Abstract
The sensing properties of monolayer arsenic phosphorus (AsP) for the adsorption of SF6, H2O, O2, and SF6 decomposition gases (SO2 and H2S) are theoretically investigated by the first-principle calculations. We calculate the adsorption energy, equilibrium distance, Mulliken charge transfer, and electron localization function (ELF) to explore whether AsP is suitable for detecting SF6 decomposition gases. By comparing the adsorption performance of SF6, H2O, O2, and H2S gases, we have revealed that the SO2 gas molecules could form stable chemisorption with AsP monolayer. The results demonstrate that AsP is highly sensitive and selective to SO2 gas molecules with robust adsorption energy and apparent charge transfer. Furthermore, the current-voltage (I–V) curves reveal that only the adsorption of SO2 can largely modify the resistance of AsP. Our results show that gas sensors based on AsP monolayer could be better than that of black phosphorene (BP) to diagnose the state of online gas-insulated switchgear (GIS).
Similar content being viewed by others
Introduction
Sulfur hexafluoride (SF6) is widely used in gas-insulated switchgear (GIS) due to its excellent thermal conductivity, high dielectric strength, arc-extinguishing properties, and chemical inertness1,2. However, trace amounts of O2 and H2O are unavoidable impurities in GIS3. With time going by, the internal insulation defects and aging in GIS equipment may cause partial discharge, which will decompose SF6 into SO2, H2S, and other decomposition products4,5. These decomposition products will further accelerate insulation deterioration in GIS, and even affect the normal work of the electric equipment. Therefore, the online detection of the SF6 decomposition gases in GIS is essential and significant to reduce unnecessary losses caused by the breakdown of GIS equipment. The sensing methods for SF6 decomposition gases include gas chromatography, mass spectrometry, infrared (IR) spectroscopy, ion mobility spectrometry, and metal oxides sensors and so on6,7,8. However, these methods are not suitable for online detection because most of them require sophisticated instruments, well-trained operators, or special operating environment.
Gas sensors based on two-dimensional (2D) materials have drawn considerable attention due to their prominent advantages such as simple, cost-effective, and portable as well as high precision and sensitivity9,10. A lot of 2D materials, such as graphene, phosphorene, MoTe2 and so on, have been applied to detect the SF6 decomposition gases11,12,13. Arsenic phosphorus (AsP) monolayer, which is a phosphorene analogue formed from a 1:1 stoichiometric mixture of P and As. Surprisingly, the electron mobility of AsP monolayer along the armchair direction (~10000 cm2 V−1 s−1) is 1 order of magnitude larger than that of the black phosphorene (BP)14. It’s well known that higher carrier mobility is beneficial to gas sensor applications. More importantly, AsxP1−x (x = 0~0.83) was successfully synthesized recently by using alloying strategy15. It has been reported that Si-doped AsP displays an excellent sensitivity for H2S molecules16. However, there is no previous work reported whether monolayer AsP is suitable for detecting SF6 decomposition gases. Therefore, we firstly investigate the sensing performances of AsP for detecting the main decomposition gases of SF6 (SO2 and H2S) with consideration of the background gas (SF6, H2O, and O2) by using First-Principles.
Results
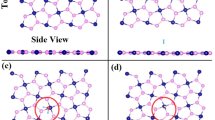
The most stable configurations of the different gas molecules adsorption on AsP monolayer are illustrated in Fig. 1, and the corresponding Ea, d0 and Q are listed in Table 1. The positive sign of Q means charge transfer from monolayer AsP to the adsorbates. As listed in Table 1, the equilibrium distance of SF6, SO2, H2S, H2O, and O2 on the AsP monolayer (3.09, 2.59, 3.07, 2.51, and 2.80 Å, respectively) are larger than P-F (1.75 Å), P-S (2.14 Å), P-H (1.43 Å), and P-O (1.74 Å) bonds17. The Ea of the most energetically favorable structures for SF6, SO2, H2S, H2O, and O2 molecules adsorbed on AsP are −0.480, −1.031, −0.069, −0.433, and −0.342 eV, respectively. Clearly, the adsorption energy of H2S on AsP is significantly smaller than the others, indicating that the AsP monolayer is not suitable for sensing this molecule. The Ea value of SO2 adsorption on AsP monolayer is also larger than that of SO2 adsorption on BP (−0.748 eV)18, indicating that a higher level sensitivity for SO2 detection with AsP than that with BP.
Charge transfer is another important factor to estimate the sensitivity of gas sensors. To further explore the adsorption properties between gas molecules and the AsP monolayer, the electron difference densities (EDD) are shown in Fig. 2. The Mulliken charge transfer results for SF6/H2S-AsP systems show that the charge is depleted on gas molecules and accumulated on the AsP surface, while the other three systems are exactly reversed. When SF6 and H2S molecules are adsorbed on AsP surface, they usually act as charge donors and provide 0.053 and 0.065 e to the AsP monolayer, respectively. H2O and O2 act as a charge acceptor and obtains 0.012 and 0.013 e from monolayer AsP. However, the charge transfer (Q) for these gas molecules is largely smaller than SO2-AsP system (0.151 e transfer from AsP to SO2 molecule). When we look into the EDD of SO2-AsP system, a much more significant charge transfer is observed. These results indicate that the electrostatic interactions between SF6/H2S/H2O/O2-AsP systems are obviously weaker than SO2-AsP system. Thus, the AsP monolayer is not suitable for detecting these four molecules.
To further obtain insight into the charge redistribution of the adsorption system, we plot the electron localization function (ELF) slices in Fig. 3. There is no obvious electron localization overlap between SF6, H2S, H2O, and O2 gas molecules and the AsP monolayer, which means the physisorption feature for these molecules adsorbed on AsP monolayer. The ELF of the SF6-, H2S-, H2O-, and O2-AsP systems do not have electron sharing area between gas molecules and the AsP monolayer, and thus the AsP monolayer is not sensitive to these molecules. For the SO2-AsP system, the electrons are slightly shared between SO2 molecule and AsP monolayer, revealing that the surface charge of the AsP monolayer is largely redistributed after SO2 adsorption. This is consistent with the result of the Mulliken charge transfer. For SO2 adsorption, it would be more reasonable to treat it as chemisorption due to the large binding energy, electron transfer and also slightly overlapped electron distribution as shown in Fig. 3(b). To further explore the adsorption mechanisms of SO2 molecules adsorbed on AsP monolayer, we plot the total electronic densities of states (DOS) and projected density of states (PDOS) in Fig. 4. Obviously, the main electronic level contributions of SO2 to the total system localize between −4 and −1.3 eV in the valence band, 1 and 1.3 eV in the conduction band, which is away from the Fermi level. The electrons are slightly shared between AsP monolayer and SO2 molecule, which reveals the intensity of the interaction between the SO2 molecule and the AsP monolayer. These findings imply that the strong adsorption of SO2 on AsP monolayer is mostly due to the electron Coulomb interaction between the lonely paired electrons of SO2 and the empty orbital of P atom, without hybridization19. Thus, we can deduce that gas sensors based on AsP are sensitive and selective to SO2 gas in the background of the SF6 decomposition gases.
To further verify the validity of our work, we calculate the I–V response of AsP sensor before and after the gas adsorption, as shown in Fig. 5(a). Armchair direction is chosen for transport calculation because the mobility along the armchair direction is significantly larger than the zigzag direction. There is no current (about 0.1 nA) passing through the devices when the bias voltage is smaller than 1.0 V due to the existence of band gap of pure AsP. When bias over 1.0 V, the current starts to increase dramatically. However, for the SO2 adsorption, with the increase of the bias voltage from 1.2 to 2.2 V, the current is clearly smaller than other cases. The reduction of current indicates the resistance of AsP is increased after the SO2 adsorption, which can be easily measured in the experiment. It should be emphasized that the increased resistance is caused by the larger charge transfer between the AsP monolayer and SO2 molecule. To gain deeper insight into the resistance change of AsP caused by the different adsorbates, we plot the current ratios before and after adsorption of gas in Fig. 5(b). It can be found that the current ratios for SO2 adsorption are significantly lower than that for H2S and SF6 adsorption. The value of the current reduction is about 21.3% for SO2 adsorption under a bias of 2.2 V, while the current reductions are 2.9% and 0.8% for H2S and SF6 adsorption respectively under the same bias. The current reduction ratio for SO2 adsorption is about seven times as that for H2S and SF6 adsorption, which can be easily distinguished by the magnification. The current is slightly enhanced after the H2O and O2 adsorption under a bias of 2.2 V. The resistance in AsP monolayer is highly selective and sensitive to SO2 in SF6 decomposition gases, which further demonstrates that it can be an excellent sensing material for online GIS diagnosis.
(a) The current-voltage (I–V) curves along the armchair direction of AsP monolayer before and after SO2, H2S, H2O, O2 and SF6 adsorption. The inset is the top view of the two-probe system of AsP monolayer with SO2 adsorption. (b) Current ratios of the two-probe systems with and without gas molecule adsorption.
Discussion
It was reported that BP could also be used for SO2 gas detection in SF6 decomposition gases11. However, the maximum current reduction is about 7% for SO2 adsorption, and not more than 1.5% for H2S and SF6 adsorption. For comparison purposes, we estimate the sensor response (S) with the formula:
where ΔR is the resistance change after SO2 adsorption and R is the prior resistance of AsP monolayer. It should be emphasized that it is different to compare S directly in experiments because the sensitivities of 2D materials are affected by many factors, such as their thicknesses, detecting areas and so on. Nonetheless, our AsP sensor shows better response to SO2 than BP in the theoretical calculations, indicating that AsP monolayer could do better than BP in fabricating high sensitivity SO2 sensors for application in online GIS diagnosis, at least comparable to BP.
Methods
The first principles calculations based on density functional theory (DFT) are performed using the Atomistix Tool Kit (ATK) codes at room temperature (T = 300 K)20. The generalized gradient approximation (GGA) with Perdew-Burke-Ernzerhof (PBE) exchange-correlation potential is adopted21. Fritz Haber Institute (FHI) pseudopotential using Troullier-Martins scheme with a double-ζ basis set is employed22. Spin polarization is only included during the calculations of the adsorption of O2 because it is a paramagnetic molecule. We use the Grimme’s DFT-D2 dispersion correction approach for van der Waals (vdW) corrections thanks for its higher accuracy23. The vacuum region is set to more than 15 Å to avoid the effect of interaction springing from the adjacent layers. A well conserved Monkhorst-Pack 8 × 8 × 1 k-point mesh is adopted for geometry optimization and electronic properties calculations with a density mesh cutoff energy of 300 Ry. Previously reported optimized lattice constants for monolayer AsP (a = 3.5 Å and b = 4.65 Å) are considered in this work14. We take a 3 × 3 supercell of monolayer AsP. The current-voltage (I–V) characteristics are calculated by using the nonequilibrium Green’s function (NEGF) method24. The k-point sampling is set to 5 × 1 × 100, and the mesh cutoff is set to 200 Ry for I–V calculation. The current of two-probe systems are calculated by the Landauer–Bütiker formula:
where T(E, Vb) is the electron transport coefficient calculated from the Green’s functions, f and u are the Fermi-Dirac distribution function and the electrochemical potential, respectively. The subscripts “r” and “l” represent the right and left electrode.
To find the most stable configurations, we consider four different sites for each gas adsorbed on monolayer AsP, which are set up at the top of upper As/P atom, the middle of As-P bond, and the center of the puckered hexagon. The moderate distance (2.5 Å) between a single molecule and monolayer AsP layer is adopted for each initial adsorption case. On the basis of the above settings, all the configurations are fully optimized and relaxed until the force and stress tolerance are mitigated to less than 0.05 eV/Å and 0.001 eV/Å3, respectively. To study the interactions between monolayer AsP and targeted gas molecules, the adsorption energy (Ea), the Mulliken charge transfer (Q) and the adsorption distance (d0) are systematically calculated. The adsorption energy is defined as:
where Egas, EAsP, and Etotal are the total energy of gas molecule, AsP monolayer, and gas molecule-AsP system, respectively. The adsorption distance is defined as the equilibrium’s nearest atoms between AsP monolayer and gas molecules.
Conclusion
In conclusion, we have investigated the sensing properties of AsP monolayer for two main SF6 decomposition gas molecules (SO2, H2S) and three background gas molecules (SF6, H2O, and O2) adsorption by using the first-principles calculations. The results demonstrate that SF6, H2S, H2O, and O2 gas molecules show physical adsorption on AsP monolayer, while AsP monolayer strongly adsorb SO2 molecules via robust chemical bonds. It is found that the Ea and Q values of SO2 molecule adsorbed on AsP monolayer are obviously larger than the others, which may allow it as a desirable gas sensor for detecting SO2. The I–V curves demonstrate that the resistance of AsP monolayer is only largely affected by SO2 adsorption, indicating that the gas sensors based on AsP are highly sensitive and selective to SO2. Therefore, we can deduce that AsP is a promising candidate for high sensitivity and selectivity SO2 sensing applications in online GIS diagnosis for SF6 decomposition gases.
References
Kim, K. H., Ingole, P. G., Kim, J. H. & Lee, H. K. Experimental investigation and simulation of hollow fiber membrane process for SF6 recovery from GIS. Polym. Adv. Technol. 24, 997–1004 (2013).
Cai, T., Wang, X.-P., Huang Yun, G. & Du Shuang, Y. Infrared Spectrum Analysis of SF6 and SF6 Decomposition. Spectroscopy and Spectral Analysis 30, 2967–2970 (2010).
Fu, Y. et al. Theoretical study of the neutral decomposition of SF6 in the presence of H2O and O2 in discharges in power equipment. Journal of Physics D-Applied Physics 49 (2016).
Beyer, C., Jenett, H. & Klockow, D. Influence of reactive SFx gases on electrode surfaces after electrical discharges under SF6 atmosphere. Ieee Transactions on Dielectrics and Electrical Insulation 7, 234–240 (2000).
Chu, F. Y. SF6 Decomposition in Gas-Insulated Equipment. Ieee Transactions on Electrical Insulation 21, 693–725 (1986).
Dong, M., Zhang, C., Ren, M., Albarracin, R. & Ye, R. Electrochemical and Infrared Absorption Spectroscopy Detection of SF6 Decomposition Products. Sensors 17 (2017).
Zhang, X., Tian, S., Xiao, S., Huang, Y. & Liu, F. Partial Discharge Decomposition Characteristics of Typical Defects in the Gas Chamber of SF6 Insulated Ring Network Cabinet. Ieee Transactions on Dielectrics and Electrical Insulation 24, 1794–1801 (2017).
Zhang, X., Zhang, J., Jia, Y., Xiao, P. & Tang, J. TiO2 Nanotube Array Sensor for Detecting the SF6 Decomposition Product SO2. Sensors 12, 3302–3313 (2012).
Wang, J. et al. A Reusable and High Sensitivity Nitrogen Dioxide Sensor Based on Monolayer SnSe. Ieee Electron Device Letters 39, 599–602 (2018).
Lee, S. W., Lee, W., Hong, Y., Lee, G. & Yoon, D. S. Recent advances in carbon material-based NO2 gas sensors. Sensors and Actuators B-Chemical 255, 1788–1804 (2018).
Yang, A.-J. et al. Phosphorene: A Promising Candidate for Highly Sensitive and Selective SF6 Decomposition Gas Sensors. Ieee Electron Device Letters 38, 963–966 (2017).
Wang, D.-W. et al. MoTe2: A Promising Candidate for SF6 Decomposition Gas Sensors With High Sensitivity and Selectivity. Ieee Electron Device Letters 39, 292–295 (2018).
Zhang, X., Yu, L., Wu, X. & Hu, W. Experimental Sensing and Density Functional Theory Study of H2S and SOF2 Adsorption on Au-Modified Graphene. Advanced Science 2 (2015).
Shojaei, F. & Kang, H. S. Electronic Structure and Carrier Mobility of Two-Dimensional alpha Arsenic Phosphide. Journal of Physical Chemistry C 119, 20210–20216 (2015).
Liu, B. et al. Black Arsenic-Phosphorus: Layered Anisotropic Infrared Semiconductors with Highly Tunable Compositions and Properties. Adv. Mater. 27, 4423–4429 (2015).
Zhang, Y., Tan, C., Yang, Q., Ye, H. & Chen, X.-P. Arsenic Phosphorus Monolayer: A Promising Candidate for H2S Sensor and NO Degradation With High Sensitivity and Selectivity. Ieee Electron Device Letters 38, 1321–1324 (2017).
Pyykko, P. & Atsumi, M. Molecular Single-Bond Covalent Radii for Elements 1–118. Chemistry-a European Journal 15, 186–197 (2009).
Yang, Q. et al. First-Principles Study of Sulfur Dioxide Sensor Based on Phosphorenes. Ieee Electron Device Letters 37, 660–662 (2016).
Dong, H., Wang, L., Zhou, L., Hou, T. & Li, Y. Theoretical investigations on novel SiC5 siligraphene as gas sensor for air pollutants. Carbon 113, 114–121 (2017).
Soler, J. M. et al. The SIESTA method for ab initio order-N materials simulation. Journal of Physics-Condensed Matter 14, 2745–2779 (2002).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 77, 3865–3868 (1996).
Blum, V. et al. Ab initio molecular simulations with numeric atom-centered orbitals. Comput. Phys. Commun. 180, 2175–2196 (2009).
Grimme, S., Antony, J., Ehrlich, S. & Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 132 (2010).
Brandbyge, M., Mozos, J. L., Ordejon, P., Taylor, J. & Stokbro, K. Density-functional method for nonequilibrium electron transport. Physical Review B 65 (2002).
Acknowledgements
This work was supported by the National Key R&D Program of China (2017YFB0402900), the Key Project of Jiangsu Province, China (Grant No. BE2016174), the National Science Foundation of China (No. 61634002, 11604124 and 61604080), the Natural Science Foundation of Jiangsu Province (No. BK20150158 and BK20160883), the Fundamental Research Funds for Central Universities (No. JUSRP51628B), and the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX18_0030).
Author information
Authors and Affiliations
Contributions
C.D.J., L.H., Z.R. and Z.Y.D. conceptualized the study, W.J., Y.G.F., X.J.J. and C.Q. calculated the data and W.J., L.J.M. and C.D.J. wrote the main manuscript text.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jin, W., Guofeng, Y., Junjun, X. et al. High Sensitivity and Selectivity of AsP Sensor in Detecting SF6 Decomposition Gases. Sci Rep 8, 12011 (2018). https://doi.org/10.1038/s41598-018-30643-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30643-y
This article is cited by
-
PH3 gas adsorption on S and Mo vacancy MoS2 monolayer: a first principle study
Journal of Nanoparticle Research (2023)
-
Simulation of gas sensing mechanism of porous metal oxide semiconductor sensor based on finite element analysis
Scientific Reports (2021)
-
Highly Sensitive and Precise Analysis of SF6 Decomposition Component CO by Multi-comb Optical-feedback Cavity Enhanced Absorption Spectroscopy with a 2.3 μm Diode Laser
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.