino acid sequences of 80122 and Chiifu were the same, as were those of 80425 and 2079. In addition, the nucleotide/amino acid sequences of 80186, BP058, and R-o-18 were the same, and differed in two bases/amino acids from 80124.
There was one G insertion at the exon 1/intron 1 junction of BraA.eIF(iso)4E.a in 80122, 80124, BP058, and 2079 that was predicted to result in premature protein termination (Tables S3 and 3), thereby conferring resistance to TuMV. Two splice variants in BraA.eIF(iso)4E.a have been reported in the 80122 line, namely 80122-1, which retained intron 1, resulting in a premature stop codon at position 234 bp and 80122-2, which has an extra G at the end of exon 1, resulting in a premature stop codon52. The nucleotide/amino acid sequences of 80186 and Chiifu were identical (Tables S3 and 3) and differed from the 80425 line in a single nucleotide/amino acid. Compared to 80186, R-o-18 exhibited seven nucleotide variations, which consisted of five synonymous and two non-synonymous changes. The BraA.eIF(iso)4E.c sequence was the same among lines 80122, 80425, 80186, 80124, BP058, 2079, and Chiifu, but differed from that of the R-o-18 line, which included five nucleotide variations consisting of one synonymous and four non-synonymous amino acid changes (Tables S4 and 4). The sequence analysis of BraA.eIF4E.a, BraA.eIF4E.c, BraA.eIF(iso)4E.a, and BraA.eIF(iso)4E.c indicated that the eIF4E genes were more variable than the eIF(iso)4E genes.
The B. rapa eIF4E genes do not interact with TuMV-VPg
Compared with the eIF(iso)4E gene, the eIF4E genes exhibited a high level of sequence variability. Two different BraA.eIF4E.a alleles were identified, namely, BraA.eIF4E.a-1 (80124, BP058, 2079, and 80186) and BraA.eIF4E.a-2 (80122, 80425, Chiifu, and R-o-18). Four different BraA.eIF4E.c alleles were also detected, namely, BraA.eIF4E.c-1 (80122 and Chiifu), BraA.eIF4E.c-2 (80425 and 2079), BraA.eIF4E.c-3 (80186, BP058 and R-o-18), and BraA.eIF4E.c-4 (80124). The BraA.eIF4E.c sequences from 80186, 80124, BP058, and R-o-18 were highly similar, and 80186 and 80124 were selected as representative lines for further analysis. The BraA.eIF4E.a and BraA.eIF4E.c genes complemented Col-0::dSpm, and TuMV could possibly interact with them for replication and multiplication. To confirm this, Y2H and BiFC assays were conducted between BraA.eIF4E.a/BraA.eIF4E.c and TuMV-C4/UK1/CDN1 VPgs.
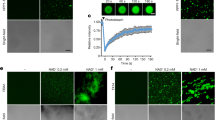
The results from the Y2H assays suggested that the positive control murine p53 interacts with the SV40 large T antigen, and the TuMV-C4/UK1/CDN1 VPgs could interact with the positive control LSP [Arabidopsis eIF(iso)4E], but not with BraA.eIF4E.a-1, BraA.eIF4E.a-2, BraA.eIF4E.c-1, BraA.eIF4E.c-2, BraA.eIF4E.c-3, or BraA.eIF4E.c-4 (Fig. 1A). BiFC was also used in B. rapa protoplast cells to study protein-protein interactions, and the results corroborated those generated by the Y2H assay (Fig. 1B). The empty vector pGADT7 did not interact with the empty vector pGBKT7, and each partner also did not interact with the empty vectors (data not shown).
TuMV C4, TuMV UK1, and TuMV CDN1 VPgs do not interact with BraA.eIF4E.a or BraA.eIF4E.c. (A) The interaction was confirmed by yeast two-hybrid (Y2H) assays. Negative control: the empty vectors pGADT7 and pGBKT7 (data not shown); positive controls: the murine p53 and SV40 large T antigen from the Matchmaker GAL4 two-hybrid system 3; TuMV-VPg and Arabidopsis eIF(iso)4E (lsp); assay controls: each partner and empty vector (data not shown). (B) The interactions were confirmed by bimolecular fluorescence complementation (BiFC). P: positive controls (the combination of bZIP63YN and bZIP63YC); N: negative controls (YNE-empty and YCE-empty vectors); the assay controls: each partner and empty vectors (data not shown).
Different TuMV isolates interact with different eIF(iso)4Es in B. rapa
Compared with the eIF4E genes, the eIF(iso)4E genes were well conserved and showed a low degree of variability. Five different BraA.eIF(iso)4E.a alleles were identified, namely, BraA.eIF(iso)4E.a-1 (80122-CDS, retaining the entire intron 1, resulting in a premature stop codon at position 234 bp), BraA.eIF(iso)4E.a-2 (80122-CDS, with an extra G at the end of exon 1 that is predicted to result in a premature stop codon), BraA.eIF(iso)4E.a-3 (80425), BraA.eIF(iso)4E.a-4 (80186), and BraA.eIF(iso)4E.a-5 (R-o-18). The sequences from 80425, 80186, and R-o-18 were also highly similar, and thus 80425 and 80186 were selected as representative lines for further investigation. Two different BraA.eIF(iso)4E.c alleles were also detected, namely, BraA.eIF(iso)4E.c-1 (80122, 80425, 80186, 80124, BP058, 2079, and Chiifu) and BraA.eIF(iso)4E.c-2 (R-o-18).
The Y2H and BiFC analyses suggested that TuMV-CDN1 VPg largely interacts with BraA.eIF(iso)4E.c-1, but not with BraA.eIF(iso)4E.c-2 or BraA.eIF(iso)4E.a-1, BraA.eIF(iso)4E.a-2, BraA.eIF(iso)4E.a-3, or BraA.eIF(iso)4E.a-4 (Fig. 2A,B). We previously showed that TuMV-C4 VPg interacts with BraA.eIF(iso)4E.a, but not with BraA.eIF(iso)4E.c, whereas TuMV-UK1 VPg only interacts with BraA.eIF(iso)4E.c, but not BraA.eIF(iso)4E.a53. In Arabidopsis, TuMV VPg could only interact with LSP [A. thaliana harbors a single copy of eIF(iso)4E]. However, in B. rapa three eIF(iso)4E copies and different TuMV isolates were able to interact with various eIF(iso)4Es.
TuMV CDN1 VPgs interacts with BraA.eIF(iso)4E.c, but not with BraA.eIF(iso)4E.a. (A) The results are from the Y2H. Negative control: the empty vectors pGADT7 and pGBKT7 (data not shown); positive controls: the murine p53 and SV40 large T antigen from the Matchmaker GAL4 two-hybrid system 3; TuMV-VPg and Arabidopsis eIF(iso)4E (lsp); assay controls: each partner and empty vector (data not shown). (B) Verification of the results using BiFC. P: positive controls (the combination of bZIP63YN and bZIP63YC); N: negative controls (YNE-empty and YCE-empty vectors); each partner and empty vector were used as controls (data not shown).
Specific single nucleotide polymorphisms (SNPs) affect the interaction between eIF(iso)4E and VPg
Some amino acid changes seem to be involved in strain-specific interactions between eIF(iso)4E and TuMV VPg. The Y2H and BiFC analysis indicated that TuMV-UK1/CDN1 VPg could interact with BraA.eIF(iso)4E.c-1 (Chiifu) but not with BraA.eIF(iso)4E.c-2 (R-o-18). Between BraA.eIF(iso)4E.c-1 (Chiifu) and BraA.eIF(iso)4E.c-2 (R-o-18), five differing bases (nt T106C, C155T, T239C, C449A, and C546T) were identified (Table S4), which were predicted to result in the substitution of four amino acids (F36L, A52V, I80T, and P150Q) (Table 4). Thus, primers were designed at the four loci, and site-directed mutagenesis (using eIF(iso)4E of Chiifu as template) was successfully implemented based on the overlap-extension PCR to detect which locus was essential for the interactions. Y2H and BiFC analyses indicated that the amino acid substitution F36L (nt T106C) in BraA.eIF(iso)4E.c played a critical role in the interaction between TuMV-UK1 VPg and BraA.eIF(iso)4E.c-1 (Chiifu), and the amino acids A52V (nt C155T), I80T (nt T239C), and P150Q had little influence (Fig. 3A,B). Compared to TuMV-UK1 VPg, TuMV-CDN1 VPg in the Y2H and BiFC assays performed differently in the interaction: amino acids F36L (nt T106C), A52V (nt C155T), and I80T (nt T239C) in BraA.eIF(iso)4E.c were the key elements; and P150Q did not play an essential role in the observed interaction (Fig. 3A,B). Taken together, the amino acid F36L (nt T106C) in BraA.eIF(iso)4E.c is a key site of the protein that generally affects the interaction between BraA.eIF(iso)4E.c-1 and TuMV-UK1/CDN1 VPg.
Specific SNPs affect the interaction between eIF(iso)4E and TuMV VPg. Variations between BraA.eIF(iso)4E.c-1 and BraA.eIF(iso)4E.c-2 could affect the interaction as confirmed by Y2H (A) and by BiFC. (B,C) Multiple sequence alignment of TuMV C4 VPg TuMV UK1 VPg and TuMV CDN1 VPg. Five amino acid substitutions were identified between TuMV C4 and CDN1, whereas four amino acid substitutions were detected between TuMV C4 and UK1. Variations between TuMV-C4 VPg and TuMV-CDN1 VPg could affect the interaction as indicated by Y2H (D) and by BiFC. (E) Y2H: negative control, the empty vectors pGADT7 and pGBKT7 (data not shown); positive controls, the murine p53 and SV40 large T antigen from the Matchmaker GAL4 two-hybrid system 3; TuMV-VPg and Arabidopsis eIF(iso)4E (lsp); assay controls: each partner and empty vector (data not shown). BiFC: P-positive controls (the combination of bZIP63YN and bZIP63YC); N-negative controls (YNE-empty and YCE-empty vectors); the assay controls were each partner and empty vectors (data not shown).
TuMV-C4 VPg could interact with BraA.eIF(iso)4E.a-3 (80425); while TuMV-CDN1 VPg could not. Sequence analysis of TuMV-C4 VPg and TuMV-CDN1 VPg identified five base/amino acid changes (nts A154C, G289A, A301G, A313G, and A349G; aas I52L, E97K, N101D, N105D, and I117V) (Fig. 3C). Five mutants (using TuMV-C4 VPg as a template) were obtained by overlap-extension PCR. The Y2H and BiFC analyses indicated that the interaction between TuMV-C4 VPg and BraA.eIF(iso)4E.a-3 (80425) was mediated by the amino acid substitutions I52L, E97K, and N105D of TuMV VPg, and the amino acid substitution N101D and I117V did not affect this particular interaction (Fig. 3D,E). Compared to BraA.eIF(iso)4E.a-3 (80425), BraA.eIF(iso)4E.c-1 (Chiifu) exhibited differences in this interaction. In the trials, the amino acid substitution I52L was essential for the interaction, whereas the other four sites did not affect the interaction (Fig. 3D,E). Taken together, the amino acid substitution I52L in TuMV-C4 VPg plays a critical role in the interaction between BraA.eIF(iso)4E.a and TuMV VPg. Therefore, various amino acids in eIF(iso)4E were essential to the interaction, whereas a few amino acids in TuMV VPg were also significant in the interaction.
Analysis of the mechanism underlying the interaction of TuMV VPg-eIF(iso)4E
Amino acid sequence alignment of BraA.eIF(iso)4E.c-1 (Chiifu) and BraA.eIF(iso)4E.c-2 (R-o-18) identified four substitutions: Phe/Leu-36, Ala/Val-52, Ile/Thr-80, and Pro/Gln-150 (Table 4). Furthermore, a three-dimensional (3D) structural model of the BraA.eIF(iso)4E.c-1 protein was constructed (Fig. 4A) to identify the special conformation of the four sites. The 36th amino acid in BraA.eIF(iso)4E.c is highly conserved and is pivotal in establishing the structural stability of the protein. The amino acid Phe is highly conserved in plant eIF4Es and eIF(iso)4Es, whereas in human and yeast, this site is occupied by a Leu residue54. The 36th amino acid in BraA.eIF(iso)4E.c changed from Phe to Leu, and Phe is an aromatic amino acid that contains a benzene ring. However, Leu is an aliphatic amino acid that is linear and does not contain a benzene ring (Fig. 4B). Thus, the two amino acid substitutions may influence the biochemical functions and the structure of the protein. Compared to the 36th amino acid, the 52th, 80th, and 150th amino acids are not conserved in the protein. The 52th amino acids changed from Val to Ala, the 80th changed from Ile to Thr, and the 150th changed from Pro to Gln, which are all aliphatic amino acids (Fig. 4B).
Analysis of BraA.eIF(iso)4E.c-1 protein domain tertiary structure. (A) The three-dimensional structural model was built based on the wheat eIF(iso)4E protein. (B) Four amino acid variations were detected between the BraA.eIF(iso)4E.c-1 and BraA.eIF(iso)4E.c-2 proteins. (C) The location of the three different amino acid variations is depicted in the structural model of the entire protein. (D) The 36th amino acid changed from phenylalanine to leucine. (E) The 52th amino acids changed from valine to alanine. (F) The 80th changed from isoleucine to threonine. (G) The 150th changed from proline to glutamine.
The structure of the BraA.eIF(iso)4E protein includes eight β-strands, three α-helices, and three extended loops. There is a large cavity at its cap-binding site that undergoes conformational changes in the cap-binding loops. The 36th amino acid is located in the middle of the first β-strands region and may play an essential role in protein structure and function (Fig. 4A). In addition, the 36th amino acid is located in the cap-free structure of BraA.eIF(iso)4E protein, thereby suggesting that it plays an essential role in the allosteric regulation of the BraA.eIF(iso)4E protein.
Discussion
In a previous study, the BraA.eIF4Es and BraA.eIF(iso)4Es from the B. rapa ‘RLR22’ line could not interact with the TuMV isolates51. The two copies of eIF4E (BraA.eIF4E.a and BraA.eIF4E.c) and two copies of eIF(iso)4E [BraA.eIF(iso)4E.a and BraA.eIF(iso)4E.c] were transformed into Col-0::dSpm, which had a transposon knocked out of the eIF(iso)4E gene. However, this resulted in a change from complete susceptibility to complete resistance to TuMV, and all four Brassica transgenes complemented the A. thaliana eIF(iso)4E knockout. These changes conferred susceptibility to both mechanical and aphid challenge with TuMV51,52. In this study, the Y2H and BiFC assays also showed that the TuMV-C4/UK1/CDN1 isolates did not interact with BraA.eIF4Es, but rather with BraA.eIF(iso)4Es. The interaction of the eIFs and TuMV VPgs differed between B. rapa and A. thaliana. BraA.eIF4Es and BraA.eIF(iso)4Es from the B. rapa ‘RLR22’ line could not interact with the TuMV isolates in vitro, but could interact with the TuMV isolates in the Col-0::dSpm, which was somewhat misleading. Genomic analyses of diploid B. rapa have indicated that it evolved from a hexaploid ancestor and then underwent a whole genome triplication event55, which resulted in more gene copies in B. rapa than in A. thaliana. These genomic changes have formed more complex compounds that are related to the TuMV infection process, and the same genes induce different results in B. rapa and A. thaliana.
Thus, different parts of eIF(iso)4E and various amino acids in VPg influence this particular interaction. Sequence comparison between BraA.eIF(iso)4E.a and BraA.eIF(iso)4E.c identified various amino acid substitutions that may affect the interactions (Fig. S1). Comparison of the TuMV-C4 and TuMV-UK1 VPgs identified four amino acids substitutions, namely, F89L, N105D, P114S, and M119V (Fig. 3C). These amino acid changes, particularly those involving residues 89 and 114, may influence the interaction. Thus, it is possible that different strains of TuMV interact with various eIF(iso)4E proteins to influence protein translation. Some amino acids indicated evidence of positive selection, which may have contributed to virus resistance in eIF4E and eIF(iso)4E. For example, in wheat, the G107R substitution in the cap-binding pocket plays a key role in both VPg interactions and cap-binding, whereas the L79R change that is located within an external loop influences VPg, but not cap-binding56.
BraA.eIF(iso)4E.a interacted with the TuMV-C4 VPg, whereas BraA.eIF(iso)4E.a-1 and −2 in 80122 showed loss of function and could not interact with the TuMV-C4 VPg. Thus, BraA.eIF(iso)4E.a-1 and −2 are resistant alleles for TuMV-C4, and the deleted parts of their proteins (from 70th to 200th amino acids) are essential to this particular interaction. TuMV-UK1 VPg interacted with BraA.eIF(iso)4E.c-1, but not with BraA.eIF(iso)4E.c-2. Four amino acid variations (L36F, V52A, T80I, and Q150P) between BraA.eIF(iso)4E.c-1 and BraA.eIF(iso)4E.c-2 influence this interaction. Thus, BraA.eIF(iso)4E.c contains a resistance locus for TuMV-UK1. In A. thaliana, structural data implicate Trp-46 and Trp-92 in eIF(iso)4E in cap recognition and when Trp-46 or Trp-92 is changed to Leu, eIF(iso)4E loses the ability to form a complex with both VPgs57. eIF4E and eIF(iso)4E belong to class I of the eIF4E family, and the novel cap-binding protein nCBP belongs to class II58. Members from class I have conserved Trp-43 and Trp-56, while those from class II have both residues substituted by Trp or Phe56. The co-evolution of the VPg of TuMV and eIF(iso)4E in B. rapa may have resulted in variations in both proteins. Further investigation into the co-evolutionary relationship between TuMV and B. rapa suggests that the amino acids of eIF(iso)4E that influence its interaction with the VPgs of different TuMV isolates are highly variable.
Accordingly, the TuMV isolate resistance loci could be screened to identify resistance genes and could be cloned to generate plants with broad-spectrum resistance. Furthermore, the 3D structural model of the BraA.eIF(iso)4E.c protein indicates that the 36th amino acid is highly conserved and plays an important role in stabilizing the protein structure. However, the main purpose of building a structural protein model was to provide the location of the mutation in a 3D structural context. This may provide information on whether the mutation is buried within the hydrophobic core, lying on the surface, located near an active site, proximal to an interface, or close to a site of post-translational modification. This model may also be used as a guide in predicting the effect of specific mutations on protein function.
In Arabidopsis, TuMV VPg can only interact with LSP [eIF(iso)4E]. Mutations involving LSP (i.e., lsp mutants) exhibit premature termination, thereby conferring loss of function and resistance to TuMV18,48. In B. rapa, retr02 [BraA.eIF(iso)4E.a] has been identified as a recessive resistance gene for TuMV C416,59. The product of the resistance gene retr02 is also involved in premature protein termination and is thus unable to interact with TuMV C4 VPg, thereby resulting in resistance to TuMV C452. Thus, the introduction of mutations in the BraA.eIF(iso)4E.a gene may confer plant resistance to TuMV C4, e.g., Retr02, and mutations in the BraA.eIF(iso)4E.c gene induce resistance to TuMV UK1. In addition, mutations in both BraA.eIF(iso)4E.a and BraA.eIF(iso)4E.c confer resistance to both TuMV C4 and TuMV UK1. It is also possible to determine whether TuMV isolates affect such mutants; and when these are resistant, the range of activity (broad resistance). This strategy could be used to create new varieties that may be used in breeding as well as in marker-assisted selection.
Materials and Methods
Plant materials and TuMV isolates
The following B. rapa accessions were used in this study: 80122 (BP8407), 80425 (Ji Zao Chun), 80186 (Er Qing), 80124(89B), Chiifu, 2079, R-o-18, and BP058, which are highly inbred lines. The lines 80122, 80186, 80124, Chiifu, 2079, and BP058 are resistant to TuMV C4, whereas 80425 and R-o-18 are susceptible to the TuMV isolate16,59. The CDS sequences of eIF4E and eIF(iso)4E genes from 8 lines were submitted to GenBank (Table 5).
Three representative pathotypes, namely TuMV isolates C4 [HQ46217] from China, CDN1 [D83184] from Canada, and UK1 [NC_002509] from the UK, were maintained in the susceptible mustard (B. juncea) cultivar Tender Green.
Identification of eIF4E and its isoform eIF(iso)4E in B. rapa
The B. rapa genome has been sequenced, and the Brassica database (BRAD) includes predicted genes and associated annotations (InterPro, KEGG2, SWISS-PROT), B. rapa genes orthologous to those in A. thaliana, and genetic markers and maps for B. rapa.
Sequences representing the complete set of eIF4E and eIF(iso)4E genes in A. thaliana were acquired from The Arabidopsis Information Resource (TAIR) database (www.arabidopsis.org) and were used to search the data from the B. rapa ssp. pekinensis cv. Chiifu genome V1.5 and its set of annotated genes (http://Brassicadb.org) for homologous genes. Estimation of the number of eIF4E and eIF(iso)4E genes in the genome of B. rapa was conducted by analysis of expressed sequence tag (EST) data downloaded from the NCBI EST database and mRNA sequencing data (unpublished data). Full-length eIF4E and eIF(iso)4E protein sequences of A. thaliana genes were retrieved from the TAIR database and the UniProt protein database (www.uniprot.org).
Cloning and sequencing of eIF4E and its isoform eIF(iso)4E
Total RNAs were extracted from the leaves of the B. rapa lines (80122, 80425, 80186, 80124, Chiifu, 2079, R-o-18, and BP058) using TRIzol reagent (Invitrogen, Carlsbad, CA, USA), and first-strand cDNA was synthesized with a polydT primer using a Prime ScriptTM RT-PCR kit (TaKaRa, Dalian, China) according to the manufacturer’s instructions. The cDNAs were used as templates for PCR.
Generic primers (Table S5) were designed using the eIF4E gene reference sequence from the B. rapa genome, which encompassed the majority of the ORF. PCR was performed on the cDNAs using KOD Hot Start DNA polymerase (TOYOBO, Osaka, Japan). The PCR products were sequenced to analyze the allelic variability of the genes.
Y2H
Interactions between proteins were assayed with a Gal4-based Y2H system, as described by the manufacturer (Clontech, Mountain View, CA, USA). Yeast strains and plasmid vectors were obtained from Clontech Laboratories (Clontech). A bait plasmid, pGBKT7, was used to fuse the VPg to the DNA-binding domain of BD. A prey plasmid, pGADT7, was used to express the eIF4E genes (Clontech). Gene-specific primers were designed to introduce restriction enzyme sites (Table S6). The DNA sequences encoding VPg from TuMV-C4 and TuMV-UK1 were amplified using forward primer Bio120213 (NdeI site) and reverse primer Bio120214 (XmaI site). BraA.eIF4E.a was amplified using forward primer Bio120850 (EcoRI site) and reverse primer Bio120851 (XhoI site), BraA.eIF4E.c was amplified using forward primer Bio120852 (EcoRI site) and reverse primer Bio120853 (XhoI site), BraA.eIF(iso)4E.c was amplified using forward primer Bio120854 (EcoRI site) and reverse primer Bio120855 (XhoI site), and BraA.eIF(iso)4E.a was amplified using forward primer Bio120075 (EcoRI site) and reverse primer Bio120076 (XhoI site). The eIF(iso)4E sequences from Arabidopsis Col-0 were amplified using forward primer Bio120582 (EcoRI site) and reverse primer Bio120583 (XhoI site), and cloned into pGADT7 as a positive control for the interaction in the yeast two-hybrid assay. The amplified fragments were digested with EcoRI/XhoI and NdeI/XmaI and cloned into the corresponding restriction sites of pGADT7 and pGBKT7, respectively. All constructs were confirmed by sequencing.
The Matchmaker GAL4 two-hybrid system (Clontech) was used according to the manufacturer’s protocols. pGADT7:4E and pGBKT7:VPg constructs were transformed into AH109 yeast strains. After yeast transformation, colonies were grown on various selective media lacking leucine, tryptophan, histidine, and adenine (SD-LW/SD-LWH/SD-LWHA). Plates were incubated at 30 °C and growth was checked 3–5 days after inoculation. Each assay was performed in triplicate. The empty vectors pGADT7 and pGBKT7 were used as negative controls; the interaction between murine p53 and SV40 large T antigen (controls from the Matchmaker GAL4 two-hybrid system 3) was used as a positive control; and TuMV-VPg and Arabidopsis eIF(iso)4E (LSP) were also used as positive controls. In addition, each partner and empty vectors were used as assay controls.
BiFC
Molecular techniques were performed using standard protocols60,61,62. The eIF(iso)4E genes were amplified using the primers above, which contained the BamHI and XhoI sites, and the eIF(iso)4E genes PCR products and pSPYNE empty vector were digested by BamHI and XhoI. Then, the recombinant vector eIF(iso)4E-pSPYNE was constructed using T4 ligase. Similarly, the TuMV VPg genes were amplified using specific primers (Table S7), which contained the ClaI and XhoI sites, and the TuMV VPg gene PCR products and pSPYCE empty vector were digested by the ClaI and XhoI sites. The primer pair Bio120901/Bio120902 was used to amplify BraA.eIF4E.a, Bio120903/Bio120904 for BraA.eIF4E.c, Bio120905/Bio120906 for BraA.eIF(iso)4E.c, Bio120907/Bio120908 for BraA.eIF(iso)4E.a, Bio120909/Bio120910 for LSP, and Bio120911/Bio120912 for VPg. The recombinant vector TuMV VPg-pSPYCE was constructed using T4 DNA ligase. The recombinant vectors were confirmed by sequencing. Each experiment was performed in triplicate. The positive controls included the combination of bZIP63YN and bZIP63YC, and the negative controls were the YNE-empty and YCE-empty vectors. In addition, each partner and empty vector was used as controls.
The B. rapa protoplasts were prepared and cultured as described elsewhere62. The fresh leaves were obtained from Chinese cabbage plants at the four-leaf stage. The reagents used in the assays were 1.5% cellulase R10; 0.4% mecerozyme R10; 0.4 M D-mannitol; 20 mM KCl; 20 mM MES (pH 5.7); 10 mM CaCl2; 0.1% BSA; 5 mM β-mercaptoethanol, and the assays were conducted as indicated in the protocol62. The recombinant vectors [eIF(iso)4E-pSPYNE, TuMV VPg-pSPYCE, bZIP63YN, bZIP63YC, YNE-empty and YCE-empty, constructed in BiFC assays] were transfected into the protoplasts, the plant cells were cultured in the dark, and the fluorescence signals were assessed using a laser confocal scanning microscope after 24-h.
Equipment and settings
The three-dimensional (3D) structural of BraA.eIF(iso)4E.c-1 protein was modeled by Phyre263, and the model was displayed by Swiss-PdbViewer64.
In BiFC assays, the fluorescence signals were assessed in the protoplasts by laser confocal scanning microscope (LCSM)65. Fluorophores Compatible with the ZOE Fluorescent Cell Imaging System, YFP channel, excitation: 480/17 nm; emission: 517/23 nm; CytoTrack YFP 511/525; VivaFix 498/521 Cell Viability Assay. And the images were combined by Adobe Photoshop CS6 (https://helpx.adobe.com/creative-suite/kb/cs6-install- instructions.html).
Accession numbers
HQ446217 (TuMV C4), D83184 (TuMV CDN1), NC_002509 (TuMV UK1).
References
Ward, C. Q. & Shukla, D. D. Taxonomy of potyviruses: current problems and some solutions. Intervirology. 32, 269–296 (1991).
Kang, B. C., Yeam, I., Frantz, J. D., Murphy, J. F. & Jahn, M. M. Thepvr1 locus in Capsicum encodes a translation initiation factor eIF4E that interacts with Tobacco etch virus VPg. The Plant Journal. 42, 392–405 (2005).
Riechmann, J. L., Lain, S. & Garcia, J. A. Highlights and prospects of potyvirus molecular biology. Journal of General Virology. 73, 1–16 (1992).
Tomlinson, J. A. Epidemiology and control of virus diseases of vegetables. Annals of Applied Biology. 110, 661–681 (1987).
Walsh, J. A. & Jenner, C. E. Turnip mosaic virus and the quest for durable resistance. Molecular Plant Pathology. 3, 289–300 (2002).
Basso, J., Dallaire, P., Charest, P. J., Devantier, Y. & Laliberté, J. F. Evidence for an internal ribosome entry site within the 5′ non-translated region of turnip mosaic potyvirus RNA. The Journal of General Virology. 75, 3157–3165 (1994).
Siaw, M. F., Shahabuddin, M., Ballard, S., Shaw, J. G. & Rhoads, R. E. Identification of a protein linked to the 50 terminus of tobacco vein mottling virus RNA. Virology. 142, 134–43 (1985).
Plante, D. et al. Turnip mosaic virus VPg does not disrupt the translation initiation complex but interferes with cap binding. Physiological and Molecular Plant Pathology. 64, 219–226 (2004).
Gómez, P., Hernández, A. M. R., Moury, B. & Aranda, M. A. Genetic resistance for the sustainable control of plant virus diseases: breeding, mechanisms and durability. European Journal of Plant Pathology. 125, 1–22 (2009).
Dangl, J. L. Plant pathogens and integrated defence responses to infection. Nature 411, 826–833 (2001).
Carrington, J. C., Kasschau, K. D., Mahajan, S. K. & Schaad, M. C. Cell-to-cell and long-distance transport of viruses in plants. The Plant Cell. 8, 1669–1681 (1996).
Wang, A. & Krishnaswamy, S. Eukaryotic translation initiation factor 4E-mediated recessive resistance to plant viruses and its utility in crop improvement. Molecular Plant Pathology. 13, 795–803 (2012).
Fraser, R. S. S. The genetics of plant-virus interactions: implications for plant breeding. Euphytica. 63, 175–185 (1992).
Ayme, V. et al. Different mutations in the Genome-Linked Protein VPg of Potato virus Y confer virulence on the pvr2 3 resistance in pepper. Molecular Plant–Microbe Interactions. 9, 557–563 (2006).
Rusholme, R. L., Higgins, E. E., Walsh, J. A. & Lydiate, D. J. Genetic control of broad-spectrum resistance to turnip mosaic virus in Brassica rapa (Chinese cabbage). Journal of General Virology. 88, 3177–3186 (2007).
Qian, W. et al. Mapping and candidate-gene screening of the novel Turnip mosaic virus resistance generetr02 in Chinese cabbage (Brassica rapa L.). Theoretical and Applied Genetics. 126, 179–188 (2013).
Hashimoto, M., Neriya, Y., Yamaji, Y. & Namba, S. Recessive resistance to plant viruses: potential resistance genes beyond translation initiation factors. Frontiers in Microbiology, 7, e1001119 (2016).
Lellis, A. D., Kasschau, K. D., Whitham, S. A. & Carrington, J. C. Loss-of-susceptibility mutants of Arabidopsis thaliana reveal an essential role for eIF(iso)4E during potyvirus infection. Current Biology. 12, 1046–1051 (2002).
Ruffel, S. et al. A natural recessive resistance gene against potato virus Y in pepper corresponds to the eukaryotic initiation factor 4E (eIF4E). The Plant Journal. 32, 1067–1075 (2002).
Nieto, C. et al. An eIF4E allele confers resistance to an uncapped and non-polyadenylated RNA virus in melon. The Plant Journal. 48, 452–462 (2006).
Truniger, V., Nieto, C., González-Ibeas, D. & Aranda, M. A. Mechanism of plant eIF4E-mediated resistance against a Carmovirus (Tombusviridae): cap-independent translation of a viral RNA controlled in cis by an(a) virulence determinant. The Plant Journal. 56, 716–727 (2008).
Yoshii, M., Yoshioka, N., Ishikawa, M. & Naito, S. Isolation of an Arabidopsis thaliana mutant in which accumulation of cucumber mosaic virus coat protein is delayed. The Plant Journal. 13, 211–219 (1998).
Sato, M., Nakahara, K., Yoshii, M., Ishikawa, M. & Uyeda, I. Selective involvement of members of the eukaryotic initiation factor 4E family in the infection of Arabidopsis thaliana by potyviruses. FEBS Letters. 579, 1167–1171 (2005).
Gao, Z. et al. The potyvirus recessive resistance gene, sbm1, identifies a novel role for translation initiation factor eIF4E in cell-to-cell trafficking. The Plant Journal. 40, 376–385 (2004).
Albar, L. et al. Fine genetic mapping of a gene required for Rice yellow mottle virus cell-to-cell movement. Theoretical and Applied Genetics. 107, 371–378 (2003).
Albar, L. et al. Mutations in the eIF(iso)4G translation initiation factor confer high resistance of rice to Rice yellow mottle virus. The Plant Journal. 47, 417–426 (2006).
Stein, N. et al. The eukaryotic translation initiation factor 4E confers multiallelic recessive Bymovirus resistance in Hordeum vulgare. The Plant Journal. 42, 912–922 (2005).
Kanyuka, K. et al. Evidence that the recessive bymovirus resistance locusrym4 in barley corresponds to the eukaryotic translation initiation factor 4E gene. Molecular Plant Pathology. 6, 449–458 (2005).
Nicaise, V. et al. The eukaryotic translation initiation factor 4E controls lettuce susceptibility to the potyvirus Lettuce mosaic virus. Plant Physiology. 132, 1272–1282 (2003).
Ruffel, S., Gallois, J. L., Lesage, M. L. & Caranta, C. The recessive potyvirus resistance gene pot-1 is the tomato orthologue of the pepper pvr2-eIF4E gene. Molecular Genetics and Genomics. 274, 346–353 (2005).
Choi, S. H., Nakahara, K. S., Andrade, M. & Uyeda, I. Characterization of the recessive resistance genecyv1 of Pisum sativum against Clover yellow vein virus. Journal of general plant pathology. 78, 269–276 (2012).
Andrade, M., Abe, Y., Nakahara, K. S. & Uyeda, I. The cyv-2 resistance to Clover yellow vein virus in pea is controlled by the eukaryotic initiation factor 4E. Journal of General Plant Pathology. 75, 241–249 (2009).
Lee, J. H. et al. Single nucleotide polymorphisms in a gene for translation initiation factor (eIF4G) of rice (Oryza sativa) associated with resistance to Rice tungro spherical virus. Molecular Plant–Microbe Interactions. 23, 29–38 (2010).
Bruun-Rasmussen, M. et al. The same allele of translation initiation factor 4E mediates resistance against two Potyvirus spp. In Pisum sativum. Molecular Plant–Microbe Interactions. 20, 1075–1082 (2007).
Naderpour, M., Lund, O. S., Larsen, R. & Johansen, E. Potyviral resistance derived from cultivars of Phaseolus vulgaris carrying bc-3 is associated with the homozygotic presence of a mutated eIF4E allele. Molecular Plant Pathology. 11, 255–263 (2010).
Shopan, J. et al. Eukaryotic translation initiation factor 2B-beta (eIF2Bβ), a new class of plant virus resistance gene. The Plant Journal 90, 929–940 (2017).
Mayberry, L. K., Allen, M. L., Dennis, M. D. & Browning, K. S. Evidence for variation in the optimal translation initiation complex: plant eIF4B, eIF4F, and eIF(iso)4F differentially promote translation of mrnas. Plant Physiology 150, 1844–1854 (2009).
Kaye, N. M., Emmett, K. J., Merrick, W. C. & Jankowsky, E. Intrinsic RNA binding by the eukaryotic initiation factor 4F depends on a minimal RNA length but not on the m7G cap. Journal of Biological Chemistry 284, 17742–50 (2009).
Gallie, D. R. & Browning, K. S. eIF4G functionally differs from eIFiso4G in promoting internal initiation, cap-independent translation, and translation of structured mRNAs. Journal of Biological Chemistry. 276, 36951–36960 (2001).
Keller, K. E., Johansen, I. E., Martin, R. R. & Hampton, R. O. Potyvirus genome-linked protein (VPg) determines Pea seed borne mosaic virus pathotype-specific virulence in Pisum sativum. Molecular Plant–Microbe Interactions. 11, 124–130 (1998).
Masuta, C., Nishimura, M., Morishita, H. & Hataya, T. A single amino acid change in viral genome-associated protein of Potato virus Y correlates with resistance breaking in ‘Virgin A Mutant’ tobacco. Phytopathology. 89, 118–123 (1999).
Moury, B. et al. Mutations in potato virus Y genome linked protein determine virulence towards recessive resistances in Capsicum annuum and Lycopersicum hirsutum. Molecular Plant–Microbe Interactions. 17, 322–329 (2004).
Nicolas, O., Dunnington, S. W., Gotow, L. F., Pirone, T. P. & Hellmann, G. M. Variations in the VPg protein allow a potyvirus to overcome a gene resistance in tobacco. Virology. 237, 452–459 (1997).
Rajamaki, M. L. & Valkonen, J. P. Viral genome-linked protein (VPg) controls accumulation and phloem-loading of a potyvirus in inoculated potato leaves. Molecular Plant–Microbe Interactions. 15, 138–149 (2002).
Schaad, M. C., Lellis, A. D. & Carrington, J. C. VPg of tobacco etch potyvirus is a host genotype-specific determinant for long distance movement. Journal of Virology. 71, 8624–8631 (1997).
Wittmann, S., Chatel, H., Fortin, M. G. & Laliberte, J. F. Interaction of the viral protein genome linked of turnip mosaic potyvirus with the translational eukaryotic initiation factor (iso) 4E of Arabidopsis thaliana using the yeast two-hybrid system. Virology. 234, 84–92 (1997).
Léonard, S. et al. Complex formation between potyvirus VPg and translation eukaryotic initiation factor 4E correlates with virus infectivity. Journal of Virology. 74, 7730–7737 (2000).
Duprat, A. et al. The Arabidopsis eukaryotic initiation factor (iso)4E is dispensable for plant growth but required for susceptibility to potyviruses. The Plant Journal. 32, 927–934 (2002).
Gallois, J. L. et al. Single amino acid changes in the turnip mosaic virus viral genome-linked protein (VPg) confer virulence towards Arabidopsis thaliana mutants knocked out for eukaryotic initiation factors eIF(iso)4E and eIF(iso)4G. Journal of general virology. 91, 288–293 (2010).
Kim, J. et al. Transgenic Brassica rapa plants over-expressing eIF(iso)4E variants show broad-spectrum turnip mosaic virus (TuMV) resistance. Molecular Plant Pathology 15, 615–626 (2014).
Jenner, C. E., Nellist, C. F., Barker, G. C. & Walsh, J. A. Turnip mosaic virus (TuMV) is able to use alleles of both eIF4E and eIF(iso)4E from multiple loci of the diploid Brassica rapa. Molecular Plant–Microbe Interactions. 23, 1498–505 (2010).
Nellist, C. F. et al. Multiple copies of eukaryotic translation initiation factors in Brassica rapa, facilitate redundancy, enabling diversification through variation in splicing and broad-spectrum virus resistance. The Plant Journal 77, 261–268 (2014).
Li, G. L. et al. Analysis of the protein interaction of eIF(iso)4E.a/c with TuMV-C4/UK1 in Brassica rapa ssp. chinensis. Acta Horticulturae Sinica, 44, 1299–1308 (2017).
Monzingo, A. F. et al. The structure of eukaryotic translation initiation factor-4E from wheat reveals a novel disulfide bond. Plant physiology. 143, 1504–1518 (2007).
Wang, X. et al. The genome of the mesopolyploid crop species Brassica rapa. Nature genetics. 43, 1035–1039 (2011).
Dinkova, T. D., Martinez-Castilla, L. & Cruz-Espíndola, M. A. The Diversification of eIF4E Family Members in Plants and Their Role in the Plant-Virus Interaction. Evolution of the Protein Synthesis Machinery and ItsRegulation. Springer International Publishing. 187–205 (2016).
Miyoshi, H. et al. Binding analyses for the interaction between plant virus genome-linked protein (VPg) and plant translational initiation factors. Biochimie. 88, 329–340 (2006).
Joshi, B., Lee, K., Maeder, D. L. & Jagus, R. Phylogenetic analysis of eIF4E-family members. BMC Evolutionary Biology. 5, 1–20 (2005).
Li, G. L. et al. Development of gene-based markers for the Turnip mosaic virus resistance generetr02 in Brassica rapa. Plant Breeding. 135, 466–470 (2016).
Sambrock, J. & Russel, D. W. Molecular cloning: a laboratory manual (3rd edition). Immunology 49, 895–909 (2001).
Kudla, J., Xu, Q., Harter, K., Gruissem, W. & Luan, S. Genes for calcineurin b-like proteins in Arabidopsis are differentially regulated by stress signals. Proceedings of the National Academy of Sciences of the United States of America 96, 4718–23 (1999).
Ulrich, T. H., Chowdhury, J. B. & Widholm, J. M. Callus and root formation from mesophyll protoplasts of Brassica rapa. Plant Science Letters 19, 347–354 (1980).
Kelley, L. A. & Al, E. The phyre2 web portal for protein modeling, prediction and analysis. Nature Protocols 10, 845–858 (2015).
Guex, N. & Peitsch, M. C. Swiss-model and the swiss-pdbviewer: an environment for comparative protein modeling. Electrophoresis 18, 2714–23 (1997).
Minsky, M. Memoir on inventing the confocal scanning microscope. Scanning 10, 128–138 (2011).
Acknowledgements
We thank Professor John A. Walsh for providing the TuMV-UK1/CDN1 isolates as well as for his suggestions relating to the preparation of the manuscript. We would like to thank LetPub (www.letpub.com) for providing linguistic assistance during the preparation of this manuscript. This work was funded by the National Natural Science Foundation of China (31772302) and the National Key Research and Development Program of China (2017YFD0101802). This work was performed at the Key Laboratory of Biology and Genetic Improvement of Horticultural Crops, Ministry of Agriculture, Beijing, China.
Author information
Authors and Affiliations
Contributions
G.L., W.Q. and R.S. drafted manuscript; G.L., S.Z., F.L., S.Z., H.Z. and W.J. performed experiments; Z.F. and X.W. revised the manuscript; G.L., W.Q. and R.S. designed the research and approved final version of manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Li, G., Qian, W., Zhang, S. et al. Variability in eukaryotic initiation factor iso4E in Brassica rapa influences interactions with the viral protein linked to the genome of Turnip mosaic virus. Sci Rep 8, 13588 (2018). https://doi.org/10.1038/s41598-018-31739-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31739-1
Keywords
This article is cited by
-
Simultaneously induced mutations in eIF4E genes by CRISPR/Cas9 enhance PVY resistance in tobacco
Scientific Reports (2022)
-
Mapping and identification of a new potential dominant resistance gene to turnip mosaic virus in Brassica rapa
Planta (2022)
-
Bulked segregant RNA-sequencing (BSR-seq) identified a novel rare allele of eIF4E effective against multiple isolates of BaYMV/BaMMV
Theoretical and Applied Genetics (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.