Abstract
Obesity, often represented by higher body mass index (BMI), is not yet fully understood as a potential risk factor for poor clinical outcomes of prostate cancer (PCa) after radical prostatectomy (RP). This study aimed to evaluate the relationship between BMI and biochemical recurrence (BCR)-free survival in RP patients. This study retrospectively reviewed a total of 2.997 PCa patients who underwent RP between 2006 and 2017. The patients were stratified into three BMI groups according to the WHO recommendations for Asian men: normal weight (<23 kg/m2), overweight (≥23 to <27.5 kg/m2) and obese (≥27.5 kg/m2). Multivariable logistic regression analyses were undertaken to evaluate the factors influencing the BCR rates including BMI. Multivariable Cox regression analyses and Kaplan-Meier analyses were performed to test the association of obesity with BCR-free survival. The final pathologic results showed obese patients had greater positive surgical margin rates (13.9%, p < 0.001), extraprostatic invasion (19.9%, p < 0.001), advanced pathological Gleason score (GS) ≥ 8 (50.8%, p = 0.017), and lymph node invasion (LNI) (14.5%, p = 0.021) than overweight and normal weight patients. According to Kaplan-Meier analyses, obese patients, especially with BMI ≥ 27.5, were more likely to have lower BCR-free-survival. Multivariate Cox analysis revealed that diabetes mellitus, LNI status, pT, pathologic GS, extraprostatic invasion, margin positivity and obesity with BMI ≥ 27.5 kg/m2 were significantly associated with BCR-free survival after RP. Obesity (higher BMI) was significantly associated with BCR after RP. BMI ≥ 27.5 kg/m2 was an independent predictor of BCR-free survival.
Similar content being viewed by others
Introduction
As the prevalence of obesity has increased rapidly during the past two decades, its potential impact on the mortality risk of certain cancers including prostate cancer (PCa) has been emphasized1,2. Moreover, PCa has become a major clinical issue in many countries. Approximately 40% of PCa patients have radical prostatectomy (RP) for their definitive treatment as the current treatment guidelines for organ-confined PCa suggest definitive therapeutic modalities including RP or radiation therapy (RT)3. Obesity as a potential risk factor for poor clinical outcomes of PCa after RP is not yet fully understood, but several explanations have been proposed and the microenvironmental changes in obese patients is one of them. Microenvironmental changes in obese patients may increase the incidence of PCa and produce negative cancer outcomes, such as increase of biochemical recurrence (BCR) or cancer-specific mortality after RP4. Furthermore, some studies have presented poor longitudinal cancer control results among obese patients5,6. Despite various publications of study results supporting the association of obesity with PCa outcomes, several studies were unable to show body mass index (BMI) as a potential hazard of PCa, as they failed to detect profoundly poor outcomes of PCa in a cohort with metabolic syndrome and high BMI7,8.
For predicting adverse clinical outcomes after RP, prostate specific antigen (PSA), clinical stages and Gleason scores (GS) are the most frequently used predictors. In the present study, we hypothesized that BMI might be significantly associated with BCR and BCR-free survival as a negative predictor in PCa patients post-RP. Therefore, this study researched a cohort of RP patients to evaluate the relationship between BMI and BCR-free survival.
Methods
Study population and definitions
After obtaining approval from the Seoul National University Bundang Hospital Institutional Review Board (IRB No. B-1712-439-103), written informed consent for medical research was obtained from all patients and their legal guardians before their inclusion in this research. All study procedures including data collection and management were performed in accordance with relevant guidelines and regulations. In turn, the medical records of patients with PCa were retrospectively reviewed. The patients included in this study underwent open radical prostatectomy (ORP), conventional laparoscopic radical prostatectomy (LRP) or robot-assisted laparoscopic radical prostatectomy (RALP) as their primary treatment for PCa at a single large-volume institution between January, 2006 and May, 2017. Four experienced staff surgeons performed all of the RP procedures without a preference for any specific surgical modality. Among the final study cohort, there were no patients who had received preoperative external beam radiation therapy (EBRT) or neoadjuvant androgen deprivation therapy (ADT). The patients with inadequate preclinical data or follow-up loss were excluded from the research design, which resulted in a total of 2,997 included patients out of 3,325 potentially eligible patients in the present study (Fig. 1). Magnetic resonance imaging (MRI) and bone scan were applied to all candidates during preoperative evaluation and no patient showed evidence of metastasis. Lymph node dissection was performed for the bilateral external and internal iliac vessels, and the obturator fossa according to the extension of lymph node involvement observed in the preoperative evaluation.
Age, BMI, year of RP, PSA, biopsy and pathological GS, clinical tumor stage (cT), pathological tumor stage (pT), margin positivity, surgical methods (ORP vs. LPR vs. RALP), extraprostatic invasion (EPI) status, continence recovery after RP, and potency were included as research variables. The present study used the continence definition of ≤1 pad per day, and the erectile function of the cohort was evaluated by using the International Index of Erectile Function (IIEF-5) questionnaire. The follow-up time (years) was calculated as the time form the date of prostatectomy to the last hospital visit. To evaluate the effect of BMI on PCa outcomes, BMI was stratified into three categories according to the World Health Organization (WHO) recommendations for Asian men as follows: normal weight (<23 kg/m2), overweight (≥23 to <27.5 kg/m2) and obese (≥27.5 kg/m2). According to the recent National Comprehensive Cancer Network (NCCN) guidelines9, the patients were also sorted into three preoperative risk groups as follows: low-risk = T1 to T2a, GS ≤ 6/Gleason grade group 1, and PSA < 10 ng/mL; intermediate-risk = T2b to T2c, or GS 3 + 4 = 7/Gleason grade group 2, or GS 4 + 3 = 7/Gleason grade group 3, or PSA 10–20 ng/mL; and high-risk = > T3a, or GS 8/Gleason grade group 4, or GS 9–10/Gleason grade group 5, or PSA > 20 ng/mL. BCR was defined as two consecutive postoperative PSA values ≥ 0.2 ng/ml.
Statistical analysis
We analyzed the associations between clinical and pathological variables according to different BMI categories by using the Wilcoxon rank sum test. Chi-square tests and Student t-tests were performed to evaluate the differences between subgroups, including the patients with and without diabetes mellitus (DM). In multivariable logistic regression analysis, the effect of obesity and other clinicopathological variables on BCR rates and PCa specific survival (PCSM) were analyzed using unadjusted (crude) and adjusted competing risks. The adjusted data was balanced according to clinicopathological variables such as age, PSA, history of DM, pathological GS, pT, LNI, PSM, and surgical methods. Multivariable Cox regression analyses and Kaplan-Meier analyses were performed to test the effect of obesity on BCR-free survival. For continuous variables, means and interquartile ranges were reported and proportions were supplied for categorical variables. All statistical tests were performed using the SPSS package version 22.0 (SPSS Inc., Chicago, IL, USA), and p values < 0.05 were considered significant.
Results
Baseline characteristics
A total of 2,997 men with PCa who underwent RP were included in this study. The clinicopathological characteristics of the study subjects are presented in Table 1. Among the patients, normal weight (<23 kg/m2), overweight (≥23 to <27.5 kg/m2) and obese (≥27.5 kg/m2) men were 867 (29.9%), 1,799 (60.0%) and 331 (11.1%), respectively. The overall median follow-up time was 3.3 years, and no significant difference in follow-up time was observed between the BMI groups (p = 0.054). No significant preference of a specific surgical type was observed among the different BMI groups, and LRP was the least performed surgical method in all three BMI groups. The number of patients who underwent RALP, LRP, and ORP were 2,250 (75.1%), 23 (0.8%) and 724 (24.1%), respectively. The mean age of the patients was 66.0 ± 6.8 years with a range of 37–82 years and no significant differences were observed between the BMI groups. Other preoperative clinicopathological factors, including the mean baseline PSA level and biopsy GS, were similar among the BMI groups. Regarding pre- and postoperative clinicopathological factors, obese men showed higher rates of stage ≥cT3 (12.0%, p = 0.420) and pathologic GS ≥ 8 (50.8%, p = 0.017), although the percentage of patients with advanced cT was not significantly different among the groups. Moreover, obese patients had greater positive surgical margins (PSM) (13.9%, p < 0.001) and positive LNI rates (14.5%, p = 0.021) in their final pathological results than overweight and normal weight patients. The obese group also had a significantly higher percentage of patients confirmed with stage ≥ pT3b (17.8%, p = 0.002) compared to 13.8% of overweight and 7.0% of normal weight patients.
The EPI rates of the obese and overweight groups were 19.9% and 15.6%, respectively and these values were markedly higher than the 11.1% EPI rate of the normal weight group (p < 0.001). The obese group (BMI ≥ 27.5 kg/m2) had a significantly higher percentage of patients with DM (26%), compared to 16.9% and 8.3% of the patients in the overweight and normal weight groups, respectively (p < 0.001).
Among the study cohort, 593 patients (19.8%) experienced BCR during the follow-up period. The obese group showed profoundly higher BCR rates (30.9%) than the overweight (18.6%) and normal weight group (17.8%) (p < 0.001). In terms of surgical modalities, obese patients presented the highest BCR rates in all three surgical types (ORP 31.6%, LRP 100%, RALP 30.0%). Particularly, only two patients were categorized as obese among the 23LRP received patients, and both these patients underwent BCR.
In terms of continence recovery after RP, 2,136 patients among the study cohort (71.3%) had their continence recovery within ≤3 months from RP, which was defined as early continence recovery in the present study. Moreover, the obese group had a significantly lower rate of early continence recovery (63.7%) compared to the overweight (68.9%) and normal weight (79.0%) groups. In addition, the obese group showed more diminished baseline erectile function than the other two weight groups (total IIEF-5 score: obese = 18.93 ± 4.35 vs. overweight = 20.11 ± 5.12 vs. normal weight = 21.45 ± 3.43, p < 0.001). The total IIEF-5 score measured at 6 months after RP demonstrated that the obese group had the greatest average decrease in potency compared to the other BMI groups (obese = 8.49 ± 6.80 vs. overweight = 11.64 ± 7.28 vs. normal weight = 13.22 ± 4.79).
The results of subgroup analysis according to the presence of DM or age greater than 55 years are presented in Table 2. Diabetic patients had a significantly higher rate of obese patients (18.6%) compared to nondiabetic patients (9.6%) (p < 0.001). Moreover, diabetic patients showed significantly more adverse pathologic features compared with nondiabetic patients, as evidenced by the higher rates of pathological GS ≥ 8, stage ≥ pT3a, EPI, LNI, and PSM in diabetic patients (Table 2). Diabetic patients also showed a higher BCR rate than DM-negative patients (26.2% vs. 19.3%, p = 0.001). When evaluating the clinicopathological characteristics of patients by stratifying the cohorts into two subgroups, age > 55 and age ≤ 55, the younger group with age ≤55 had significantly higher proportions of poor oncologic factors including pathological GS ≥ 8, pathological stage ≥ pT3a, EPI, LNI, PSM, and BCR rates (Table 2).
Multivariate analysis of BCR and PCSM
To evaluate the factors influencing BCR rates after RP, multivariate analyses were performed. Among the preoperative risk factors, PSA, DM, BMI, intermediate- and high-risk groups were significantly related to BCR (Table 3). In addition, pathological GS, pT, LNI, and PSM were independent predictors of BCR among the postoperative factors. In particular, all pathological stages that were more advanced than pT2a, achieved independent predictor status for BCR (Table 3). BMI ≥ 23 kg/m2 was also significantly associated with BCR in the multivariate analysis adjusted for clinicopathological variables including age, PSA, DM, pathological GS, pT, LNI, PSM and surgical methods (Table 3). In the multivariate analyses performed with unadjusted data, PCSM was significantly predicted by several factors such as BMI, intermediate- and high-preoperative risk groups, pT and LNI. Obesity with BMI ≥ 27.5 kg/m2 showed a significant association with PCSM, even in the multivariate analysis with post-adjustment data (Table 3).
Predictors of BCR-free survival
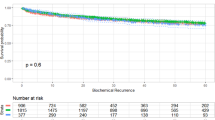
According to Kaplan-Meier analyses performed by sorting patients into three BMI categories, obesity was significantly correlated with BCR-free survival after RP, especially in the patients with BMI ≥ 27.5 kg/m2 (p < 0.001, Fig. 2). Upon categorizing the cohort into preoperative risk groups, low-risk and intermediate-risk patients (Fig. 3A,B) showed more significant differences in BCR-free survival between each BMI group, compared with the high-risk patients (Fig. 3C). Obesity with BMI ≥ 27.5 kg/m2 showed a significant impact on 5-year BCR-free survival among the low-risk group (75.6%), and the impact was greater for the intermediate- (81.4%) and high-risk groups (86.3%), as shown in Fig. 3.
According to multivariate Cox analyses, DM, LNI, pT, pathologic GS, EPI and PSM were statistically significant predictors of BCR-free survival after RP, but age and surgical methods were not (Table 4). Furthermore, obesity with BMI ≥ 27.5 kg/m2 was also an independent predictor of BCR-free survival (hazard ratio (HR) = 1.145; 95% confidence interval (CI): 1.109–1.183; p < 0.001; Table 3).
Discussion
Although many previous studies showed that obesity was associated with both aggressiveness of PCa at the time of diagnosis and decreased BCR-free survival10,11,12,13, the relationship between BMI and BCR is still debatable, as a significant number of prior studies failed to show an association between increased risk of BCR and higher BMI14,15. Thus, this study evaluated whether obesity (higher BMI) is a predictor of BCR and BCR-free survival after RP. The present study results presented that BMI was a significant independent predictor of poor outcomes in terms of BCR and BCR-free survival. Moreover, we found that comorbid DM was associated with BCR and BCR-free survival. Recent studies suggested that higher BMI was associated with an elevated risk of higher GS or higher cancer stage16,17, which coincides with our research results, in which the obese group (BMI ≥ 27.5 kg/m2) contained a significantly greater percentage of men with biopsy GS ≥ 8, stage ≥ cT3, stage ≥ pT3b and pathologic GS ≥ 8. Obese patients also showed a significant association with adverse surgical outcomes such as EPI, LNI and PSM in the current study. According to the results of the multivariate and Kaplan-Meier analyses, it could be postulated that obesity-associated pathological variables, such as higher GS, increased PSM and LNI, simultaneously influenced BCR-free survival to decrease in obese patients. Interestingly, a recent study showed that PCa patients primarily treated with EBRT had increases in BCR, metastases and cancer-specific mortality as BMI increased11. Since, no patients included in this study underwent neoadjuvant EBRT, the effect of radiation therapy on the cohort was minimal from the beginning of the analysis.
There are several explanations for poor oncologic outcomes in obese patients, and technical difficulties of performing surgery on obese men may be one of them. Previous studies have suggested that obesity increases the chance of capsular incision by 30%, and hinders the ideal performance of RP18. Other surgical challenges, such as more difficult lymph node dissection or nerve sparing, might also induce poor outcomes in oncological features. Furthermore, as BCR and BCR-free survival after RP were similar among the different surgical methods in this study, no technical inferiority of a specific surgical method was observed within the cohort. We believe this might be a result of the technical maturity of the surgeons who participated in the present study. Another possible explanation for the association of obesity with poor oncologic outcomes focuses on biological factors. First, obese patients show increased serum insulin, insulin like growth factor-1, interleukin-6 and leptin levels19,20. In contrast, some studies showed decreased adiponectin and serum testosterone levels in obese males21,22,23. Moreover, a retrospective study by Ma and colleagues analyzed the relationship between cancer-specific survival and C-peptide level, which directly represents insulin level, in PCa patients5. According to their research, the effect of BMI on PCa-specific survival was partly controlled by C-peptide level. However, the validity of these factors still needs to be evaluated in further studies.
In this study, the mean age of the patients was similar between BMI groups. However, the obese group showed a 26.0% DM prevalence, which was significantly higher than the other BMI groups. These outcomes are similar to the results of a previous epidemiological study that showed middle to old age men (age > 54 years) had a greater prevalence of DM in the obese or overweight population24. In addition, a prior study reported that the increased prevalence of DM diminished the adverse effects of obesity on BCR in PCa patients7. However, our findings showed diabetic patients had higher BCR rates than nondiabetic patients and 95.7% of the DM positive patients had hemoglobin A1c (HgbA1c) ≥ 6.5%. In our previous study published in 201525, we showed that poor glycemic control with HgbA1c ≥ 6.5% was negatively associated with BCR rates after RP. Therefore, a relatively high proportion of patients with inadequate glycemic control in DM-positive group might have caused poor prognosis in terms of BCR. Ji et al. recently reported that PCa patients with age ≤55 or >75 years are likely to have more aggressive disease compared to the patients between the ages of 55 and 75. In this study, we observed similar results as the patients aged ≤55 years had more adverse pathologic features, including stage ≥ pT3b and higher BCR rate than the patients > 55 years old. Howeverm further studies are needed to confirm the association between specific age groups and PCa aggressiveness.
Yamoah et al.26 reported in their study that the intermediate- and high-risk groups had a worse 7-year freedom from biochemical failure among obese patients (BMI ≥ 30 kg/m2) compared with the low-risk group. This study showed similar results by presenting 75.6% 5-year BCR-free survival in the high-risk group among obese patients (BMI ≥ 27.5 kg/m2), whereas the intermediate- and low-risk groups showed 86.3% and 81.4%, respectively. However, in terms of overall BCR-free survival, the high-risk group had a smaller difference between each BMI group with no statistical significance (p = 0.203). According to the corresponding study results, we believe obese patients with NCCN high-risk should be more carefully approached than normal weight patients during the planning of appropriate treatment option, to provide the best oncological outcomes.
A prior meta-analysis study described that an increase of BMI by 5 kg/m2 caused the risk of PCSM to be increased by 20%15. Another previous study presented that higher BMI was associated with greater PCSM in RP patients (HR 1.58 for BMI ≥ 35 kg/m2)27. Our results also suggested obesity with higher BMI might be a risk factor for PCSM and the findings were similar to the prior research outcomes. However, as either more aggressive PCa or less aggressive treatment can cause a variation of PCSM25, further evaluations that account for the effect of adjuvant therapy are required to confirm the association between higher BMI and PCSM.
There have been few studies that evaluated the association of obesity with poor oncologic outcomes of PCa in the Asian population. As this study assessed a purely Asian population with PCa, the difference between our study results and other studies focused on Western ethnic groups might be the result of variances in obesity prevalence.
For further evaluation, we also conducted additional analyses regarding the relationship between BMI and continence recovery after RP. According to our results, obese men had a significantly lower rate of early continence recovery after RP compared to normal or overweight patients. This result is similar to the previous studies28,29, including the study of Bacon et al.28, which presented normal weight patients having a significantly higher continence recovery rates than overweight and obese patients at 12 months post RP (continence rate: 70% vs 68% vs 57%, p = 0.03). Due to the corresponding results concerning continence recovery, we believe that obese patients should be consulted for the higher risk of post-RP at the begging of surgical planning. Moreover, anti-incontinence surgical techniques including bladder neck preservation should be actively considered when RP is performed.
Some previous studies suggested an association between obesity and erectile function29,30. In a study of Wiltz et al.29, they showed that obese patients had poorer preoperative potency than normal weight and overweight males. Ahlering et al.31 also presented that obese patients had persistently lower potency rates compared with other weight groups during 24 months follow-up after RALP. Our study results showed that both pre- and post-RP erectile function was poorer in obese patients, and these results are similar to the outcomes from the previous results30,31. We believe higher risks of endothelial dysfunction and a greater rates of medical comorbidities, including DM caused profound erectile dysfunction in obese patients.
This study had several limitations. It was a retrospective cross-sectional study based on the medical records from a single institution. Thus, the treatments of the study cohort, such as postoperative ADT type and duration, and RT protocols, were heterogeneous, and a further research is needed to confirm whether the results are applicable to the general population or other ethnic groups. We used only BMI to represent obesity. BMI may not be useful in distinguishing pure adipose tissue from lean body mass in patients with relatively larger proportions of muscle mass because BMI encompasses lean body mass in its definition. Thus, other obesity indicators such as the amount of visceral adipose tissue, which is often represented by waist circumference, need to be included in further studies. Moreover, further studies with longer follow-up periods and molecular-level evaluation of the effects of obesity on oncological outcomes are needed.
Conclusions
In conclusion, this study analyzed the effects of obesity (higher BMI) on BCR and BCR-free survival after RP in Asian population with PCa. Our study results demonstrated that obesity was significantly associated with BCR in PCa patients post-RP. Obesity (BMI ≥ 27.5 kg/m2) was a strong independent predictor of BCR-free survival. However, confirmation of the obesity influence on PCa remains debatable, and further studies, including consideration of pathophysiologic mechanisms at the molecular-level, are warranted.
References
Ligibel, J. A. et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 32, 68–74 (2014).
Calle, E. E., Rodriguez, C., Walker-Thurmond, K. & Thun, M. J. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 348, 1625–38 (2003).
Welch, H. G. & Albertsen, P. C. Prostate cancer diagnosis and treatment after the introduction of prostate-specific antigen screening. 1986–2005. J Natl Cancer Inst. 101, 1325–29 (2009).
Lee, A. & Chia, S. J. Prostate cancer detection: the impact of obesity on Asian men. Urol Oncol. 33(266), e17–e22 (2015).
Ma, J. et al. Prediagnostic body mass index, plasma peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 9, 1039–47 (2008).
Gong, Z., Agalliu, I., Lin, D. W., Stanford, J. L. & Kristal, A. R. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 109, 1192–202 (2007).
Post, J. M. et al. The metabolic syndrome and biochemical recurrence following radical prostatectomy. Prostate Cancer. 2011, 245642 (2011).
Jaggers, J. R. et al. Metabolic syndrome and risk of cancer mortality in men. Eur J Cancer. 45(10), 1831–8 (2009).
The 2017 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2, 10–12 (2017).
Vidal, A. C. et al. Obesity increases the risk for high-grade prostate cancer: results from the REDUCE study. Cancer Epidemiol Biomarkers Prev. 23, 2936–42 (2014).
Wang, L. S. et al. Impact of obesity on outcomes after definitive dose escalated intensity-modulated radiotherapy for localized prostate cancer. Cancer. 121(17), 3010–7 (2015).
De Nunzio, C. et al. Abdominal obesity as risk factor for prostate cancer diagnosis and high grade disease: a prospective multicenter Italian cohort study. UrolOncol. 31, 997–1002 (2013).
Tomaszewski, J. J. et al. Obesity is not associated with aggressive pathologic features or biochemical recurrence after radical prostatectomy. Urology. 81(5), 992–6 (2013).
Asmar, R., Beebe-Dimmer, J. L., Korgavkar, K., Keele, G. R. & Cooney, K. A. Hypertension, obesity and prostate cancer biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis. 16(1), 62–6 (2013).
Cao, Y. & Ma, J. Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila). 4(4), 486–501 (2011).
Amling, C. L. et al. Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol. 22(3), 439–45 (2004).
Wright, M. E. et al. Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer. 109(4), 675–84 (2007).
Freedland, S. J. et al. Obesity and capsular incision at the time of open retropubic radical prostatectomy. J Urol. 174(5), 1798–801 (2005).
Mistry, T., Digby, J. E., Desai, K. M. & Randeva, H. S. Obesity and prostate cancer: a role for adipokines. Eur Urol. 52(1), 46–53 (2007).
Hoda, M. R. & Popken, G. Mitogenic and anti-apoptotic actions of adipocyte-derived hormone leptin in prostate cancer cells. BJU Int. 102, 383 (2008).
Allott, E. H., Masko, E. M. & Freedland, S. J. Obesity and prostate cancer: weighing the evidence. Eur Urol. 63(5), 800–9 (2013).
Agalliu, I. et al. The impact of obesity on prostate cancer recurrence observed after exclusion of diabetics. Cancer Causes Control. 26(6), 821–30 (2015).
Ohwaki, K., Endo, F. & Hattori, K. Visceral adipose tissue measured by computed tomography and high-grade prostate cancer after radical prostatectomy. International Journal of Obesity. 39(11), 1659–61 (2015).
Kwon, J. W. et al. Effects of age, time period, and birth cohort on the prevalence of diabetes and obesity in Korean men. Diabetes Care. 31(2), 255–60 (2008).
Ji, G. et al. Are the pathological characteristics of prostate cancer more aggressive or more indolent depending upon the patient age? Biomed research international. 2017, 1438027 (2017).
Yamoah, K. et al. The impact of body mass index on treatment outcomes for patients with low-intermediate risk prostate cancer. BMC Cancer. 16, 557 (2016).
Chalfin, H. J. et al. Obesity and long-term survival after radical prostatectomy. J Urol. 192(4), 1100–4 (2014).
Bacon, C. G. et al. A prospective study of risk factors for erectile dysfunction. J Urol. 176(1), 217–21 (2006).
Wiltz, A. L. et al. Robotic radical prostatectomy in overweight and obese patients: oncological and validated-functional outcomes. Urology. 73(2), 316–22 (2009).
Blanker, M. H. et al. Correlates for erectile and ejaculatory dysfunction in older Dutch men: a community based study. J Am Geriatr Soc. 49, 436 (2001).
Ahlering, T. E., Eichel, L., Edwards, R. & Skarecky, D. W. Impact of obesity on clinical outcomes in robotic prostatectomy. Urology. 65, 740–4 (2005).
Acknowledgements
This research was neither funded nor supported by any institution or organization. The results published here belong to the medical assets of Seoul National University Bundang Hospital. The authors wish to acknowledge all participating investigators.
Author information
Authors and Affiliations
Contributions
Conceived, designed, supervised experiments: Y.D.Y., S.K.H. Performed the experiments: Y.D.Y., S.K.H., S.S.B., S.E.L. Analyzed the data: Y.D.Y., S.S.B., S.K.H. Wrote the manuscript: Y.D.Y.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yu, Y.D., Byun, SS., Lee, S.E. et al. Impact of Body Mass Index on Oncological Outcomes of Prostate Cancer Patients after Radical Prostatectomy. Sci Rep 8, 11962 (2018). https://doi.org/10.1038/s41598-018-30473-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30473-y
This article is cited by
-
Obesity and biochemical recurrence in clinically localised prostate cancer: a systematic review and meta-analysis of 86,490 patients
Prostate Cancer and Prostatic Diseases (2022)
-
Overweight and obesity as risk factors for biochemical recurrence of prostate cancer after radical prostatectomy
International Journal of Clinical Oncology (2022)
-
Prediction of a positive surgical margin and biochemical recurrence after robot-assisted radical prostatectomy
Scientific Reports (2021)
-
The impact of robotic colorectal surgery in obese patients: a systematic review, meta-analysis, and meta-regression
Surgical Endoscopy (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.