Abstract
Estrogen is a potent vasodilator through activation of endothelial nitric oxide synthase (eNOS). Arginine and its homologue homoarginine are substrates for NOS, while asymmetric dimethylarginine (ADMA) is a NOS inhibitor. Healthy, never-pregnant women aged 18 to 40 years (n = 158) were categorized according to use of hormonal contraception into non-users (n = 76), users of estrogen contraceptives (EC-users, n = 58) and users of progestins-only contraceptives (PC-users, n = 24). Plasma homoarginine, arginine, ADMA and SDMA concentrations were assayed using a LC-MS/MS method. Compared to non-users, EC users had higher plasma homoarginine (median (interquartile range) 1.63 (1.24, 2.04) vs. 2.39 (2.05, 2.85) µmol/L, p < 0.001), lower arginine (80.8 (72.4, 94.3) vs. 72.1 (62.9, 85.1) µmol/L, p = 0.008) and ADMA (0.52 (0.46, 0.59) vs. 0.48 (0.42, 0.54) µmol/L, p = 0.003) concentrations. The lowest median plasma homoarginine concentration (1.34 (0.92, 1.75) µmol) was seen in PC-users. No differences were seen in SDMA concentrations according to use of hormonal contraception. In healthy, never-pregnant women aged 18 to 40 years, use of estrogen containing contraception was associated with significantly higher plasma concentrations of homoarginine and lower plasma concentrations of arginine and ADMA as compared to non-users, while the lowest plasma homoarginine concentrations were seen in progestin-only users. Whether the observed changes in relation to use of hormonal contraception have an impact on cardiovascular status, should be evaluated in an intervention study.
Similar content being viewed by others
Introduction
Estrogen is a potent vasodilator, and conditions with high estrogen concentrations, as during the latter part of the follicular phase of the menstrual cycle, use of oral contraception (OC) and pregnancy, are all associated with increased endothelial dependent vasodilatation1,2,3. Estrogen mediates its effect on the vascular endothelium partly via activation of endothelial nitric oxide synthase (eNOS)4. The resulting increase in circulating nitric oxide (NO) is crucial for endothelial function, including arteriolar relaxation, an important determinant of blood pressure5. L-Arginine and its homolog L-homoarginine are competitive substrates of NOS6, whereas asymmetric dimethylarginine (ADMA), a guanindine (NG)-dimethylated derivate of arginine, is a NOS inhibitor7. ADMA and the stereoisomer symmetric dimethylarginine (SDMA) may also indirectly reduce NO synthesis by inhibiting cellular uptake of arginine8. Accordingly, the ratio between arginine and ADMA is regarded as a marker of NOS activity7. A favourable cardiovascular risk profile has been related to high circulating homoarginine9 and low ADMA concentrations10.
Higher arginine and lower ADMA concentrations are reported in women using OC compared to non-users11, while lower arginine and ADMA concentrations are reported in pregnant women12. A marked increase in homoarginine concentrations are observed in women using OC13 and and in pregnant women during the second and third trimester. In pregnancy, homoarginine concentrations are reported to be more strongly correlated to brachial artery flow-mediated dilatation than arginine12.
We investigated systemic amino acids involved in NO regulation in healthy, never-pregnant women aged 18 to 40 years. The purpose of the study was to evaluate plasma homoarginine, arginine, ADMA and SDMA concentrations in relation to use of hormonal contraception.
Results
Demographics
The population included never-pregnant women with a mean (range) age of 25.3 (18–40) years. The participants were healthy, well-educated, with a median (IQR) BMI of 21.8 (20.6, 23.7). The majority (124/158, 78%) had an omnivore diet, and a minority (21/158, 30%) were regular users of both multivitamins/minerals and omega 3 fatty acids/cod oil supplements.
Demographic data according to reported current use of contraceptives (non-users, n = 76, EC-users, n = 58 and PC-users, n = 24) are given in Table 1. Apart from a higher consumption of alcohol in women who used hormonal contraceptives compared to non-users, there were no significant differences in demographic data among the three groups (Table 1).
Plasma homoarginine, arginine, ADMA and SDMA concentrations according to use of hormonal contraceptives
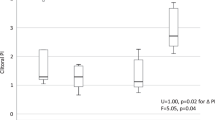
There were significant differences in the plasma concentrations of homoarginine, arginine and ADMA, but not in SDMA, according to use of hormonal contraception (Table 2, Fig. 1). Plasma homoarginine concentrations were higher in EC-users (+47%, p < 0.001) and lower in PC-users (−18%, p = 0.005), as compared to non-users (Table 2). The majority of the EC-users (13/58, 78%) had homoarginine concentrations >2.0 µmol/L, while the majority of the non-users (56/76, 74%) and almost all PC-users (23/24, 96%) had concentrations <2.0 µmol/L.
EC-users had lower median arginine and ADMA concentrations compared to non-users (p = 0.008 and p = 0.003, for arginine and ADMA respectively), and lower ADMA concentrations compared to PC-users (p = 0.004).
EC-users had higher median homoarginine/ADMA (hArg/ADMA) ratio compared to both non-users and PC-users (p < 0.001), while the lowest ratio was seen in PC-users. No significant differences in median arginine/ADMA (Arg/ADMA) ratios were observed between the groups (Table 2).
Use of hormonal contraception was the strongest positive predictor of homoarginine and the hArg/ADMA ratio, and the strongest negative predictor of ADMA concentrations, in a multiple linear regression model which additionally included age, BMI, use of alcohol and tobacco (based on cotinine levels ≥85 nmol/L) (Table 3).
Discussion
In healthy, never-pregnant women aged 18 to 40 years, the use of estrogen containing contraception was associated with significantly higher plasma concentrations of homoarginine, higher hArg/ADMA ratio and lower concentrations of arginine and ADMA as compared to non-users. The lowest homoarginine concentrations were seen in progestin-only users. No difference in Arg/ADMA ratio was seen according to use of hormonal contraception.
Premenopausal women who do not use hormonal contraception, have the highest circulating estrogen concentrations in the latter part of the follicular phase, just prior to ovulation14. Oral contraceptive pills are classically prescribed as a constant supply of estrogen for 21 days, followed by 7 days of placebo15. The transdermal patch or vaginal ring is worn for 21 days when it delivers a continuous estrogen and progestin formulation and is then removed for 7 days16. As a result, women who use combination contraceptives have a constant high estrogen concentration for 21 of a 28 days cycle, and a lower estrogen concentration during the 7 days with placebo. The various forms and doses of progestins in progestin-only contraceptives differ in their inhibition of ovarian activity17. The estrogen levels differ accordingly, but are reported to be lower or remain comparable to normal early or mid-follicular phase levels17,18,19. In pregnant women, the estrogen concentrations increase continuously during pregnancy with the highest concentrations observed in the last trimester20.
Homoarginine is endogenously synthesized by L-arginine:glycine amidinotransferase (AGAT)13, and gene expression is shown to be modulated by estrogen in chick liver cells21. Conditions with higher estrogen concentrations are associated with higher circulating homoarginine concentrations, as confirmed in the present and previous studies on oral oestrogen contraceptive users13 and also in pregnant women12.
The majority (78%) of the EC-users in our population had high homoarginine concentrations (>2 µmol/L), but in one quarter of the sample the concentration was below 2 µmol/L. One quarter of the non-users had a high homoarginine concentration (>2 µmol/L). The observed variations could be explained by cyclic changes in estrogen concentrations in our population. Assuming this was a representative sample of premenopausal women, one would expect one quarter of the EC-users to be in their placebo-phase (7/28 days) with low estrogen concentrations and one quarter of the non-users to be in their late follicular phase with high estrogen concentrations.
The lowest homoarginine concentrations were seen in PC-users. This might be explained by lower estrogen levels in PC-users compared to non-users and EC-users; however, as various progestins have different effects on ovarian activity, this may also reflect specific progestin subtypes and doses. Progestins might also have an independent effect on endogen homoarginine production, but this is currently unknown.
Arginine concentrations are reported to be higher in in the follicular phase compared to the luteal phase11, although one study did not observe any changes in arginine concentrations during the menstrual cycle12. Lower ADMA concentrations have been reported in conditions associated with higher estrogen concentrations, as in pregnancy22, after ovarian hyperstimulation23 and after hormone therapy in postmenopausal women24, which are observations in agreement with the results in the present study. We observed no differences in SDMA according to hormonal contraceptive use.
In our population of fertile women, the hArg/ADMA ratio was the strongest predictor of hormonal contraception use, with the highest ratio seen in EC-users, followed by non-users and PC-users. Association of hArg/ADMA ratios with higher estrogen levels are also reported by Valtonen et al. Nonpregnant women had a hArg/ADMA ratio of 5, while the values increased from 6 to 10 during pregnancy12. The hArg/ADMA ratio is considered to be mainly determined by circulating concentrations of homoarginine, which shows a larger variation than ADMA that is more closely controlled25.
We observed no differences in Arg/ADMA ratio. The Arg/ADMA ratio was reported to be higher in OC-users compared to non-users in one study11, while no change was seen in postmenopausal women after hormone replacement24. Slightly higher Arg/ADMA ratios have been found in pregnant women in their second and third trimester, although this was not related to improved endothelial function22. The Arg/ADMA ratio may reflect the capacity of NOS catalysed NO formation from arginine; however, as the ratio is mainly determined by large fluctuations in circulating concentrations of arginine, the utility of this ratio has been questioned25.
Physiological states associated with high estrogen concentrations are associated with increased endothelial dependent vasodilatation1,2,3. In premenopausal women with variant angina, the frequency of ischemic episodes was lowest in the late part of the follicular phase26, associated with the highest estrogen concentrations in the menstrual cycle20. Use of estrogen containing contraceptives has also been shown to cause vasodilatation2,3,27. These estrogen effects are probably mediated by increased expression and activation the endothelial isoform of NO synthase (eNOS)4, which uses both arginine and homoarginine as substrates for NO production6.
A higher cardiovascular risk has been related to high ADMA10, high SDMA28 and low circulating homoarginine concentrations9,25. High circulating homoarginine concentrations have also been associated with cardiovascular risk factors like hypertension, obesity and insulin resistance13. A 10 year follow-up study of young Finnish adults reported no effect of lifetime exposure of higher homoarginine levels on cardiovascular disease risk13, so the role for homoarginine as a biomarker for cardiovascular status still remains uncertain in young adults13.
ADMA and homoarginine are considered to have opposite effects on NO production, with ADMA serving as an inhibitor of NOS and homoarginine as a substrate29. The vascular effects of ADMA and SDMA have however been questioned, as ADMA and presumably also SDMA, are considered to be weak inhibitors of eNOS25. Homoarginine has a low affinity for NOS30, but is considered an alternative substrate to arginine and has been shown to increase NO availability31. Homoarginine is however, also reported to reduce NO production by acting as an inhibitor rather than a substrate for the three NO synthase isoforms (eNOS, neuronal and inducible NOS)32. Homoarginine may compete with arginine at the substrate binding site33 and has been shown to impair cellular arginine transport34 by inhibiting the cationic amino acid transporter (CAT-1), thereby reducing intracellular arginine availability for NOS35, and possibly also for other enzymatic reactions. However, high concentrations of L-homoarginine (1 mmol/L) were used to inhibit arginine uptake34 and it is unlikely that such levels can be reached in humans. Therefore, the exact mechanisms for homoarginine inhibition of NO production are still not clear.
Arginine is an important amino acid in pregnancy and serves both as a building block for proteins and is additionally hydrolyzed by the enzyme arginase to ornithine and converted into the polyamines putrescine, spermine, and spermidine36,which are key regulators of placental angiogenesis, trophoblast growth, and embryogenesis37. Arginine is reported to be abundant in the amniotic fluid in early pregnancy38, and a predictor for birth weight, length, and head circumference39.
The study included 158 healthy never-pregnant premenopausal women, of whom approximately 50% used hormonal contraception, which was a sufficient population size to detect statistically significant differences in concentrations of arginine metabolites studied. As we did not have information about the menstrual phase, sex hormone concentrations, or endothelial dependent vasodilatation, we were unable to relate our data to estrogen concentrations and vascular effects more directly, which are all limitations of this study.
Conclusion
In healthy, never-pregnant women aged 18 to 40 years, use of estrogen containing contraception was associated with significantly higher plasma concentrations of homoarginine and lower concentrations of arginine and ADMA compared to non-users. The lowest homoarginine concentrations were seen in progestin-only users.
Whether the observed changes in arginine and its metabolites in relation to use of hormonal contraception have an impact on cardiovascular health should be evaluated in an intervention study.
Material and Methods
Study population and design
Between June 2012 and March 2015, healthy, never-pregnant women aged 18 to 40 years were recruited among employees and students at Haukeland University Hospital and the University of Bergen, Norway.
Ethical approval of the protocol was granted by the Regional Committee for Medical Research Ethics West (2011/2447). All methods were performed in accordance with the relevant guidelines and regulations. Written informed consent was obtained from all women.
The dataset generated and analyzed for the current study are available from the corresponding author on reasonable request.
Clinical data
The women completed a questionnaire concerning age, body weight, health status, years of completed education, diet, and the use of multiple micronutrient supplements (MMN), alcohol and tobacco. Regular use of supplements was defined as use more than three days per week and the definition of a regular tobacco user was based on a plasma cotinine concentration >85 nmol/L40.
Use of hormone containing contraceptives
Use of hormonal contraception, including oral contraceptives, hormone implants and injections, was recorded. Progestins-only contraceptives contain different forms and doses of progestins, while combination contraceptives additionally contain ethinylestradiol (range 20–35 µg)15.
Women who did not use hormonal contraception were defined as non-users, women who used estrogen containing contraceptives were defined as the EC-users, whereas women who used progestin-only contraceptives were defined as the PC-users.
Blood sampling and analysis
Non-fasting blood samples were obtained by antecubital venipuncture and collected into EDTA Vacutainer Tubes (Becton Dickinson), placed in ice water, and plasma was separated within 4 hours. The samples were stored at −80 °C until analysis. Plasma concentrations of arginine, asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine were assayed using a LC-MS/MS method41 by the laboratory of Bevital AS (www.bevital.no).
Statistical analysis
Results are presented as mean and standard deviation (SD), compared by Student’s t-test or ANOVA, and median and interquartile range (IQR), compared by Mann-Whitney U test or Kruskal Wallis test. Chi-square test was used for categorical data. Multiple linear regression models were used to assess the association of plasma homoarginine, arginine and ADMA with age, BMI, use of hormonal contraception, alcohol and tobacco.
The R Foundation for Statistical Computing (version 3.3) was used for graphical illustrations of the relation between use of hormonal contraception and homoarginine, arginine and ADMA. The SPSS statistical program (version 24) was used for statistical analyses. Two-sided p-values < 0.05 were considered statistically significant.
References
Khalil, A. & Hardman, L. The role of arginine, homoarginine and nitric oxide in pregnancy. Amino acids 47, 1715–1727, https://doi.org/10.1007/s00726-015-2014-1 (2015).
Limberg, J. K. et al. Greater Beta-Adrenergic Receptor Mediated Vasodilation in Women Using Oral Contraceptives. Frontiers in physiology 7, 215, https://doi.org/10.3389/fphys.2016.00215 (2016).
Thompson, A. K., Przemska, A., Vasilopoulou, D., Newens, K. J. & Williams, C. M. Combined oral contraceptive pills containing desogestrel or drospirenone enhance large vessel and microvasculature vasodilation in healthy premenopausal women. Microcirculation (New York, N.Y.: 1994) 18, 339–346, https://doi.org/10.1111/j.1549-8719.2011.00094.x (2011).
Chambliss, K. L. & Shaul, P. W. Estrogen modulation of endothelial nitric oxide synthase. Endocrine reviews 23, 665–686, https://doi.org/10.1210/er.2001-0045 (2002).
Mendelsohn, M. E. Protective effects of estrogen on the cardiovascular system. The American journal of cardiology 89, 12E–17E; discussion 17E–18E (2002).
Hecker, M., Walsh, D. T. & Vane, J. R. On the substrate specificity of nitric oxide synthase. FEBS letters 294, 221–224 (1991).
Bode-Boger, S. M., Scalera, F. & Ignarro, L. J. The l-arginine paradox: Importance of the l-arginine/asymmetrical dimethylarginine ratio. Pharmacol Ther 114, 295–306 (2007).
Gore, M. O. et al. Symmetrical dimethylarginine predicts mortality in the general population: observations from the Dallas heart study. Arteriosclerosis, thrombosis, and vascular biology 33, 2682–2688, https://doi.org/10.1161/ATVBAHA.113.301219 (2013).
Atzler, D., Schwedhelm, E. & Choe, C. U. L-homoarginine and cardiovascular disease. Curr Opin Clin Nutr Metab Care 18, 83–88, https://doi.org/10.1097/MCO.0000000000000123 (2015).
Willeit, P. et al. Asymmetric dimethylarginine and cardiovascular risk: systematic review and meta-analysis of 22 prospective studies. Journal of the American Heart Association 4, e001833, https://doi.org/10.1161/JAHA.115.001833 (2015).
Valtonen, P. et al. ADMA concentration changes across the menstrual cycle and during oral contraceptive use: the Cardiovascular Risk in Young Finns Study. Eur J Endocrinol 162, 259–265, https://doi.org/10.1530/EJE-09-0658 (2010).
Valtonen, P. et al. Serum L-homoarginine concentration is elevated during normal pregnancy and is related to flow-mediated vasodilatation. Circ J 72, 1879–1884, doi:JST.JSTAGE/circj/CJ-08-0240 (2008).
Seppala, I. et al. The biomarker and causal roles of homoarginine in the development of cardiometabolic diseases: an observational and Mendelian randomization analysis. Sci Rep 7, 1130, https://doi.org/10.1038/s41598-017-01274-6 (2017).
Somerville, B. W. Daily variation in plasma levels of progesterone and estradiol throughout the menstrual cycle. American journal of obstetrics and gynecology 111, 419–426 (1971).
Wright, K. P. & Johnson, J. V. Evaluation of extended and continuous use oral contraceptives. Therapeutics and clinical risk management 4, 905–911 (2008).
Shufelt, C. L. & Bairey Merz, C. N. Contraceptive hormone use and cardiovascular disease. Journal of the American College of Cardiology 53, 221–231, https://doi.org/10.1016/j.jacc.2008.09.042 (2009).
D’Arpe, S. et al. Ovarian function during hormonal contraception assessed by endocrine and sonographic markers: a systematic review. Reproductive biomedicine online 33, 436–448, https://doi.org/10.1016/j.rbmo.2016.07.010 (2016).
Duijkers, I. J., Heger-Mahn, D., Drouin, D. & Skouby, S. A randomised study comparing the effect on ovarian activity of a progestogen-only pill (POP) containing desogestrel and a new POP containing drospirenone in a 24/4 regimen. The European journal of contraception & reproductive health care: the official journal of the European Society of Contraception 20, 419–427, https://doi.org/10.3109/13625187.2015.1044082 (2015).
Duijkers, I. J., Klipping, C., Verhoeven, C. H. & Dieben, T. O. Ovarian function with the contraceptive vaginal ring or an oral contraceptive: a randomized study. Human reproduction 19, 2668–2673, https://doi.org/10.1093/humrep/deh493 (2004).
Tulchinsky, D., Hobel, C. J., Yeager, E. & Marshall, J. R. Plasma estrone, estradiol, estriol, progesterone, and 17-hydroxyprogesterone in human pregnancy. I. Normal pregnancy. Am J Obstet Gynecol 112, 1095–1100 (1972).
Zhu, Y. & Evans, M. I. Estrogen modulates the expression of L-arginine:glycine amidinotransferase in chick liver. Molecular and cellular biochemistry 221, 139–145 (2001).
Saarelainen, H. et al. Subtle changes in ADMA and l-arginine concentrations in normal pregnancies are unlikely to account for pregnancy-related increased flow-mediated dilatation. Clinical physiology and functional imaging 28, 120–124, https://doi.org/10.1111/j.1475-097X.2007.00784.x (2008).
Cevik, D., Unay, O., Durmusoglu, F., Yurdun, T. & Bilsel, A. S. Plasma markers of NO synthase activity in women after ovarian hyperstimulation: influence of estradiol on ADMA. Vascular medicine (London, England) 11, 7–12, https://doi.org/10.1191/1358863x06vm636oa (2006).
Verhoeven, M. O., Hemelaar, M., Teerlink, T., Kenemans, P. & van der Mooren, M. J. Effects of intranasal versus oral hormone therapy on asymmetric dimethylarginine in healthy postmenopausal women: a randomized study. Atherosclerosis 195, 181–188, https://doi.org/10.1016/j.atherosclerosis.2006.09.018 (2007).
Tsikas, D., Bollenbach, A., Hanff, E. & Kayacelebi, A. A. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): the ADMA, SDMA and hArg paradoxes. Cardiovascular diabetology 17, 1, https://doi.org/10.1186/s12933-017-0656-x (2018).
Kawano, H. et al. Menstrual cyclic variation of myocardial ischemia in premenopausal women with variant angina. Annals of internal medicine 135, 977–981 (2001).
Meendering, J. R., Torgrimson, B. N., Miller, N. P., Kaplan, P. F. & Minson, C. T. Ethinyl estradiol-to-desogestrel ratio impacts endothelial function in young women. Contraception 79, 41–49, https://doi.org/10.1016/j.contraception.2008.07.025 (2009).
Emrich, I. E. et al. Symmetric dimethylarginine (SDMA) outperforms asymmetric dimethylarginine (ADMA) and other methylarginines as predictor of renal and cardiovascular outcome in non-dialysis chronic kidney disease. Clinical research in cardiology: official journal of the German Cardiac Society 107, 201–213, https://doi.org/10.1007/s00392-017-1172-4 (2018).
Tsikas, D. & Kayacelebi, A. A. Do homoarginine and asymmetric dimethylarginine act antagonistically in the cardiovascular system? Circ J 78, 2094–2095, https://doi.org/10.1253/circj.CJ-14-0484 (2014).
Moali, C., Boucher, J. L., Sari, M. A., Stuehr, D. J. & Mansuy, D. Substrate specificity of NO synthases: detailed comparison of L-arginine, homo-L-arginine, their N omega-hydroxy derivatives, and N omega-hydroxynor-L-arginine. Biochemistry 37, 10453–10460, https://doi.org/10.1021/bi980742t (1998).
Marz, W. et al. Homoarginine, cardiovascular risk, and mortality. Circulation 122, 967–975, https://doi.org/10.1161/CIRCULATIONAHA.109.908988 (2010).
Alesutan, I. et al. Augmentation of phosphate-induced osteo-/chondrogenic transformation of vascular smooth muscle cells by homoarginine. Cardiovascular research 110, 408–418, https://doi.org/10.1093/cvr/cvw062 (2016).
Bretscher, L. E., Li, H., Poulos, T. L. & Griffith, O. W. Structural characterization and kinetics of nitric-oxide synthase inhibition by novel N5-(iminoalkyl)- and N5-(iminoalkenyl)-ornithines. The Journal of biological chemistry 278, 46789–46797, https://doi.org/10.1074/jbc.M306787200 (2003).
Bogle, R. G., Moncada, S., Pearson, J. D. & Mann, G. E. Identification of inhibitors of nitric oxide synthase that do not interact with the endothelial cell L-arginine transporter. British journal of pharmacology 105, 768–770 (1992).
Chafai, A., Fromm, M. F., Konig, J. & Maas, R. The prognostic biomarker L-homoarginine is a substrate of the cationic amino acid transporters CAT1, CAT2A and CAT2B. Scientific reports 7, 4767, https://doi.org/10.1038/s41598-017-04965-2 (2017).
Wu, G., Bazer, F. W., Hu, J., Johnson, G. A. & Spencer, T. E. Polyamine synthesis from proline in the developing porcine placenta. Biology of reproduction 72, 842–850, https://doi.org/10.1095/biolreprod.104.036293 (2005).
Mandal, S., Mandal, A., Johansson, H. E., Orjalo, A. V. & Park, M. H. Depletion of cellular polyamines, spermidine and spermine, causes a total arrest in translation and growth in mammalian cells. Proc Natl Acad Sci USA 110, 2169–2174, https://doi.org/10.1073/pnas.1219002110 (2013).
Bazer, F. W., Johnson, G. A. & Wu, G. Amino acids and conceptus development during the peri-implantation period of pregnancy. Adv Exp Med Biol 843, 23–52, https://doi.org/10.1007/978-1-4939-2480-6_2 (2015).
Bjorke-Jenssen, A., Ueland, P. M. & Bjorke-Monsen, A. L. Amniotic Fluid Arginine from Gestational Weeks 13 to 15 Is a Predictor of Birth Weight, Length, and Head Circumference. Nutrients 9, doi:10.3390/nu9121357 (2017).
Kim, S. Overview of Cotinine Cutoff Values for Smoking Status Classification. International journal of environmental research and public health 13, doi:10.3390/ijerph13121236 (2016).
Midttun, O., Kvalheim, G. & Ueland, P. M. High-throughput, low-volume, multianalyte quantification of plasma metabolites related to one-carbon metabolism using HPLC-MS/MS. Analytical and bioanalytical chemistry 405, 2009–2017, https://doi.org/10.1007/s00216-012-6602-6 (2013).
Acknowledgements
We thank all participants for their willingness to participate in the study and the laboratory staff at Bevital AS, Bergen, Norway for analyzing the results. The study was supported by grants from the Foundation to promote research into functional vitamin B12-deficiency. The sponsor of the study had no role in study design, data collection, data analysis, data interpretation, writing of the report or in the decision to submit the paper for publication. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Contributions
T.H. and C.H.F. conducted research, analyzed the data and wrote the manuscript, P.M.U. was responsible for the laboratory analyses, prepared the figure and wrote the paper. A.L. investigated and wrote the manuscript. G.F.T.S. edited and reviewed the manuscript. K.V. and A.-L.B.-M. conceived, designed and performed the study, analyzed the data and wrote the paper. All authors approved the final version.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Helm, T., Varsi, K., Fløtre, C.H. et al. Plasma Homoarginine Concentrations According to Use of Hormonal Contraception. Sci Rep 8, 12217 (2018). https://doi.org/10.1038/s41598-018-30708-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30708-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.