Abstract
Heat waves have profoundly impacted biota globally over the past decade, especially where their ecological impacts are rapid, diverse, and broad-scale. Although usually considered in isolation for either terrestrial or marine ecosystems, heat waves can straddle ecosystems of both types at subcontinental scales, potentially impacting larger areas and taxonomic breadth than previously envisioned. Using climatic and multi-species demographic data collected in Western Australia, we show that a massive heat wave event straddling terrestrial and maritime ecosystems triggered abrupt, synchronous, and multi-trophic ecological disruptions, including mortality, demographic shifts and altered species distributions. Tree die-off and coral bleaching occurred concurrently in response to the heat wave, and were accompanied by terrestrial plant mortality, seagrass and kelp loss, population crash of an endangered terrestrial bird species, plummeting breeding success in marine penguins, and outbreaks of terrestrial wood-boring insects. These multiple taxa and trophic-level impacts spanned >300,000 km2—comparable to the size of California—encompassing one terrestrial Global Biodiversity Hotspot and two marine World Heritage Areas. The subcontinental multi-taxa context documented here reveals that terrestrial and marine biotic responses to heat waves do not occur in isolation, implying that the extent of ecological vulnerability to projected increases in heat waves is underestimated.
Similar content being viewed by others
Introduction
Recent dramatic ecological shifts in response to climate extremes have had profound societal impacts1 and have galvanized recognition of extreme climate events, rather than gradual, mean change, as the most conspicuous hand of climate change1,2. Of particular concern are short and extreme temperature anomalies spanning days to weeks, referred to collectively here as “heat waves”3. A terrestrial heat wave event can be identified as three or more consecutive days where the maximum temperature is over the 90th percentile4, whereas a marine heat wave is usually defined as a discrete, prolonged, anomalously warm water event in a particular location5. The land area affected by heat waves is expected to double by 2020 and quadruple by 20406. In combination with droughts, heat waves are also predicted to increase in frequency and magnitude, with climate models pointing at land–atmosphere coupling as a key reason for this exacerbation7,8. Increases in frequency are not restricted to terrestrial heat waves; marine heat waves now occur 4–5 times more often than in the 1980s9.
Heat waves can produce profound physiological consequences for flora and fauna2,10,11, triggering mortality, abrupt demographic and community-level disruptions, and ecosystem reconfigurations12,13,14 – as also reflected in well-documented spikes in human mortality during recent heat waves1,12. Despite the potential subcontinental scale of heat waves15, studies of heat wave effects on biota almost exclusively focus on physiological or phenological change to a single species or ecosystem type10,16,17,18. This precludes broader evaluation of their impacts, which could disrupt biota across taxonomic and evolutionary lineages, multiple trophic levels, and fundamentally different ecosystem types. In terrestrial ecosystems, research has focused on the combined effects of drought and heat on tree die-off17, with more recent studies pointing to the specific importance of heat waves16. However in marine ecosystems, heat waves alone are recognised as obvious and dramatic drivers of extensive coral bleaching and mortality19,20,21. Long-term, gradual climate-driven changes in growth chronology have been reported within individuals from both terrestrial and marine organism systems22. But, despite the potential for heat waves to straddle terrestrial and marine ecosystems concurrently15,23 their ability to simultaneously trigger ecological responses in both ecosystems at the ecoregion and sub-continental scale has received limited attention. Furthermore, although heat waves are explicitly discussed in terms of impacts on human populations in the most recent climate assessment reports, their full and detailed effects on ecosystems is lacking1,12. Given these gaps in reporting and knowledge, the full extent of ecological vulnerability to projected heat waves may be underestimated.
Following a heat wave event in early 2011, which straddled both the marine and terrestrial ecosystems of Western Australia, we aimed to document, using a meta-analytic framework, the pervasive ecological effect of a climate change-induced extreme event, highlight the breadth of taxa affected, and quantify demographic change in the abundance and mortality rates. We predicted that the heat wave could cause the loss of foundation species, demographic shifts, as well as alter the composition, structure and function of ecosystems, and change species distributions across a wide range of taxa. That is, overall, some taxa would respond positively, others negatively. Organisms present prior to the event (sessile species, long-lived vagile taxa) were assumed a priori to be negatively impacted, whereas vagile consumers that were not present or rare pre-heat wave were presumed to be neutral or increasing following a heat wave event.
Results
Climatic event
The terrestrial maximum temperatures extending from 25°S at Shark Bay to 34°S at Cape Leeuwin for all of March 2011, were 2 °C higher than the long-term March average over the period 1971–2000 baseline (Fig. 1a). Minimum temperatures were also higher than average (see Fig. S1). At finer temporal and spatial scales, weekly maximum temperature near the Western Australian city of Perth (32°S, 116°E) exceeded long-term means by ~5 °C (Fig. 1b). The number of heat wave days in 2011 was the highest, and the Standardized Precipitation Evapotranspiration Index (SPEI)24 was the lowest on record since 1960 (Fig. S2a,b). The terrestrial heat wave coincided with a drought characterized by an extremely dry 2010 winter (40–50% below the average rainfall25) and a 30-year pronounced drying trend of reduced winter rainfall (14% decline)26.
Temperature anomalies for Western Australia in early 2011. (a) Maximum temperature anomaly over land from gridded observations63 for March 2011 relative to March 1971–2000. Sea Surface Temperature (SST) anomaly from a combined in-situ and satellite derived product67 for March 2011 relative to 1971–2000. (b), Weekly mean maximum temperatures during 2011 (red dots), and the mean over 1971–2000 (blue dots) near Perth (32 °S, 116 °E) as shown by the black dot in panel (a,c) Weekly mean SST during 2011 and the mean over 1990–2010 off the coast of Western Australia (−32.5 °S, 115.5 °E). Figure was created with the NCAR Command Language (Version 6.4.0) [Software]. (2017). Boulder, Colorado: UCAR/NCAR/CISL/TDD. http://dx.doi.org/10.5065/D6WD3XH5.
The conditions of the terrestrial heat wave were mirrored in marine conditions, and coincided with a near-record strength Leeuwin Current and one of the strongest La Niña events on record3. Sea surface temperatures (SST) were abnormally high27 and also nested within a long-term increase in mean SST for Western Australia28. The SSTs for March 2011 were 2–2.5 °C higher compared with the long-term March average over the period 1971–2011 (Fig. 1a) and close to the coast, weekly temperatures were 3–3.5 °C above long term averages (1990–2010) for that time of year (Fig. 1c).
Biotic response
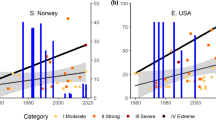
The subcontinental heat wave event triggered statistically significant, abrupt, and synchronous (i.e. occurring within 1–2 seasons) biotic disruptions in both terrestrial and marine ecosystems, including mortality, demographic shifts and altered species distributions (Fig. 2a,b). Of 19 terrestrial quantitative contrasts, 17 (89%) were significant and all were in the expected direction (Fig. 2a). Of 20 marine quantitative contrasts, 14 (70%) were significant and all but one were in the hypothesised direction (Fig. 2b).
Heat wave-impacted organisms in terrestrial and marine ecosystems in Western Australia, 2011. (a,b) consistent with expected, (c–f) examples from species that decreased, and (g,h) increased. Blue dots and confidence interval (CI) lines denote prior to heat wave, orange dots and CI lines denote following the heat wave. Note that these are different types of responses (e.g. cover change, mortality) and are not connected in a cascade or food web, but are separate examples. Inset photos are an example of each type: (c) 12 species of trees plus 3 shrubs (photo credit: K. Ruthrof), (d) 3 species of seagrass/kelp (T. Wernberg), (e) 1 species of cockatoo (L. Valentine), (f) 1 species of penguin (B. Cannell), (g) 1 species of wood boring insect (G. Matusick) and, (h) 3 species of fish (T. Wernberg).
When further refined into trophic levels, primary producers in both terrestrial (Fig. 2c) and marine (Fig. 2d) ecosystems showed decreases in both survival and cover, in response to the heat wave. Consumers were subdivided a priori into groups to differentiate those expected to decrease in abundance following a heat wave (sessile organisms, long-lived vertebrates) from those expected to increase in abundance or distribution (e.g. tropical fish). Consumers expected to be negatively affected by the heat wave did so for both the terrestrial (Fig. 2e) and marine (Fig. 2f) ecosystems (abundance of the endangered cockatoo Calyptorhynchus latirostris, and breeding success of penguins Eudyptula minor declined). Similarly, consumers expected to increase in abundance did so for both the terrestrial (Fig. 2g) and marine (Fig. 2h) ecosystems (outbreaks of the wood boring insect Phoracantha semipunctata in response to dying trees) reinforcing support for the pervasive effect of the heat wave in both biomes. Further underscoring the scale of the event, taxa from a breadth of lineages were impacted by the heat wave (Table 1; species-specific responses by study are provided in Extended Data Table 1), providing additional evidence for the permeating effects of the heat wave.
The magnitude of changes documented was substantial. Mean shrub and tree mortality (including Beaufortia elegans, Eucalyptus marginata and Corymbia calophylla) was 19% following the heat wave (Fig. 2a), greatly exceeding the magnitude of background mortality (e.g. for Eucalyptus marginata, reported locally: 0.02–2.42%/yr29) and for forests elsewhere30. Annual counts of endangered Carnaby’s Black Cockatoo (Calyptorhynchus latirostris), have to date, not recovered from the substantial decline (60%) observed (Fig. 2; Extended Data Table 1). Our quantitative results are reinforced by other qualitative studies indicating sub-lethal heat wave impacts, including partial tree canopy dieback31, coral bleaching32, decline in health status of turtles (Chelonia mydas)18, and altered lobster (Panulirus cygnus) behaviour33.
Discussion
The results of our meta-analytic approach illustrate a broad range of diverse and pervasive biotic disruptions caused by a heat wave, reflecting strong spatial coherence between climate signal and ecological response. These include loss of foundation, habitat-forming species such as trees (e.g. Eucalyptus marginata and Corymbia calophylla16), corals (Acropora34), algae35, critical reductions in ionic vertebrate species such as an endangered cockatoo (Calyptorhynchus latirostris36), and penguins (Eudyptula minor37), increased abundance of fishes (e.g. Labracinus lineatus35), and an outbreak of wood boring insects (Phoracantha semipunctata38). Specifically, negative impacts of the heat wave were anticipated for taxa where individuals were physically present during the heat wave. For example, all sessile organisms, as well as longer-lived vertebrates such as Little Penguins (Eudyptula minor) and Carnaby’s Black Cockatoos (Calyptorhynchus latirostris). In contrast, a number of species increased dramatically from low numbers (tropical fish and wood boring beetles). The attribute of a species population being present prior to the heat wave provided a clear and useful way in which to consider heat wave response syndromes39. Qualitative studies describing responses to the heat wave also reinforced our quantitative results (Extended Data Table 1). Ecological changes triggered by heat waves like those we document here are likely to cause fundamental disruptions in the structure40, function41, and distribution of species leading to phase shifts42 or cascades43 to alternate ecological states with long-term consequences for ecosystem services44. For example, following the heat wave discussed in this study, 36% of seagrass meadows were damaged in Shark Bay, a world heritage listed area, and an estimated 2–9 Tg CO2 may have been released to the atmosphere44. Similarly, the 2003 heat wave in Europe resulted in significant disruptions to productivity across multiple forest ecosystems45,46, and the heat wave in 2012 in the NW Atlantic Ocean which led to marine species shifting their geographic distribution and seasonal cycles23. Additional flow-on effects include changes to key ecological processes, such as herbivory14,35,42,47, which maintained altered habitats, reduced resilience of tree populations to pests and pathogens38, altered forest structure40,48, and increased proximal predicted rate of wildfire spread49. As the frequency and spatial extent of heat waves continues to increase1,50, recovery times and persistence opportunities for many of these species may be further impacted.
Collectively, these results show a consistent, synchronous large spatial and taxonomic response throughout a terrestrial-marine ecoregion. We infer this from the spatial coherence of observations and consistency of findings across taxa, trophic groups, and ecosystems types within 1–2 seasons following the heat wave. Our results do not enable us to evaluate more detailed questions about the precise causality of responses to the heat wave, or the longer-term impacts or resilience. The rapid response of many taxa to heat stress indicate changes in abundance and condition are likely a direct consequence of the heat wave. However, for species with protracted change we are unable to determine if this is a direct response to the heat wave or an indirect response to changes in resource availability. Our results are consistent with some expected cross-taxa sensitivities, particularly for terrestrial woody plant species for which mortality increased with lifeforms progressing towards more mesic-affiliated taxa, from heathland shrubs (~2% mortality) to heathland trees (~18%) to woodland trees (~25%) to forest trees (~49%; Table 1). The coherence of marine and terrestrial responses to the heat wave also are suggestive that tree mortality patterns, while likely predisposed by drought, appear to have been triggered by the heat wave itself. Observations of changes in crown health corresponded with a prolonged heat wave in late February 201116. These findings provide a foundation on which to build future experimental and observational studies regarding the specific nature of heat wave impacts, while simultaneously providing evidence for the potential of heat waves to trigger pervasive and spatially extensive biotic disruptions.
Understanding the coherence of the marine and terrestrial heat wave requires an appreciation of large-scale atmospheric and oceanic climatic drivers. Sea surface temperatures (SSTs) along the west coast of Australia are linked to the El Niño Southern Oscillation (ENSO), which influences the strength of southerly flowing currents via the Indonesian Flow Through. La Nina years are associated with a stronger Leeuwin current and warmer tropical SSTs at high latitude temperate reefs51,52. During the 2010–2011 Austral summer, an exceptionally strong La Nina event and northerly winds resulted in a surge in the Leeuwin current and abnormally high SSTs temperatures along the ocean margins of southwestern Australia53. In the terrestrial system, the warmer and dryer than average conditions in southwestern Australia leading up to the 2010–2011 event, follow a long-term warming and drying trend. While the warming trend has been largely attributed to increased anthropogenic greenhouse gas emissions26,54, the cause of the consistent reduction in rainfall since the 1970s is not as clear, with numerous studies identifying several factors as likely contributors. This includes natural variability55, changes in ocean temperatures56,57, land-use change58, a southern shift of storm tracks59, as well as snowfall increases in coastal east Antarctica60. The latest global climate projections61 and recent high resolution regional climate projections62 show a statistically significant decline in winter rainfall across southwestern Australia linked to few rain bearing fronts traversing the region, consistent with a southern shift of storm tracks59. However, compound terrestrial and marine heat waves in this region, and the degree to which they could be linked along a given terrestrial-marine continental boundary, remains uncertain. Nonetheless, the coherence of the marine and terrestrial patterns seen in the 2011 event is striking, and future evaluation of potential linkages is warranted.
The biotic disruptions that we document are notable in each of five aspects, in that our results: (1) build from a focus on single or co-dominant species studies10,16,17,18 to document responses across broad taxonomic lineages; (2) extend beyond physiological1,11 or phenological1 change to demographic disruptions; (3) move beyond focus on gradual ecological changes12,22,30 to specifically assess abrupt ecological change; (4) quantify changes from individual locations16,17,18 to spanning up to sub-continental scale; and (5) break down silos across historic disciplinary boundaries between marine13,14,18,19,35,42,47 and terrestrial11,16 ecology. That our results simultaneously show biotic responses in all five of these aspects provides evidence for the pervasive ecological vulnerability to a climate change-induced extreme event. We have documented and highlighted the breadth of taxa affected and quantified demographic change in abundance and mortality rates. Collectively, our results imply that the full extent of ecological vulnerability to projected heat waves is grossly underestimated.
Methods
We evaluated the ecological responses associated with a subcontinental heat wave in 2010–2011 across an ecoregion of adjoining terrestrial and marine ecosystems along the Western Australian coast. To characterize physical heat wave conditions in both terrestrial and marine ecosystems, we compared 2011 temperature conditions with long-term averages (1971–2000). Impact on terrestrial and marine biota (N = 30 taxa) was then assessed via assimilating published reports and summarising using a meta-analytic framework.
Climatic Data. The daily maximum and minimum temperature and precipitation dataset used in this study is the Australian Bureau of Meteorology’s gridded observational product. The dataset has a resolution of 5 km and represents an interpolation from a network of weather stations across Australia, employing topography-resolved analysis methods to minimize uncertainty in fitting a surface to observations63. This dataset was chosen as it is the most reliable and widely used by numerous studies which focus on heat waves in Australia, e.g.4,64,65,66.
A terrestrial heat wave event within the climate record (1960–2014) was identified as three consecutive days during Austral summer months (November to March) where the maximum temperature was over the 90th percentile threshold (based on a 15-day analysis window). These calculations followed the ‘CTX90pct’ method4, a method well suited to capturing trends in heat waves in Australia. Heat wave days are calculated as the sum of all summer days that were identified in a heat wave event for a particular summer period (i.e. November 2010 to March 2011 was assigned as 2011 summer).
Sea Surface Temperature (SST) data are from Reynolds et al.67. This dataset combines in-situ observations and remotely sensed estimates to produce a SST dataset at a 1 by 1 degree resolution and is suitable for monitoring of weather and climate on a weekly time-scale since the 1990s. Whilst there are other blended in-situ and remotely sensed SSTs datasets such as that of Rayner et al.68, these are only available on a monthly time-scale, which was judged too coarse for capturing heat wave events. Therefore the dataset of Reynolds et al.67 was chosen because of its higher frequency and previous use in heat wave studies, e.g5,69.
Biotic Data. Our aim was to quantify pre- to post-heat wave demographic change in the abundance or mortality rates of individual taxa (see Extended Data Table 1 for detailed description of metrics). To quantify the magnitude of ecological impacts from the heat wave, data were sourced from peer-reviewed publications, government reports, and unpublished sources (Extended Data Table 1). Authors used the peer-reviewed literature, local knowledge, and professional contacts to identify data sources and develop a dataset of heat wave disruptions, encompassing 45 records of 30 terrestrial and marine taxa from within the impacted ecoregion. For marine data, a workshop was held shortly after the heat wave event33 and evidence of impacts, both quantitative and qualitative, were compiled. We relied on this report and participants’ subsequent publications for marine-based data. For terrestrial impacts, most data were unpublished and contributed by co-authors. To merit inclusion, data on heat wave impacts had to include information from October 2010 to July 2011 (start of the marine heat wave and end of the terrestrial heat wave responses), include an unimpacted contrast (typically prior measurement), and be from the impacted region of Western Australia (marine: latitudes 20–34 °S; terrestrial: 25–34 °S; Fig. 1, Fig S1). All such available data were included: taxa and data were not selected on the basis of an observed impact and were treated as a sample of taxa measured during the climate event. How the taxa examined were selected for survey in the first place varied for each study, typically species were keystone species, ecosystem dominants or threatened taxa. To be included, quantitative data had to report a pre to post comparison (10 studies) or a space for time substitution (1 study; Extended Data Table 1).
Data were extracted from studies recording taxon, location, mean, error, and sample size for pre and post heat wave measurements. Data were demographic in nature, including mortality rates of individual plants or changes in population abundance (cover of plants, mortality rates of individual plants, counts of animals; Extended Data Table 1). Where data included multiple pre-heat wave measurements we combined prior data or retained only the previous year (where time scales >2 years prior). Where studies included multiple taxa, spanned a major geographic divide (island groups) or distance (>100 km), we recorded each comparison separately (Extended Data Table 1). Taxa affected were categorized in relation to their ecosystem function (producers: photosynthetic organisms including algae, seagrass, coral, shrubs, and trees; consumers: non-photosynthetic organisms). We further divided organisms based on their pre-heat wave prevalence/abundance and life history attributes of distribution (tropical/temperate) and disturbance response (positive/negative). This resulted in taxa classified in terms of expected positive (increaser) or negative (decreaser) response to a heat wave. In all cases, we relativised data within contrasts to set the pre-heat wave levels to an equivalent point to facilitate comparisons.
Impact data varied among studies depending on the initial design of the survey and the nature of the taxon in question. All parameters had a direct bearing on population response, and included: survival, abundance, range change or fecundity. Biotic disruption records were aggregated by trophic levels (primary vs. secondary), producers or consumers where taxa were classified a priori expected to decrease in abundance following the heat wave from those expected to increase in abundance or distribution (range extension). We expressed all biotic disruptions as percent change post-event, and normalized all pre heat wave values to zero, thereby permitting assessment of heat wave impacts and comparison across taxa and disparate units of measurement (similar to other meta-analytic studies where the outcome measures from different experiments are standardised and put on the same scale70). Means and 95% confidence intervals by functional group were calculated using a mean weighted by the number of independent sample units in order to facilitate pre to post comparisons. Lack of confidence interval overlap was interpreted as strong evidence of a difference between groups, and confidence interval overlap but not including the mean was interpreted as moderate evidence71. The datasets generated during and/or analysed during the current study are available from the corresponding author on request.
References
IPCC. Managing the risks of extreme events and disasters to advance climate change adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change. 582 (Cambridge University Press, Cambridge, UK, and New York, NY, USA, 2012).
Smith, M. D. The ecological role of climate extremes: current understanding and future prospects. J. Ecol. 99, 651–655, https://doi.org/10.1111/j.1365-2745.2011.01833.x (2011).
Pearce, A. F. & Feng, M. The rise and fall of the “marine heat wave” off Western Australia during the summer of 2010/2011. J. Mar. Syst. 111, 139–156, https://doi.org/10.1016/j.jmarsys.2012.10.009 (2013).
Perkins, S. E. & Alexander, L. V. On the measurement of heat waves. J. Clim. 26, 4500–4517, https://doi.org/10.1175/jcli-d-12-00383.1 (2013).
Hobday, A. et al. A hierarchical approach to defining marine heatwaves. Progress in Oceanography 141, 227–238, https://doi.org/10.1016/j.pocean.2015.12.014 (2016).
Coumou, D. & Robinson, A. Historic and future increase in the global land area affected by monthly heat extremes. Environ. Res. Lett. 8, 6, https://doi.org/10.1088/1748-9326/8/3/034018 (2013).
Rasmijn, L. M. et al. Future equivalent of 2010 Russian heatwave intensified by weakening soil moisture constraints. Nat. Clim. Chang. 8, 381–+, https://doi.org/10.1038/s41558-018-0114-0 (2018).
Miralles, D. G., Gentine, P., Seneviratne, S. I. & Teuling, A. J. Land–atmospheric feedbacks during droughts and heatwaves: state of the science and current challenges. Annals of the New York Academy of Sciences, Special Issue: Climate Sciences, 1–17, https://doi.org/10.1111/nyas.13912 (2018).
Hughes, T. P. et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359, 80–+, https://doi.org/10.1126/science.aan8048 (2018).
Smale, D. A. & Wernberg, T. Extreme climatic event drives range contraction of a habitat-forming species. Proc. R. Soc. B-Biol. Sci. 280, 9, https://doi.org/10.1098/rspb.2012.2829 (2013).
Teskey, R. et al. Responses of tree species to heat waves and extreme heat events. Plant Cell Environ. 38, 1699–1712, https://doi.org/10.1111/pce.12417 (2015).
IPCC. Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. 1132 (Cambridge University Press, Cambridge, UK and New York, NY, USA, 2014).
Ainsworth, T. D. et al. Climate change disables coral bleaching protection on the Great Barrier Reef. Nature 352, 338–342, https://doi.org/10.1126/science.aac7125 (2016).
Wernberg, T. et al. Climate-driven regime shift of a temperate marine ecosystem. Science 353, 169–172, https://doi.org/10.1126/science.aad8745 (2016).
Feudale, L. & Shukla, J. Role of Mediterranean SST in enhancing the European heat wave of summer 2003. Geophys. Res. Lett. 34, 4, https://doi.org/10.1029/2006gl027991 (2007).
Matusick, G., Ruthrof, K. X., Brouwers, N. C., Dell, B. & Hardy, G. S. Sudden forest canopy collapse corresponding with extreme drought and heat in a mediterranean-type eucalypt forest in southwestern Australia. European Journal of Forest Research 132, 497–510, https://doi.org/10.1007/s10342-013-0690-5 (2013).
Allen, C. D. et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manage. 259, 660–684, https://doi.org/10.1016/j.foreco.2009.09.001 (2010).
Thomson, J. A. et al. Extreme temperatures, foundation species, and abrupt ecosystem change: an example from an iconic seagrass ecosystem. Glob. Change Biol. 21, 1463–1474, https://doi.org/10.1111/gcb.12694 (2015).
Hughes, T. P. et al. Global warming and recurrent mass bleaching of corals. Nature 543, 373–+, https://doi.org/10.1038/nature21707 (2017).
Brown, B. E. Coral bleaching: causes and consequences. Coral Reefs 16, S129–S138, https://doi.org/10.1007/s003380050249 (1997).
Glynn, P. W. Coral-reef bleaching in the 1980s and possible connections with global warming. Trends Ecol. Evol. 6, 175–179, https://doi.org/10.1016/0169-5347(91)90208-f (1991).
Ong, J. J. L. et al. Evidence for climate-driven synchrony of marine and terrestrial ecosystems in northwest Australia. Glob. Change Biol. 22, 2776–2786, https://doi.org/10.1111/gcb.13239 (2016).
Mills, K. E. et al. Fisheries Management in a Changing Climate Lessons from the 2012 Ocean Heat Wave in the NorthwestAtlantic. Oceanography 26, 191–195, https://doi.org/10.5670/oceanog.2013.27 (2013).
Vicente-Serrano, S. M., Begueria, S. & Lopez-Moreno, J. I. A multiscalar drought index sensitive to global warming: the standardized precipitation evapotranspiration index. J. Clim. 23, 1696–1718, https://doi.org/10.1175/2009jcli2909.1 (2010).
BOM. Western Australia in 2010: A very dry year in southwest Western Australia. Commonwealth of Australia 2011, Bureau of Meteorology Available from, http://www.bom.gov.au/climate/current/annual/wa/archive/2010.summary.shtml. Tuesday, 4 January 2011 - Annual Climate Summary for Western Australia - Product code IDCKGC21R0. Accessed 04 July 2018, 2011).
Bates, B. C., Hope, P., Ryan, B., Smith, I. & Charles, S. Key findings from the Indian Ocean Climate Initiative and their impact on policy development in Australia. Clim. Change 89, 339–354, https://doi.org/10.1007/s10584-007-9390-9 (2008).
Feng, M. et al. Decadal increase in Ningaloo Nino since the late 1990s. Geophys. Res. Lett. 42, 104–112, https://doi.org/10.1002/2014gl062509 (2015).
Zinke, J. et al. Corals record long-term Leeuwin current variability including Ningaloo Nino/Nina since 1795. Nat. Commun. 5, 9, https://doi.org/10.1038/ncomms4607 (2014).
Abbott, I. & Loneragan, O. Ecology of jarrah (Eucalyptus marginata) in the northern jarrah forest of Western Australia. Western Australia Department of Conservation and Land Management Bulletin, I–XVI, 1–118 (1986).
van Mantgem, P. J. et al. Widespread increase of tree mortality rates in the western United States. Science 323, 521–524, https://doi.org/10.1126/science.1165000 (2009).
Matusick, G., Ruthrof, K. X. & Hardy, G. Drought and heat triggers sudden and severe dieback in a dominant mediterranean-type woodland species. Open Journal of Forestry 2, 183–186 (2012).
Moore, J. A. Y. et al. Unprecedented Mass Bleaching and Loss of Coral across 12 degrees of Latitude in Western Australia in 2010–11. PLoS One 7, 11, https://doi.org/10.1371/journal.pone.0051807 (2012).
Pearce, A. et al. The “marine heat wave” off Western Australia during the summer of 2010/11. 40 (Fisheries Research Division Western Australian Fisheries and Marine Research Laboratories, North Beach, Western Australia, 2011).
Depczynski, M. et al. Bleaching, coral mortality and subsequent survivorship on a West Australian fringing reef. Coral Reefs 32, 233–238, https://doi.org/10.1007/s00338-012-0974-0 (2013).
Wernberg, T. et al. An extreme climatic event alters marine ecosystem structure in a global biodiversity hotspot. Nat. Clim. Chang. 3, 78–82, https://doi.org/10.1038/nclimate1627 (2013).
Byrne, M., Barrett, G., Blythman, M., Finn, H. & Williams, M. Cocky Count: a community-based survey for Carnaby’s Black-Cockatoo (Calyptorhynchus latirostris) and Forest Red-tailed Black-Cockatoo (Calyptorhynchus banksii naso). (BirdLife Australia, Floreat, Western Australia, 2015).
Cannell, B. L., Chambers, L. E., Wooller, R. D. & Bradley, J. S. Poorer breeding by little penguins near Perth, Western Australia is correlated with above average sea surface temperatures and a stronger Leeuwin Current. Mar. Freshw. Res. 63, 914–925, https://doi.org/10.1071/mf12139 (2012).
Seaton, S., Matusick, G., Ruthrof, K. X. & Hardy, G. Outbreak of Phoracantha semipunctata in response to severe drought in a mediterranean eucalyptus forest. Forests 6, 3868–3881, https://doi.org/10.3390/f6113868 (2015).
Green, R. H. Sampling design and statistical methods for environmental biologists. (Wiley, 1979).
Matusick, G., Ruthrof, K. X., Fontaine, J. B. & Hardy, G. E. S. Eucalyptus forest shows low structural resistance and resilience to climate change-type drought. J. Veg. Sci. 27, 493–503, https://doi.org/10.1111/jvs.12378 (2016).
Gazol, A. et al. Beneath the canopy: Linking drought-induced forest die off and changes in soil properties. For. Ecol. Manage. 422, 294–302, https://doi.org/10.1016/j.foreco.2018.04.028 (2018).
Verges, A. et al. The tropicalization of temperate marine ecosystems: climate-mediated changes in herbivory and community phase shifts. Proc. R. Soc. B-Biol. Sci. 281, 10, https://doi.org/10.1098/rspb.2014.0846 (2014).
Rodriguez-Castaneda, G. The world and its shades of green: a meta-analysis on trophic cascades across temperature and precipitation gradients. Glob. Ecol. Biogeogr. 22, 118–130, https://doi.org/10.1111/j.1466-8238.2012.00795.x (2013).
Arias-Ortiz, A. et al. A marine heatwave drives massive losses from the world’s largest seagrass carbon stocks. Nat. Clim. Chang. 8, 338–+, https://doi.org/10.1038/s41558-018-0096-y (2018).
Jolly, W. M., Dobbertin, M., Zimmermann, N. E. & Reichstein, M. Divergent vegetation growth responses to the 2003 heat wave in the Swiss Alps. Geophys. Res. Lett. 32, 4, https://doi.org/10.1029/2005gl023252 (2005).
Martinez-Meier, A., Sanchez, L., Pastorino, M., Gallo, L. & Rozenberg, P. What is hot in tree rings? The wood density of surviving Douglas-firs to the 2003 drought and heat wave. For. Ecol. Manage. 256, 837–843, https://doi.org/10.1016/j.foreco.2008.05.041 (2008).
Wernberg, T. et al. Impacts of climate change in a global hotspot for temperate marine biodiversity and ocean warming. J. Exp. Mar. Biol. Ecol. 400, 7–16, https://doi.org/10.1016/j.jembe.2011.02.021 (2011).
Cobb, R. et al. Ecosystem Dynamics and management after forest die-off: a global synthesis with conceptual state-and-transition models. Ecosphere, 116.00463755800 (2017).
Ruthrof, K. X. et al. How drought-induced forest die-off alters microclimate and increases fuel loadings and fire potentials. Int. J. Wildland Fire 25, 819–830, https://doi.org/10.1071/wf15028 (2016).
Cowan, T. et al. More frequent, longer, and hotterheat waves for Australia in the twenty-first century. J. Clim. 27, 5851–5871, https://doi.org/10.1175/jcli-d-14-00092.1 (2014).
Pearce, A. F. & Phillips, B. F. ENSO events, the Leeuwin current, and larval recruitment of the Western Rock Lobster. Journal Du Conseil 45, 13–21 (1988).
Feng, M., Meyers, G., Pearce, A. & Wijffels, S. Annual and interannual variations of the Leeuwin Current at 32 degrees S. J. Geophys. Res.-Oceans 108, 21, https://doi.org/10.1029/2002jc001763 (2003).
Feng, M., McPhaden, M. J., Xie, S. P. & Hafner, J. La Nina forces unprecedented Leeuwin Current warming in 2011. Sci Rep 3, 9, https://doi.org/10.1038/srep01277 (2013).
IOCI. Climate variability and change in south west Western Australia (Department of Environment Perth, Western Australia, 2002).
Cai, W. J. & Cowan, T. SAM and regional rainfall in IPCC AR4 models: Can anthropogenic forcing account for southwest Western Australian winter rainfall reduction? Geophys. Res. Lett. 33, https://doi.org/10.1029/2006gl028037 (2006).
England, M. H., Ummenhofer, C. C. & Santoso, A. Interannual rainfall extremes over southwest Western Australia linked to Indian ocean climate variability. J. Clim. 19, 1948–1969, https://doi.org/10.1175/jcli3700.1 (2006).
Ummenhofer, C. C., Sen Gupta, A., Pook, M. J. & England, M. H. Anomalous rainfall over southwest Western Australia forced by Indian Ocean sea surface temperatures. J. Clim. 21, 5113–5134, https://doi.org/10.1175/2008jcli2227.1 (2008).
Pitman, A. J., Narisma, G. T., Pielke, R. A. & Holbrook, N. J. Impact of land cover change on the climate of southwest Western Australia. J. Geophys. Res.-Atmos. 109, 12, https://doi.org/10.1029/2003jd004347 (2004).
Frederiksen, J. S. & Frederiksen, C. S. Interdecadal changes in southern hemisphere winter storm track modes. Tellus Ser. A-Dyn. Meteorol. Oceanol. 59, 599–617, https://doi.org/10.1111/j.1600-0870.2007.00264.x (2007).
van Ommen, T. D. & Morgan, V. Snowfall increase in coastal East Antarctica linked with southwest Western Australian drought. Nat. Geosci. 3, 267–272, https://doi.org/10.1038/ngeo761 (2010).
Hope, P. et al. Seasonal and regional signature of the projected southern Australian rainfall reduction. Aust. Meteorol. Oceanogr. J. 65, 54–71, https://doi.org/10.22499/2.6501.005 (2015).
Andrys, J., Kala, J. & Lyons, T. J. Regional climate projections of mean and extreme climate for the southwest of Western Australia (1970–1999 compared to 2030–2059). Clim. Dyn. 48, 1723–1747, https://doi.org/10.1007/s00382-016-3169-5 (2017).
Jones, D. A., Wang, W. & Fawcett, R. High-quality spatial climate data-sets for Australia. Aust. Meteorol. Oceanogr. J. 58, 233–248 (2009).
Cai, W., Cowan, T. & Raupach, M. Positive Indian Ocean Dipole events precondition southeast Australia bushfires. Geophys. Res. Lett. 36, https://doi.org/10.1029/2009gl039902 (2009).
Purich, A. et al. Atmospheric and oceanic conditions associated with southern Australian heat waves: a CMIP5 analysis. J. Clim. 27, 7807–7829, https://doi.org/10.1175/jcli-d-14-00098.1 (2014).
Perkins, S. E., Argueso, D. & White, C. J. Relationships between climate variability, soil moisture, and Australian heatwaves. J. Geophys. Res.-Atmos. 120, 8144–8164, https://doi.org/10.1002/2015jd023592 (2015).
Reynolds, R. W., Rayner, N. A., Smith, T. M., Stokes, D. C. & Wang, W. Q. An improved in situ and satellite SST analysis for climate. J. Clim. 15, 1609–1625, https://doi.org/10.1175/1520-0442(2002)015<1609:aiisas>2.0.co;2 (2002).
Rayner, N. A. et al. Global analyses of sea surface temperature, sea ice, and night marine air temperature since the late nineteenth century. J. Geophys. Res.-Atmos. 108, 37, https://doi.org/10.1029/2002jd002670 (2003).
Feudale, L. & Shukla, J. Influence of sea surface temperature on the European heat wave of 2003 summer. Part I: an observational study. Clim. Dyn. 36, 1691–1703, https://doi.org/10.1007/s00382-010-0788-0 (2011).
Gurevitch, J., Koricheva, J., Nakagawa, S. & Stewart, G. Meta-analysis and the science of research synthesis. Nature 555, 175–182, https://doi.org/10.1038/nature25753 (2018).
Ramsey, F. L. & Schafer, D. W. The Statistical Sleuth. (Duxbury, 2002).
Acknowledgements
The authors thank Prof. D. Tissue and Dr. C. Allen for discussions and comments on the manuscript. K.X.R., G.M., G.E.St.J.H. were supported through the Western Australian State Centre of Excellence for Climate Change, Woodland and Forest Health, which is a partnership between private industry, community groups, Universities and the Government of Western Australia. D.D.B. was supported by a Sir Walter Murdoch Distinguished Collaborator Award from Murdoch University, the U.S. National Science Foundation (EF-1340649 and EF-1550756), the Consortium for Arizona-Mexico Arid Environments, and the Arizona Agriculture Experiment Station. J.K. is supported by an Australian Research Council Discovery Early Career Researcher Grant (DE170100102).
Author information
Authors and Affiliations
Contributions
K.X.R. and D.D.B. co-led the study; K.X.R. and G.M. conceived and initiated the study; K.X.R., D.D.B., J.B.F., R.H.F. and B.P.M. produced the first draft; K.X.R., J.B.F., G.M., B.P.M., N.J.E. and G.E.St.J.H. collected vegetation data; and J.B.F., J.K. and P.J.M. analysed data. All authors, including S.K.W., M.K., D.J.L., T.W., contributed to the writing of subsequent drafts of the paper.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ruthrof, K.X., Breshears, D.D., Fontaine, J.B. et al. Subcontinental heat wave triggers terrestrial and marine, multi-taxa responses. Sci Rep 8, 13094 (2018). https://doi.org/10.1038/s41598-018-31236-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31236-5
This article is cited by
-
Global field observations of tree die-off reveal hotter-drought fingerprint for Earth’s forests
Nature Communications (2022)
-
Declining pollination success reinforces negative climate and fire change impacts in a serotinous, fire-killed plant
Plant Ecology (2022)
-
Anthropogenic Disturbances and the Emergence of Native Diseases: a Threat to Forest Health
Current Forestry Reports (2022)
-
Mechanisms of woody-plant mortality under rising drought, CO2 and vapour pressure deficit
Nature Reviews Earth & Environment (2022)
-
Reply to: Spatial scale and the synchrony of ecological disruption
Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.