Abstract
The aim of this study was to examine the relationship between physical activity level and impaired left ventricular (LV) relaxation in a large sample of apparently healthy men and women. We conducted a cross-sectional study in 57,449 adults who underwent echocardiography as part of a comprehensive health examination between March 2011 and December 2014. Physical activity level was assessed using the Korean version of the International Physical Activity Questionnaire Short Form. The presence of impaired LV relaxation was determined based on echocardiographic findings. Physical activity levels were inversely associated with the prevalence of impaired LV relaxation. The multivariable-adjusted odds ratios (95% confidence interval) for impaired LV relaxation comparing minimally active and health-enhancing physically active groups to the inactive group were 0.84 (0.77–0.91) and 0.64 (0.58–0.72), respectively (P for trend < 0.001). These associations were modified by sex (p for interaction <0.001), with the inverse association observed in men, but not in women. This study demonstrated an inverse linear association between physical activity level and impaired LV relaxation in a large sample of middle-aged Koreans independent of potential confounders. Our findings suggest that increasing physical activity may be independently important in reducing the risk of impaired LV relaxation.
Similar content being viewed by others
Introduction
Heart failure is a major public health problem that affects more than 5.8 million people in the United States and more than 23 million people worldwide and it results in significant morbidity, mortality and healthcare expenditures1,2,3,4. Several epidemiological studies have reported that nearly half of subjects with heart failure have left ventricular (LV) diastolic dysfunction5,6,7. Recent studies have described a rise in the proportion of heart failure with preserved LV ejection fraction relative to heart failure with reduced LV ejection fraction in recent decades8,9. Because of the serious consequences of overt heart failure, there is substantial interest in identifying potentially modifiable risk factors that may prevent or reduce the progression of LV diastolic dysfunction. Aging, diabetes, hypertension, obesity, connective tissue disease, exercise training are risk factors known to affect LV diastolic function10,11,12.
Especially, physical activity has multiple beneficial cardiovascular effects13,14 and is associated with reduced incidence of and lower mortality from cardiovascular disease15,16. Recent epidemiological studies have reported a close link between LV diastolic dysfunction and metabolic disorders including obesity, metabolic syndrome and insulin resistance11,17, all of which are also related to reduced physical activity. Most studies of physical activity and LV dysfunction, however, have focused on small cohorts of young male endurance athletes14,18,19 or of patients with overt heart disease20,21,22,23.
Impaired LV relaxation is likely to go ahead of LV chamber stiffness or systolic dysfunction during the development of heart failure and has been assigned as a sensitive sign of LV diastolic dysfunction24. Despite the fact that exercise is associated with better LV diastolic dysfunction, the relationship between physical activity and impaired LV relaxation remains largely unexplored in general population cohorts. Therefore, the aim of this study was to examine the association between physical activity and impaired LV relaxation in a large sample of Korean men and women free of clinical cardiovascular disease.
Methods
Study population
The Kangbuk Samsung Health Study was a cohort study of Korean men and women who attended a comprehensive annual or biennial health exam at the Kangbuk Samsung Hospital Total Healthcare Centers in Seoul and Suwon, South Korea25. The study population was composed of 61,775 participants who completed a physical activity questionnaire and underwent echocardiography as part of a comprehensive screening examination from March, 2011 to December, 2014.
We excluded 4,326 participants with one or more of the following exclusion criteria: self-reported history of malignancy (N = 1,495); self-reported history of cardiovascular disease (N = 537); presence of echocardiographic abnormalities, including ejection fraction <50%, hypertrophic or dilated cardiomyopathy, ischemic cardiomyopathy, valvular replacement or valvular surgery, mitral stenosis or regurgitation, atrial fibrillation and congenital disease (N = 2,218). Because some exclusion criteria overlapped, the total number of patients eligible for the present study was 57,449.
The Institutional Review Board of the Kangbuk Samsung Hospital approved this study. The requirement for informed consent was waived as we used only anonymized retrospective data routinely collected during the health screening process.
Measurements
All examinations were conducted at the Kangbuk Samsung Hospital Total Healthcare Centers clinics in Seoul and Suwon. Data on demographic characteristics, medication use, medical history, smoking, alcohol consumption and education level were collected by self-administered, standardized questionnaires as previously described25.
We measured physical activity levels using the validated Korean version of the International Physical Activity Questionnaire (IPAQ) Short Form26,27, as previously described25. IPAQ Short Form measures the frequency and duration of walking or any other moderate to vigorous physical activity undertaken for more than 10 continuous minutes across all contexts (i.e., work, home and leisure) during a seven-day period. Physical activity levels were classified into three categories: inactive, minimally active (600 MET-minutes per week), and health-enhancing physically active (HEPA; 3000 MET-minutes per week)25. The total amount of weekday sitting time was assessed using the following single question: “During the last seven days, how much time did you usually spend sitting on a weekday?”26. The sitting time was divided into the following categories commonly found in previous studies: <5, 5–9, and ≥10 hours/day28.
The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality29. We used component 3 of the PSQI, which is concerned with the number of hours of actual nighttime sleep obtained during the past month, to assess sleep duration. We measured usual dietary intake using a 103-item, self-administered food frequency questionnaire designed and validated for use in Korea30. We calculated total energy and nutrient intake with a food composition tables developed by the Korean Nutrition Society30.
Trained nurses measured height and weight while the participants wore a lightweight hospital gown and no shoes. Body mass index (BMI) was calculated as weight divided by height squared (kg/m2). Obesity was defined as BMI >25.0 kg/m2 according to the criteria proposed for Asian populations31. Blood pressure was measured using an automated oscillometric device (53000, Welch Allyn, New York, USA) with the participants in a sitting position with the arm supported at heart level. Hypertension was defined as a systolic blood pressure ≥140 mmHg, a diastolic blood pressure ≥90 mmHg, or current use of antihypertensive medication.
Measurements for serum biochemical parameters, including total cholesterol, triglycerides, high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), insulin, glucose, hemoglobin A1c, and high-sensitivity C reactive protein (hsCRP) are described in detail elsewhere25.
Echocardiography
Conventional echocardiography was performed with ultrasound scanners (Vivid 7 and E9, General Electric, Milwaukee, WI, USA) by trained sonographers following a standardized protocol32. Linear measurements of intraventricular septum thickness (IVST), left posterior wall thickness (PWT) and diameter of the left ventricular cavity at the end of diastole and systole were obtained in M-mode in the parasternal long axis view. LV mass in grams was calculated using the following equation: LV mass = 0.8 × [1.04 × (LVEDD + IVST + PWT)3 − LVEDD3] + 0.633,34. Left ventricular mass index (LVMI) was determined as LV mass/body surface area (g/m2). The anteroposterior diameter of the left atrium (LA) was measured in all participants.
Diastolic function was evaluated using pulse-wave Doppler transmitral LV inflow in an apical four-chamber view. Early diastolic mitral inflow peak velocity (E), late diastolic peak velocity (A) during atrial contraction, and deceleration time of the E velocity were also measured. The early (E’) and late (A’) tissue velocities were obtained from Tissue Doppler imaging of the septal mitral annuls. Since E’ is primarily determined by myocardial relaxation and to a lesser extent by restoring forces and filling pressures, impaired LV relaxation was defined based on decreased E’(<7 cm/s) in this study35. In this study, only 102 participants (0.18%) had increased E/E’(>15).
Statistical analyses
The characteristics of the study participants were explored using physical activity categories. Mean values (95% confidence intervals [CIs]) of echocardiographic parameters were also estimated using physical activity categories. We conducted a logistic regression to estimate the odds ratios (with 95% CIs) for impaired LV relaxation by comparing the categories of physical activity with the reference category. We used three models with progressive adjustments. Model 1 was adjusted for age, sex, study center (Seoul, Suwon), and year of screening exam (one-year categories). Model 2 further included alcohol intake (0, <20, ≥20 g/d, or unknown), smoking (never, past, current, or unknown), educational level (high school graduate or less, community college or university graduate, graduate school or higher, and unknown), total calorie intake (quintile of total calorie intake or missing), sleep duration, family history of heart disease, history of hypertension and history of diabetes. Model 3 further adjusted for BMI, HOMA-IR, hsCRP, blood pressure and LVMI. To test for linear trends, we use the category rank as a continuous variable in the regression models. In addition, we examined the relationship between sitting time and impaired LV relaxation.
We performed stratified analyses in clinically relevant subgroups defined by sex (female vs male), age (<50 vs ≥50 years), smoking (never or ex-smoker vs current smoker), alcohol intake (<20 vs ≥20 g of alcohol per day), BMI (<25 vs ≥25 kg/m2), diabetes (no vs yes) and hypertension (no vs yes). Interactions between subgroups were tested using likelihood ratio tests to compare models with and without product terms.
All P-values were two-tailed, and P values < 0.05 were considered statistically significant. We used STATA version 14.0 (Stata Corp., College Station, TX, USA) for data analysis.
Results
The mean (SD) age, BMI, physical activity, and sitting time of study participants were 40.4 (7.6) years, 24.0 (3.2) kg/m2, 1,549.5 (3,257.9) METs, and 8.0 (3.7) hours, respectively. The correlation between physical activity level (MET) and sitting time was −0.14 (P < 0.001). The proportions of inactive, minimally active and HEPA participants were 45.0, 38.8, and 16.2%, respectively. Compared to inactive participants, those with HEPA were more likely to be older, and obese, more likely to have a history of diabetes and hypertension, more likely to have higher total calorie intake, alcohol intake, systolic and diastolic blood pressure, glucose and HDL-C levels, less likely to be smokers, and more likely to have lower levels of education total cholesterol, LDL-C, triglycerides, HOMA-IR and sleep duration (Table 1).
In models adjusted for age, sex, center and year of screening exam, E, A, E/E’, E/A ratio, LVEDD, LV mass, LVMI and LA size were positively related to physical activity level whereas heart rate and septal E’ were inversely related to physical activity level (Table 2).
The overall prevalence of impaired LV relaxation was 6.7%. After adjusting for age, sex, center and year of screening exam, the odds ratios (95% CIs) for impaired LV relaxation comparing minimally active and HEPA groups to the inactive group were 0.84 (0.78–0.91) and 0.68 (0.62–0.76), respectively (Table 3). Further adjustment for potential confounding variables did not materially alter the findings. When adjusted for potential intermediate variables, the association was still statistically significant. The respective odds ratios (95% CIs) for impaired LV relaxation comparing minimally active and HEPA groups to the inactive group were 0.84 (0.78–0.92) and 0.65 (0.58–0.73), respectively (P for trend < 0.001) (Table 3). On the other hand, sitting time was not significantly associated with impaired LV relaxation (Appendix Table 1). The interaction of physical activity and sitting time for impaired LV relaxation was not statistically significant (p for interaction = 0.36). The multivariable adjusted odds ratios (95% CIs) for impaired LV relaxation comparing HEPA groups to the minimally active group were 0.77 (0.69–0.86) (Appendix Table 2).
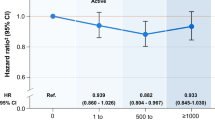
The association between physical activity and impaired LV relaxation was similar in pre-specified subgroups of study participants except those divided based on sex (Table 4 and Fig. 1). The association between physical activity and impaired LV relaxation was observed in men, but not in women (Fig. 1, P interaction < 0.001). The association between physical activity levels and impaired LV relaxation was still present in non-obese participants (BMI <25 kg/m2).
Multivariate-adjusted odds ratiosa (95% CIs) of impaired LV relaxation according to physical activity level in women and men. aEstimated from a logistic regression model. The multivariable model was adjusted for age, sex, year of screening exam, center, educational level, alcohol intake, smoking status, total calorie intake, sleep duration, family history of heart disease, history of hypertension, and history of diabetes. The P-value for the interaction of sex and physical activity levels for impaired LV relaxation was < 0.001.
Discussion
There were several novel findings in this large sample of Korean men and women. First, we observed an inverse linear association between physical activity level and impaired LV relaxation independent of potential confounders. Both minimally active levels and HEPA were inversely associated with impaired LV relaxation. Second, these associations were modified by sex. The inverse association between physical activity and impaired LV relaxation was observed only for men. Lastly, the association between physical activity level and impaired LV relaxation was still observed in non-obese participants.
Many epidemiologic studies have reported an association of inadequate physical activity with diabetes, coronary heart disease, stroke and increased cardiovascular mortality15,16,36. However, the relationship between physical activity and impaired LV relaxation in general a population sample remains largely unexplored. Most studies of the cardiac effects of physical activity have focused on LV function among elderly patients with heart problems or in small cohorts of young male endurance athletes14,18,19,20,21,22,23. A small investigation of 36 healthy participants reported that a sedentary lifestyle was related to decreased LV compliance, leading to diminished diastolic performance37. To our knowledge, this is the first study to show an inverse linear association between physical activity and the prevalence of impaired LV relaxation in a large sample of adults who were not elite athletes or patients with heart failure.
The mechanism by which physical activity was inversely related to the prevalence of impaired LV relaxation has yet to be identified. Because obesity is associated with changes in cardiac structure and function, including LV diastolic dysfunction, adiposity might represent a possible mechanism linking physical activity with impaired LV relaxation11,38,39. In this study, adjusting for BMI did not change the inverse association between physical activity levels and impaired LV relaxation. In addition, an inverse relationship between physical activity and impaired LV relaxation was still observed even in participants with a BMI <23 kg/m2, the group in which confounding from BMI should be the smallest.
Insulin resistance might also be another possible mechanism to explain the association of physical activity with impaired LV relaxation. Physical activity leads to greater insulin sensitivity due to increases in the glucose transport protein-4 levels and muscle glycogen synthase activity, decreases in serum triglyceride concentrations and increases in the muscle capillary network40. Indeed, recent epidemiological studies have described a close link between LV diastolic dysfunction and insulin resistance or metabolic syndrome17,41. In our study, however, the relationship between physical activity and impaired LV relaxation remained statistically significant after further adjusting for HOMA-IR suggesting that physical activity may directly affect LV diastolic function through biological pathways independent of obesity or insulin resistance. The positive association between physical activity and impaired LV relaxation might be explained by high blood pressure. The relationship of hypertension with LV diastolic dysfunction has been established42,43. These results did not change after adjustment for history of hypertension and systolic blood pressures.
Prolonged endurance exercise leads to a balanced enlargement of ventricular mass and dimensions14, resulting in enhanced cardiac performance without an obvious change in contractility, which thus might explain the low prevalence of impaired LV relaxation among participants with minimally active levels or HEPA levels37,44. Furthermore, prolonged exercise could have beneficial effects due to the maintenance of vascular elasticity and thus smaller arterial load37. Endurance training preserves vascular elasticity with aging and therefore might prevent changes in cardiac adaptation, such as focal proliferation of matrix or alteration of myocyte morphology45,46.
In the current analyses, the association between physical activity levels and impaired LV relaxation was statistically significant in men, but not in women. Despite conflicting results on possible sex differences in the protective effects of physical activity, several investigations have shown that there is differential remodeling in responses to aerobic exercise by sex. Although the reasons for sex-dependent effects of physical activity on impaired LV relaxation are not fully understood, there could be strong genetic modifiers of cardiac adaptation and exercise capacity47,48. A recent experimental study showed that the α1-adrenergic system is very important in determining heart size and the ability of the heart to respond to pathological and physiologic stimuli only in male mice, and this sex difference remained following ovariectomy in females49. Sex-related difference could also be related to shorter stature, hence earlier wave reflection and diastolic dysfunction50. Further research is needed to understand this sex-related difference.
Our study had some limitations that should be considered in the interpretation of our findings. First, we don’t have any information related to cardiorespiratory fitness, and because physical activity was self-reported, measurement error was inevitable. Specifically, people often have trouble recalling light-intensity exercise and tend to misreport the amount of time spent doing in such activities51. Misclassification is likely but might be non-differential. As a consequence, the true association of physical activity with LV diastolic function could be much stronger than shown here. Second, the cross-sectional design in this study limited our ability to determine temporality and prove causal relationships. Third, we have no information about LA volume or peak tricuspide velocity among the measurements recommended by the American Society of Echocardiography to assess LV diastolic function35. Future prospective studies that assess all four variables: annular E’ velocity, E/E’ ratio, LA volume, and peak tricuspide velocity are needed to examine potential causal associations of physical activity with the development of LV diastolic dysfunction. Finally, participants in our study were highly educated, young and middle-aged Koreans who regularly underwent health check-up exams, most often as a part of work-related health screening programs; thus, the results from our study may not be generalizable to other populations with different demographic composition. Despite these potential limitations, major strengths of our study were its large sample size, a comprehensive inclusion of potential confounding variables, and the availability of high-quality imaging and laboratory procedures with extensive quality control measures.
Conclusion
This study demonstrated an inverse linear association between physical activity level and impaired LV relaxation independently of potential confounders in a large sample of young and middle-aged Koreans. This association was shown primarily in men, but not in women. Our findings suggest that increasing physical activity may be independently associated with reduced risk of impaired LV relaxation.
References
Hunt, S. A. American College of, C. & American Heart Association Task Force on Practice, G. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Journal of the American College of Cardiology 46, e1–82, https://doi.org/10.1016/j.jacc.2005.08.022 (2005).
Mosterd, A. et al. The prognosis of heart failure in the general population: The Rotterdam Study. European heart journal 22, 1318–1327, https://doi.org/10.1053/euhj.2000.2533 (2001).
Cowie, M. R. et al. Survival of patients with a new diagnosis of heart failure: a population based study. Heart 83, 505–510 (2000).
Dunlay, S. M. et al. Hospitalizations after heart failure diagnosis a community perspective. Journal of the American College of Cardiology 54, 1695–1702, https://doi.org/10.1016/j.jacc.2009.08.019 (2009).
Vasan, R. S. et al. Congestive heart failure in subjects with normal versus reduced left ventricular ejection fraction: prevalence and mortality in a population-based cohort. Journal of the American College of Cardiology 33, 1948–1955 (1999).
Mosterd, A. et al. Prevalence of heart failure and left ventricular dysfunction in the general population; The Rotterdam Study. European heart journal 20, 447–455 (1999).
Senni, M. et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation 98, 2282–2289 (1998).
Roger, V. L. Epidemiology of heart failure. Circulation research 113, 646–659, https://doi.org/10.1161/CIRCRESAHA.113.300268 (2013).
Gerber, Y. et al. A contemporary appraisal of the heart failure epidemic in Olmsted County, Minnesota, 2000 to 2010. JAMA Intern Med 175, 996–1004, https://doi.org/10.1001/jamainternmed.2015.0924 (2015).
Abhayaratna, W. P., Marwick, T. H., Smith, W. T. & Becker, N. G. Characteristics of left ventricular diastolic dysfunction in the community: an echocardiographic survey. Heart 92, 1259–1264, https://doi.org/10.1136/hrt.2005.080150 (2006).
Russo, C. et al. Effect of obesity and overweight on left ventricular diastolic function: a community-based study in an elderly cohort. Journal of the American College of Cardiology 57, 1368–1374, https://doi.org/10.1016/j.jacc.2010.10.042 (2011).
Vegh, J., Hegedus, I., Szegedi, G., Zeher, M. & Bodolay, E. Diastolic function of the heart in mixed connective tissue disease. Clin Rheumatol 26, 176–181, https://doi.org/10.1007/s10067-006-0257-7 (2007).
Rerych, S. K., Scholz, P. M., Sabiston, D. C. Jr. & Jones, R. H. Effects of exercise training on left ventricular function in normal subjects: a longitudinal study by radionuclide angiography. The American journal of cardiology 45, 244–252 (1980).
Pluim, B. M., Zwinderman, A. H., van der Laarse, A. & van der Wall, E. E. The athlete’s heart. A meta-analysis of cardiac structure and function. Circulation 101, 336–344 (2000).
Sattelmair, J. et al. Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 124, 789–795, https://doi.org/10.1161/CIRCULATIONAHA.110.010710 (2011).
Manson, J. E. et al. Walking compared with vigorous exercise for the prevention of cardiovascular events in women. The New England journal of medicine 347, 716–725, https://doi.org/10.1056/NEJMoa021067 (2002).
Hwang, Y. C. et al. Metabolic syndrome and insulin resistance are associated with abnormal left ventricular diastolic function and structure independent of blood pressure and fasting plasma glucose level. International journal of cardiology 159, 107–111, https://doi.org/10.1016/j.ijcard.2011.02.039 (2012).
Scharhag, J. et al. Athlete’s heart: right and left ventricular mass and function in male endurance athletes and untrained individuals determined by magnetic resonance imaging. Journal of the American College of Cardiology 40, 1856–1863 (2002).
Perseghin, G. et al. Effect of the sporting discipline on the right and left ventricular morphology and function of elite male track runners: a magnetic resonance imaging and phosphorus 31 spectroscopy study. American heart journal 154, 937–942, https://doi.org/10.1016/j.ahj.2007.06.039 (2007).
Alves, A. J. et al. Exercise training improves diastolic function in heart failure patients. Medicine and science in sports and exercise 44, 776–785, https://doi.org/10.1249/MSS.0b013e31823cd16a (2012).
Panovsky, R. et al. The effect of regular physical activity on the left ventricle systolic function in patients with chronic coronary artery disease. Physiological research/Academia Scientiarum Bohemoslovaca 60, 869–875 (2011).
Nishi, I. et al. Effects of exercise training in patients with chronic heart failure and advanced left ventricular systolic dysfunction receiving beta-blockers. Circulation journal: official journal of the Japanese Circulation Society 75, 1649–1655 (2011).
Aggelopoulos, P. et al. Sex differences regarding the impact of physical activity on left ventricular systolic function in elderly patients with an acute coronary event. Hellenic journal of cardiology: HJC = Hellenike kardiologike epitheorese 55, 448–456 (2014).
Yamamoto, K. The time constant of left ventricular relaxation: extrication from load dependence and overestimation of functional abnormality. Circ Heart Fail 3, 178–180, https://doi.org/10.1161/CIRCHEARTFAILURE.110.941773 (2010).
Ryu, S. et al. Relationship of sitting time and physical activity with non-alcoholic fatty liver disease. Journal of hepatology 63, 1229–1237, https://doi.org/10.1016/j.jhep.2015.07.010 (2015).
Craig, C. L. et al. International physical activity questionnaire: 12-country reliability and validity. Medicine and science in sports and exercise 35, 1381–1395, https://doi.org/10.1249/01.MSS.0000078924.61453.FB (2003).
Oh, J. Y., Yang, Y. J., Kim, B. S. & Kang, J. H. Validity and reliability of Korean version of International Physical Activity Questionnaire (IPAQ) short form. Korean J Fam Med 28, 532–541 (2007).
Aadahl, M. et al. Recent temporal trends in sleep duration, domain-specific sedentary behaviour and physical activity. A survey among 25-79-year-old Danish adults. Scandinavian journal of public health 41, 706–711, https://doi.org/10.1177/1403494813493151 (2013).
Buysse, D. J., Reynolds, C. F. 3rd, Monk, T. H., Berman, S. R. & Kupfer, D. J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res 28, 193–213, 0165-1781(89)90047-4 [pii] (1989).
Ahn, Y. et al. Validation and reproducibility of food frequency questionnaire for Korean genome epidemiologic study. European journal of clinical nutrition 61, 1435–1441, https://doi.org/10.1038/sj.ejcn.1602657 (2007).
Wen, C. P. et al. Are Asians at greater mortality risks for being overweight than Caucasians? Redefining obesity for Asians. Public Health Nutr 12, 497–506, https://doi.org/10.1017/s1368980008002802 (2009).
Lang, R. M. et al. Recommendations for chamber quantification. European Heart Journal-Cardiovascular Imaging 7, 79–108 (2006).
Devereux, R. B. et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. The American journal of cardiology 57, 450–458 (1986).
de Simone, G. et al. Left ventricular mass and body size in normotensive children and adults: assessment of allometric relations and impact of overweight. Journal of the American College of Cardiology 20, 1251–1260 (1992).
Nagueh, S. F. et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography: official publication of the American Society of Echocardiography 29, 277–314, https://doi.org/10.1016/j.echo.2016.01.011 (2016).
Krishnan, S., Rosenberg, L. & Palmer, J. R. Physical activity and television watching in relation to risk of type 2 diabetes: the Black Women’s Health Study. American journal of epidemiology 169, 428–434, https://doi.org/10.1093/aje/kwn344 (2009).
Arbab-Zadeh, A. et al. Effect of aging and physical activity on left ventricular compliance. Circulation 110, 1799–1805, https://doi.org/10.1161/01.CIR.0000142863.71285.74 (2004).
Cil, H. et al. Impact of body mass index on left ventricular diastolic dysfunction. Echocardiography 29, 647–651, https://doi.org/10.1111/j.1540-8175.2012.01688.x (2012).
DeVallance, E. et al. Is obesity predictive of cardiovascular dysfunction independent of cardiovascular risk factors? International journal of obesity 39, 244–253, https://doi.org/10.1038/ijo.2014.111 (2015).
Henriksson, J. Influence of exercise on insulin sensitivity. Journal of cardiovascular risk 2, 303–309 (1995).
Ayalon, N. et al. Preclinical left ventricular diastolic dysfunction in metabolic syndrome. The American journal of cardiology 114, 838–842, https://doi.org/10.1016/j.amjcard.2014.06.013 (2014).
Erdogan, D. et al. Aortic elastic properties and left ventricular diastolic function in white-coat hypertensive individuals. Blood Press Monit 11, 191–198, https://doi.org/10.1097/01.mbp.0000209079.17246.7d (2006).
Clement, D. L., De Buyzere, M. & Duprez, D. Left ventricular function and regression of left ventricular hypertrophy in essential hypertension. Am J Hypertens 6, 14S–19S (1993).
Levine, B. D., Lane, L. D., Buckey, J. C., Friedman, D. B. & Blomqvist, C. G. Left ventricular pressure-volume and Frank-Starling relations in endurance athletes. Implications for orthostatic tolerance and exercise performance. Circulation 84, 1016–1023 (1991).
Lakatta, E. G. Cardiovascular aging research: the next horizons. Journal of the American Geriatrics Society 47, 613–625 (1999).
Harris, T. S. et al. Constitutive properties of hypertrophied myocardium: cellular contribution to changes in myocardial stiffness. American journal of physiology. Heart and circulatory physiology 282, H2173–2182, https://doi.org/10.1152/ajpheart.00480.2001 (2002).
Hoit, B. D. et al. Naturally occurring variation in cardiovascular traits among inbred mouse strains. Genomics 79, 679–685, https://doi.org/10.1006/geno.2002.6754 (2002).
Lerman, I. et al. Genetic variability in forced and voluntary endurance exercise performance in seven inbred mouse strains. Journal of applied physiology 92, 2245–2255, https://doi.org/10.1152/japplphysiol.01045.2001 (2002).
O’Connell, T. D. et al. The alpha(1A/C)- and alpha(1B)-adrenergic receptors are required for physiological cardiac hypertrophy in the double-knockout mouse. The Journal of clinical investigation 111, 1783–1791, https://doi.org/10.1172/JCI16100 (2003).
Chirinos, J. A. et al. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: the Asklepios study. Hypertension 61, 296–303, https://doi.org/10.1161/HYPERTENSIONAHA.111.00530 (2013).
Washburn, R. A. Assessment of physical activity in older adults. Research quarterly for exercise and sport 71, S79–88 (2000).
Author information
Authors and Affiliations
Contributions
Seungho Ryu designed the research; Hocheol Shin, Eliseo Guallar and Yoosoo Chang supervised the research: Seungho Ryu conducted the data analyses; Seungho Ryu wrote the draft of initial manuscript; Jeonggyu Kang, Kyung Eun Yun, Hyun-Suk Jung, Chan-Won Kim, Juhee Cho, Joao A Lima and Ki-Chul Sung critically contributed to data reanalysis and draft revision; Seungho Ryu, Yoosoo Chang, Jeonggyu Kang, Kyung Eun Yun, Hyun-Suk Jung, Chan-Won Kim, Juhee Cho, Joao A Lima, Ki-Chul Sung, Hocheol Shin and Eliseo Guallar contributed to the interpretation of the data and preparation of the manuscript. All authors were involved in the preparation of the manuscript, have read the manuscript, agree with the analyses of the data and the conclusions reached in the manuscript and are accountable for all aspects of the work.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ryu, S., Chang, Y., Kang, J. et al. Physical activity and impaired left ventricular relaxation in middle aged adults. Sci Rep 8, 12461 (2018). https://doi.org/10.1038/s41598-018-31018-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-31018-z
This article is cited by
-
The effect of physical activity level on the severity of diastolic dysfunction
BMC Sports Science, Medicine and Rehabilitation (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.