Abstract
It is unclear whether non-alcoholic fatty liver disease (NAFLD) is an independent risk factor for cardiovascular disease. We examined the independent impact of NAFLD on the progression of the coronary artery calcification (CAC) score, a well-known marker of atherosclerosis progression. We examined 1,173 asymptomatic participants who underwent repeated CAC score measurement during routine health examinations. The subjects were categorised into four groups based on the presence (+) or absence (−) of NAFLD and metabolic syndrome (MetS). The progression of CAC score was defined as either incident CAC in a CAC-free population at baseline or an increase of ≥2.5 units between the baseline and the final square roots of the CAC scores of participants with detectable CAC at baseline. CAC progression was seen in 18.6% (98/526), 28.3% (77/272), 29.1% (30/103) and 32.0% (87/272) of the subjects with NAFLD(−)/MetS(−), NAFLD(+)/MetS(−), NAFLD(−)/MetS(+) and NAFLD(+)/MetS(+), respectively. The subjects with NAFLD(+)/MetS(+) and NAFLD(+)/MetS(−) had a significantly higher risk of CAC progression than those with NAFLD(−)/MetS(−) (multivariate-adjusted odds ratio [OR]: 1.76; 95% confidence interval [CI]: 1.18–2.62 and multivariate-adjusted OR: 1.53, 95% CI: 1.05–2.23, respectively). NAFLD is an independent risk factor for CAC progression, irrespective of the presence of MetS.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is emerging as the leading cause of chronic liver disease worldwide1. Furthermore, NAFLD is not only associated with liver-related morbidity and mortality, but also with serious extrahepatic complications, including cardiovascular disease (CVD)2. However, the importance of NAFLD as an independent contributor to CVD is still uncertain because numerous cardiovascular risk factors are shared by both NAFLD and CVD2. In addition, NAFLD has been recognised as the hepatic manifestation of metabolic syndrome (MetS) and insulin resistance3. Therefore, whether NAFLD is merely a part of the systemic derangement composing MetS or whether NAFLD is an important CVD risk factor still remains unclear.
The coronary artery calcification (CAC) score, measured by multi-detector computed tomography (MDCT), reflects the overall coronary plaque burden and a high CAC score is independently and incrementally predictive of future coronary events and prognosis4. Moreover, recent studies show that CAC score progression is significantly related to higher risk of future CVD events and all-cause mortality5,6. Because atherosclerosis is a dynamic process, CAC score progression provides a better reflection of atherosclerosis progression than the baseline CAC score, making the assessment of the effectiveness of medical treatment and the risk of future CVD events possible5,7.
A recent meta-analysis demonstrated a significant positive association between NAFLD and CAC, supporting the role of NAFLD as an independent predictor of CVD8. However, to date, few studies have evaluated whether NAFLD is longitudinally associated with CAC progression, independently of MetS. In this study, we aimed to investigate the impact of NAFLD and MetS on CAC progression in an asymptomatic, middle-aged, Korean population. To clarify the independent impact of NAFLD on CAC progression, we analysed the risk of CAC progression in subjects divided into four groups based on the presence (+)/absence (−) of NAFLD and MetS.
Results
Baseline characteristics of the participants based on the presence of NAFLD and/or MetS
We divided the 1,173 participants (mean age, 54.1 ± 7.4 years; range, 33–79 years) into four sub-groups based on the presence or absence of NAFLD and/or MetS as follows: (1) subjects without either abnormality (n = 526; 44.8%); (2) subjects with NAFLD only (n = 272; 23.2%); (3) subjects with MetS only (n = 103; 8.8%) and (4) subjects with both abnormalities (n = 272; 23.2%). The baseline characteristics of the subjects in each sub-group are shown in Table 1. Overall, males predominated (81.5%). The subjects with NAFLD only or with both abnormalities were more likely to be male. Comparison of these parameters among the groups showed that the NAFLD only group, the MetS only group and the group with both abnormalities had unfavourable metabolic profiles. All three groups had higher body mass index (BMI), waist circumference (WC), triglyceride (TG), fasting plasma glucose (FPG), glycosylated haemoglobin (HbA1c), uric acid, high-sensitive C-reactive protein (hsCRP) and lower high-density lipoprotein cholesterol (HDL-C) than the group without either abnormality. The 10-year Framingham risk score (FRS) and the 10-year atherosclerotic CVD (ASCVD) risk scores in the NAFLD only group were also significantly higher than those in normal subjects (Table 1). The proportion of subjects with hypertension in the MetS only group and the group with both abnormalities was significantly higher than that in the NAFLD only group, although the NAFLD only group demonstrated a higher prevalence of hypertension than the normal subjects (Table 1). The alanine aminotransferase (ALT) level in the NAFLD only group was higher than that in the MetS only group; however, the opposite was true for the gamma-glutamyltransferase (GGT) level (Table 1). The mean age, total cholesterol and low-density lipoprotein cholesterol (LDL-C) and follow-up intervals did not differ among the four groups (Table 1).
Association between the presence of NAFLD and/or MetS and the baseline CAC score
The baseline CAC score in the NAFLD only group, the MetS only group and the group with both abnormalities was higher than that in the control group (Table 1). A baseline CAC score of > 0 was seen in 42.2% of the population. The proportion of subjects with a baseline CAC of > 0 was highest in the group with both abnormalities and lowest in the group without either abnormality (52.9% vs. 34.4%, respectively; Fig. 1). Both the NAFLD only and MetS only groups also had significantly higher proportions of detectable CAC at baseline than the control group (42.6% vs. 52.4% vs. 34.4%, respectively; Fig. 1).
Multiple logistic regression analysis was performed with the baseline CAC score as a dependent variable; odd ratios (ORs) and 95% confidence intervals (CIs) were calculated for the presence of NAFLD and/or MetS (Table 2). In an unadjusted model, subjects with NAFLD only (OR = 1.42, 95% CI = 1.05–1.91), MetS only (OR = 2.10, 95% CI = 1.37–3.22) and both NAFLD and MetS (OR = 2.14, 95% CI = 1.59–2.89) had significantly higher risks of detectable CAC than normal subjects (Table 2). However, the significance of these relationships in the NAFLD only and MetS only groups was lost after adjusting for age, sex, BMI, smoking, drinking, exercise habits, follow-up interval, LDL-C and hsCRP (Table 2).
Baseline characteristics of the participants based on the CAC score progression
Compared with non-progressors, progressors were significantly older and demonstrated higher BMI, WC and systolic and diastolic blood pressure (BP). In addition, progressors were more likely to be male, current smokers, frequent drinkers and less physically active. In addition, progressors had a less favourable risk profile, which included higher prevalence of hypertension and diabetes and higher levels of FPG, HbA1c, TG, uric acid, AST, ALT, GGT and hsCRP. The 10-year FRS and 10-year ASCVD risk scores in progressors were also significantly higher than those in non-progressors. Progressors had higher baseline CAC scores and tended to be followed-up for a longer period (Table 3).
Association between the presence of NAFLD and/or MetS and the CAC score progression
The proportion of subjects showing CAC score progression in the NAFLD only group, the MetS only group and the group with both abnormalities was significantly higher than that in the control group (28.3%, 29.1%, 32.0% and 18.6%, respectively) (Fig. 2). A statistically significant difference was found between the control and the other groups, but not among the three groups with abnormalities (Fig. 2).
Multiple logistic regression analysis was performed to compare the association of CAC progression with NAFLD and/or MetS and the results are shown in Table 4. In an unadjusted model including the whole population, the NAFLD only group (OR = 1.73, 95% CI = 1.22–2.43), the MetS group (OR = 1.80, 95% CI = 1.11–2.90) and the group with both abnormalities (OR = 2.05, 95% CI = 1.47–2.88) all showed significantly higher risks of progression of CAC than the control group (Table 4). Although the MetS only group did not show CAC progression (OR = 1.54, 95% CI = 0.90–2.63) in a model adjusted for age, sex, BMI, smoking, drinking, exercise habits, baseline CAC score, follow-up interval, LDL-C and hsCRP, the risk of CAC progression in the NAFLD only group and the group with both abnormalities was attenuated but remained statistically significant (OR = 1.53, 95% CI = 1.05–2.23 for the NAFLD only group; OR = 1.76, 95% CI = 1.18–2.62 for the group with both NAFLD and MetS) (Table 4).
Discussion
In the present study, we examined the association between NAFLD, MetS and the progression of CAC in the asymptomatic, middle-aged, Korean population. Although the baseline CAC score was not significantly different, subjects with NAFLD but not MetS had a significantly higher risk of CAC progression compared with the subjects in the control group. We observed that a higher proportion of participants showed CAC progression in the NAFLD only group (28.3%) compared with the control group (18.6%) (Fig. 2). In a logistic regression analysis, the risk of CAC progression in subjects with both NAFLD and MetS was significantly higher than that in the normal subjects (OR = 1.76, 95% CI = 1.18–2.62). More importantly, the subjects with NAFLD but not MetS also had a significantly higher risk of CAC progression (OR = 1.53, 95% CI = 1.05–2.23), whereas the subjects with MetS only did not show significant CAC progression (OR = 1.54, 95% CI = 0.90–2.63) (Table 4). Our results indicated that NAFLD was closely associated with CAC progression, irrespective of the presence of MetS.
Previous studies reported a higher risk of CAC in subjects with NAFLD5,7,9,10 and, recently, a meta-analysis showed that patients with NAFLD had a higher risk of CAC than subjects without NAFLD based on multivariable-adjusted estimates8. However, the present study did not show a significant association between the presence of NAFLD only and the baseline CAC score after adjusting for other risk factors (Table 2), which appears to conflict with prior studies reporting an independent association after adjusting for confounding variables. A possible explanation is that our population mainly included low-risk participants because we recruited our participants during their routine health examinations and excluded those with a history of CVD. Our population included only 11.3% participants with a CAC score higher than 100 at the initial examination (data not shown). Therefore, this characteristic of our population might attenuate the power of the study to discriminate differences in baseline CAC score among the groups.
Although the baseline CAC score, measured by MDCT, has been established as a surrogate marker for coronary atherosclerosis, recent studies have shown that CAC score progression is significantly associated with higher risk of future cardiovascular events and all-cause mortality and represents a useful predictor of future cardiac events; it has, therefore, been proposed for use in the assessment of the effectiveness of medical therapies5,11. Considering that atherosclerosis progression is a dynamic and continuous process, monitoring of CAC progression using serial CAC scanning may be a more useful predictor of a patient’s risk of future events than the baseline CAC score12. In light of these findings, we assessed the CAC score progression using serial MDCT scans, which were performed a mean of 3 years apart. Interestingly, subjects with NAFLD only showed a significantly higher risk for CAC progression. These results indicate that NAFLD per se still has a significant relationship with CAC progression even after adjusting for known metabolic factors as confounders.
Although the pathophysiological mechanisms on how NAFLD affects CAC progression cannot be elucidated based on the results of this study, several plausible mechanisms have been suggested13,14,15,16,17,18,19. Endothelial dysfunction of the systemic circulation, the first step in the process of coronary atherosclerosis, has been observed in NAFLD13,14. In addition, several studies have demonstrated a positive association between liver fat and prothrombotic factors, including factors VII, IX, XI and XII and the plasminogen activator inhibitor-115,16. This procoagulant imbalance in NAFLD may thus represent a causative link between NAFLD and CVD. Greater oxidative stress might also explain the high cardiovascular risk associated with NAFLD. Plasma homocysteine is a cardiovascular risk factor because of its adverse effects on cardiovascular endothelium and smooth muscle cells and high levels of plasma homocysteine have been consistently reported in NAFLD17,18. Finally, because the liver contains the largest number of macrophages and immune cells, cytokines secreted by the injured liver have been proposed to be one of the major pathogenic mechanisms generating systemic inflammation that leads to CVD19. Although pathophysiological mechanisms were not investigated in this study, the mechanisms described above could provide explanations for the association between NAFLD and CAC progression.
The most novel finding of this study is the independent association between NAFLD and CAC progression in individuals without MetS. Recently, a large meta-analysis of observational studies indicated that NAFLD is significantly associated with a higher risk of fatal and non-fatal CVD events20. However, whether NAFLD is associated with higher risk for CVD beyond the conventional cardiovascular risk factors and co-morbidities such as MetS remains uncertain. Previous studies have shown that individuals with MetS have a higher risk of cardiovascular morbidity and mortality21. Jelavic et al. demonstrated that MetS by National Cholesterol Education Program (NCEP) Adult Treatment Panel III (ATP III) as a pathophysiological concept is relevant and superior to its components in risk prediction of patients with acute ST elevation myocardial infarction urgently treated with primary percutaneous coronary intervention22. Furthermore, MetS was found to be an independent predictor of the rapid development or progression of CAC in a large retrospective longitudinal study23. NAFLD and MetS share common pathophysiological pathways and risk factors, including central obesity, hypertension, dyslipidemia and dysglycaemia24. The homeostatic model assessment of insulin resistance and hsCRP were reported to be independently associated with fatty liver index, implying that insulin resistance and subclinical inflammation have important roles in NAFLD and MetS25,26. In addition to these conventional CVD risk factors, hypercoagulation, impaired fibrinolysis, obstructive sleep apnoea, hyperuricaemia and polycystic ovary syndrome are frequently present in both NAFLD and MetS24. Furthermore, common therapeutic approaches, including lifestyle intervention, some anti-obesity and anti-diabetic medications and statins are beneficial for both NAFLD and MetS24. Thus, the common pathophysiology, risk factors and therapeutic approaches support that NAFLD is regarded as a hepatic manifestation of MetS24. Taking these findings together, it remains unclear whether NAFLD affects cardiac outcomes through the effects of metabolic risk factors it shares with MetS or NAFLD alone. Thus, clarification of whether NAFLD per se has an independent association with CVD is important. The present study is the first to show that subjects with NAFLD only, without MetS, have a higher risk of CAC progression than healthy subjects. This result suggested that NAFLD could have a harmful effect on the cardiovascular system, irrespective of the presence of MetS. Therefore, detecting and treating NAFLD in metabolically healthy patients are important.
Our findings implied that the presence of NAFLD or MetS in healthy population represented a risk factor for atherosclerosis and future CVD, implying that CV risk factors should be treated in these individuals. In patients with MetS, beyond lifestyle therapies directed toward underlying risk factors, attention must be given to metabolic risk factors, including hypertension and atherogenic dyslipidemia27. The joint guidelines of the European Association for the Study of the Liver, European Association for the Study of Diabetes and European Association for the Study of Obesity and a recent expert panel statement both suggest lifestyle management and statins for NAFLD to decrease LDL-C and CVD risk28,29. Dyslipidaemia is frequently associated with NAFLD, and patients with NASH have increased levels of small, dense LDL3 and LDL4 compared with those with simple steatosis; LDL3 and LDL4 implicate the crucial role of dyslipidaemia for CVD in NAFLD30,31. Although there is concern that patients with NAFLD and dyslipidaemia could develop liver enzyme elevation, evidence from previous studies showed that statins can be used safely to treat dyslipidaemia in patients with NAFLD30. Furthermore, in some studies, reduction and/or normalisation of liver enzymes due to statin use has been observed30,32,33,34,35.
Several therapeutic interventions for NAFLD, including anti-diabetic medications, have been proposed36. Rizvi et al. reported that liraglutide significantly reduced carotid IMT, a surrogate marker of atherosclerosis, independently of glucometabolic changes in diabetic subjects with NAFLD37. A recent meta-analysis has found a significant improvement of NAFLD in patients with type 2 diabetes mellitus treated with incretin-based therapies38. However, Smits et al. reported the conflicting data that hepatic fat contents and hepatic fibrosis scores were not altered by liraglutide or sitagliptin39. However, there are conflicting results regarding the effects of incretin-based therapies on NAFLD39,40. These discrepancies could be partly caused by the difference of statin use in study subjects, which can influence the interpretation of the results because statins can improve NAFLD40. Apart from anti-diabetic drugs, NAFLD/NASH may be improved in terms of both biochemical and histological features by statins40,41,42. Taken all previous findings together, statin treatment may be beneficial in patients with NAFLD.
This study had several limitations. First, this was a retrospective analysis without a histopathological investigation; therefore, causality cannot be established. However, the previously reported pathological mechanisms that underlie the relationship between NAFLD and CVD might explain our findings. Second, we could not obtain quantitative histories of alcohol consumption of the participants; therefore, we could not discriminate between NAFLD and alcoholic FLD. Third, prescribing statins after study enrolment was not considered in the analyses and these drugs might have contributed to the calcification of coronary plaques43. Finally, the definition of CAC progression we used might be problematic because there is no consensus regarding this yet44. Most previous studies assessed CAC progression by measuring absolute changes in CAC the scores between baseline and follow-up6,44 or mean changes in the square-root-transformed (SQRT) method5,45. The large number of zeros and skewed distribution of changes in CAC may also have compromised the precision of the cut-off value used to define progression44. However, the best CAC progression model for the prediction of mortality has been shown to be the SQRT method, which we chose to use in this study and a SQRT difference of 2.5 provides the best fit for the data5.
In conclusion, this study was the first to demonstrate that NAFLD is an independent contributor to CAC progression, irrespective of the presence of MetS. Our data suggest that special attention should be paid to those individuals with NAFLD but not MetS because they are at high risk for the development of CVD.
Methods
Ethics statement
In accordance with the ethical guidelines of the declaration of Helsinki and Korea Good Clinical Practice, this study was approved by the institutional review board of the Asan Medical Center (AMC). All participants provided written informed consent.
Study population
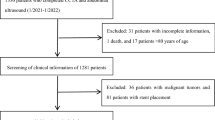
The study population consisted of 7,300 participants who underwent baseline coronary computed tomography angiography (CCTA) using a 64-slice MDCT scanner during routine health evaluation at AMC (Seoul, Republic of Korea) between January 2007 and June 2011. Of these, repeat CCTA was performed on 1,591 participants until December 2014. This analysis also used data obtained using in-person follow-up examinations conducted after the baseline examinations. Each participant completed a questionnaire that listed a history of previous medical and/or surgical diseases, medications and drinking and smoking habits. The drinking habits were categorised based on frequency (1 or 2 times/week [moderate drinker]), the smoking habits as non-current or current and the exercise habits based on frequency (2 or 3 times/week [physically active])46. A history of CVD was recorded based on each participant’s history of physician-diagnosed angina, myocardial infarction and/or cerebrovascular accidents. Participants with an FPG of ≥7.0 mmol/L and/or HbA1c level of ≥6.5% were categorised as diabetic47. In addition, participants who reported the use of anti-diabetic medications on a self-report questionnaire were considered to have diabetes48. Hypertension was recorded if subjects had a systolic and/or diastolic BP of ≥140/90 mmHg or if they were receiving anti-hypertensive medications. The 10-year FRS and 10-year ASCVD risk score were calculated to estimate the cardiovascular risk49. The 10-year ASCVD risk was estimated using the Pooled Cohort Equations for non-Hispanic whites, which was developed by the Risk Assessment Work Group50.
Participants with a history of CVD at baseline examination (n = 95) and those that were receiving statins (n = 238) were excluded. Participants who underwent percutaneous coronary intervention (n = 8) or coronary arterial bypass surgery (n = 3) after the initial examination were also excluded. Subjects that were not between 20 and 79 years were also excluded (n = 3). Finally, participants with hepatitis B surface antigen (n = 48), positive hepatitis C antibody test (n = 19) and liver cirrhosis or hepatocellular carcinoma (n = 4), as well as recipients of liver transplantation (n = 2), were excluded. Some participants met more than two exclusion criteria. After excluding ineligible subjects, 1,173 subjects, with a mean age of 54.1 years (range, 33–79 years), were enrolled in the final study population.
Clinical and laboratory measurements
Height and body mass were measured with the participants wearing light clothing and no shoes. BMI was calculated as body mass in kilograms divided by the square of the height in meters. WC (in cm) was measured mid-way between the costal margin and the iliac crest at the end of normal expiration. BP was measured on the right arm after resting for 5 min using an automatic manometer with an appropriate cuff size. After overnight fasting, early-morning blood samples were drawn from the antecubital vein into vacuum tubes and subsequently analysed by the central, certified laboratory at AMC. Measurements included concentrations of fasting glucose, insulin, hsCRP, several lipid parameters and liver enzymes.
Fasting total cholesterol, HDL-C, LDL-C, TG, uric acid, AST and ALT levels were measured using enzymatic colorimetric methods on a Toshiba 200FR Neo analyser (Toshiba Medical System Co., Ltd.). GGT was measured using the L-g-glutamyl-p-nitroanilide method (Toshiba Medical System Co., Ltd.). FPG and hsCRP were measured using the enzymatic colorimetric method on the Toshiba 200 FR auto-analyser and the immunoturbidimetric method (Toshiba Medical System Co., Ltd.), respectively. Ion-exchange high-performance liquid chromatography (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to measure the HbA1c levels. All enzyme activities were measured at 37 °C.
Definitions of NAFLD and MetS
Hepatic ultrasonography was performed to diagnose NAFLD (Ultrasound Systems IU22; Philips, Holland) by experienced radiologists who were blinded to the laboratory and clinical details of the study subjects at the time of the procedure. Fatty liver was diagnosed based on the characteristic ultrasonographic features that were consistent with ‘bright liver’ and evident contrast between hepatic and renal parenchyma, vessel blurring, focal sparing and narrowing of the lumen of the hepatic veins1.
MetS was defined based on the criteria established by the NCEP-ATP III using Asian-specific cut-off points for abdominal obesity, as recommended in the criteria51,52. An individual was classified as having MetS if the following five criteria were met: (1) WC of ≥90 cm in men and ≥80 cm in women; (2) TG ≥150 mg/dL (1.7 mmol/L); (3) HDL-C of < 40 mg/dL (1.0 mmol/L) in men and < 50 mg/dL (1.3 mmol/L) in women; (4) BP ≥130/85 mmHg or the use of anti-hypertensive medication and (5) fasting glucose ≥100 mg/dL (6.1 mmol/L) or the self-reported use of anti-diabetic medication (insulin or oral agents).
Use of MDCT to assess the CAC score
MDCT examinations were performed using either 64-slice, single-source, CT (LightSpeed VCT; GE, Milwaukee, WI, USA) or dual-source CT (Somatom Definition or Somatom Definition Flash; Siemens, Erlangen, Germany)53,54. The CAC score was calculated using an automated software program and the Agatston scoring method55 and the participants were categorised based on the cut-off points used by Greenland et al.56 (none, 0; mild, 1–100; moderate, 101–300; severe, > 300).
CAC progression was defined as (1) incident CAC, indicating a baseline Agatston score of zero but detectable CAC at the follow-up examination in a population free of CAC at baseline57,58 or (2) an increase of ≥2.5 units between the baseline and the final square root of CAC scores in participants with detectable CAC at baseline5,59,60. To eliminate the dependence of residual inter-scan variability on the baseline CAC score, square root transformation of the CAC score was performed before the estimation of CAC progression. Using the data published by Hokanson et al., ‘progressors’ were defined as individuals with a difference of ≥2.5 units between the baseline and the final square root of their CAC scores (the SQRT method)5,59,60. Expressed differently, a change of < 2.5 units between the baseline and the final square root of the CAC score was considered to be within the margin of error for CAC score estimation using MDCT, and this was thus attributed to inter-scan variability. Such participants were classified as ‘non-progressors’5,59,60.
Statistical analysis
Continuous variables with normal distribution were expressed as mean ± standard deviation, whereas continuous variables with skewed distribution were expressed as median (and interquartile range). Categorical variables were expressed as percentage. In the comparison of sub-groups reflecting the presence of NAFLD and/or MetS, one-way analysis of variance with Scheffé’s method and the Kruskal–Wallis test with the Dunn procedure were used to assess continuous variables and the Chi-squared test was used to assess categorical variables. The demographic and biochemical characteristics of the sub-groups categorised by CAC score progression were compared using the Student’s t-test or the Mann–Whitney U test for continuous variables or the Chi-squared test for categorical variables. Logistic regression analysis was performed to calculate the ORs and 95% CIs of the sub-groups defined by the presence of NAFLD and/or MetS to predict the baseline CAC and CAC progression. All statistical analyses were performed using SPSS software (version 20.0 for Windows; SPSS, Inc., Chicago, IL, USA). In the present analyses, a two-sided P-value was adopted and P < 0.05 was considered to be statistically significant.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Angulo, P. Nonalcoholic fatty liver disease. N. Engl. J. Med. 346, 1221–1231 (2002).
Satapathy, S. K. & Sanyal, A. J. Epidemiology and natural history of nonalcoholic fatty liver disease. Semin. Liver Dis. 35, 221–235 (2015).
Marchesini, G. et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes 50, 1844–1850 (2001).
Silverman, M. G. et al. Impact of coronary artery calcium on coronary heart disease events in individuals at the extremes of traditional risk factor burden: the Multi-Ethnic Study of Atherosclerosis. Eur. Heart J. 35, 2232–2241 (2014).
Budoff, M. J. et al. Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc. Imaging 3, 1229–1236 (2010).
Budoff, M. J. et al. Progression of coronary calcium and incident coronary heart disease events: MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 61, 1231–1239 (2013).
Raggi, P., Cooil, B., Ratti, C., Callister, T. Q. & Budoff, M. Progression of coronary artery calcium and occurrence of myocardial infarction in patients with and without diabetes mellitus. Hypertension 46, 238–243 (2005).
Jaruvongvanich, V., Wirunsawanya, K., Sanguankeo, A. & Upala, S. Nonalcoholic fatty liver disease is associated with coronary artery calcification: A systematic review and meta-analysis. Dig. Liver Dis. 48, 1410–1417 (2016).
Al Rifai, M. et al. The association of nonalcoholic fatty liver disease, obesity, and metabolic syndrome, with systemic inflammation and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis (MESA). Atherosclerosis 239, 629–633 (2015).
Sung, K. C. et al. Arterial stiffness, fatty liver and the presence of coronary artery calcium in a large population cohort. Cardiovasc. Diabetol. 12, 162 (2013).
Raggi, P., Callister, T. Q. & Shaw, L. J. Progression of coronary artery calcium and risk of first myocardial infarction in patients receiving cholesterol-lowering therapy. Arterioscler. Thromb. Vasc. Biol. 24, 1272–1277 (2004).
Cho, Y. K. et al. Association between serum gamma-glutamyltransferase and the progression of coronary artery calcification. Atherosclerosis 243, 300–306 (2015).
Villanova, N. et al. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology 42, 473–480 (2005).
Vanhoutte, P. M. Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ. J. 73, 595–601 (2009).
Kotronen, A. et al. Increased coagulation factor VIII, IX, XI and XII activities in non-alcoholic fatty liver disease. Liver Int 31, 176–183 (2011).
Verrijken, A. et al. Prothrombotic factors in histologically proven nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 59, 121–129 (2014).
Ganguly, P. & Alam, S. F. Role of homocysteine in the development of cardiovascular disease. Nutr. J. 14, 6 (2015).
de Carvalho, S. C. et al. Plasmatic higher levels of homocysteine in non-alcoholic fatty liver disease (NAFLD). Nutr. J. 12, 37 (2013).
Francque, S. M., van der Graaff, D. & Kwanten, W. J. Non-alcoholic fatty liver disease and cardiovascular risk: pathophysiological mechanisms and implications. J. Hepatol. 65, 425–443 (2016).
Targher, G., Byrne, C. D., Lonardo, A., Zoppini, G. & Barbui, C. Non-alcoholic fatty liver disease and risk of incident cardiovascular disease: a meta-analysis. J. Hepatol. 65, 589–600 (2016).
Isomaa, B. et al. Cardiovascular morbidity and mortality associated with the metabolic syndrome. Diabetes Care 24, 683–689 (2001).
Jelavic, M. M., Babic, Z. & Pintaric, H. The importance of two metabolic syndrome diagnostic criteria and body fat distribution in predicting clinical severity and prognosis of acute myocardial infarction. Arch. Med. Sci. 13, 795–806 (2017).
Kim, L. K. et al. Impact of metabolic syndrome on the progression of coronary calcium and of coronary artery disease assessed by repeated cardiac computed tomography scans. Cardiovasc. Diabetol. 15, 92 (2016).
Katsiki, N., Perez-Martinez, P., Anagnostis, P., Mikhailidis, D. P. & Karagiannis, A. Is Nonalcoholic Fatty Liver Disease Indeed the Hepatic Manifestation of Metabolic Syndrome? Curr. Vasc. Pharmacol. 16, 219–227 (2018).
Mazidi, M., Toth, P. P. & Banach, M. C-reactive Protein Is Associated With Prevalence of the Metabolic Syndrome, Hypertension, and Diabetes Mellitus in US Adults. Angiology 69, 438–442 (2018).
Klisic, A. et al. Bioavailable testosterone is independently associated with Fatty Liver Index in postmenopausal women. Arch. Med. Sci. 13, 1188–1196 (2017).
Grundy, S. M. et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 112, 2735–2752 (2005).
European Association for the Study of the, L., European Association for the Study of, D. & European Association for the Study of, O. EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. Diabetologia 59, 1121–1140 (2016).
Athyros, V. G. et al. The use of statins alone, or in combination with pioglitazone and other drugs, for the treatment of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis and related cardiovascular risk. An Expert Panel Statement. Metabolism 71, 17–32 (2017).
Pastori, D. et al. The efficacy and safety of statins for the treatment of non-alcoholic fatty liver disease. Dig. Liver Dis. 47, 4–11 (2015).
Sonmez, A. et al. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol 9, 576–582 (2015).
Han, K. H. et al. Evaluation of short-term safety and efficacy of HMG-CoA reductase inhibitors in hypercholesterolemic patients with elevated serum alanine transaminase concentrations: PITCH study (PITavastatin versus atorvastatin to evaluate the effect on patients with hypercholesterolemia and mild to moderate hepatic damage). J. Clin. Lipidol. 6, 340–351 (2012).
Gomez-Dominguez, E., Gisbert, J. P., Moreno-Monteagudo, J. A., Garcia-Buey, L. & Moreno-Otero, R. A pilot study of atorvastatin treatment in dyslipemid, non-alcoholic fatty liver patients. Aliment. Pharmacol. Ther. 23, 1643–1647 (2006).
Antonopoulos, S., Mikros, S., Mylonopoulou, M., Kokkoris, S. & Giannoulis, G. Rosuvastatin as a novel treatment of non-alcoholic fatty liver disease in hyperlipidemic patients. Atherosclerosis 184, 233–234 (2006).
Abel, T., Feher, J., Dinya, E., Eldin, M. G. & Kovacs, A. Safety and efficacy of combined ezetimibe/simvastatin treatment and simvastatin monotherapy in patients with non-alcoholic fatty liver disease. Med. Sci. Monit. 15, MS6–11 (2009).
Del Ben, M. et al. Modern approach to the clinical management of non-alcoholic fatty liver disease. World J. Gastroenterol. 20, 8341–8350 (2014).
Rizvi, A. A. et al. Liraglutide improves carotid intima-media thickness in patients with type 2 diabetes and non-alcoholic fatty liver disease: an 8-month prospective pilot study. Expert Opin. Biol. Ther. 15, 1391–1397 (2015).
Carbone, L. J., Angus, P. W. & Yeomans, N. D. Incretin-based therapies for the treatment of non-alcoholic fatty liver disease: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 31, 23–31 (2016).
Smits, M. M. et al. Twelve week liraglutide or sitagliptin does not affect hepatic fat in type 2 diabetes: a randomised placebo-controlled trial. Diabetologia 59, 2588–2593 (2016).
Katsiki, N., Athyros, V. G. & Mikhailidis, D. P. Non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus: Effects of statins and antidiabetic drugs. J. Diabetes Complications 31, 521–522 (2017).
Athyros, V. G. et al. Cardiovascular risk across the histological spectrum and the clinical manifestations of non-alcoholic fatty liver disease: An update. World J. Gastroenterol. 21, 6820–6834 (2015).
Katsiki, N., Mikhailidis, D. P. & Mantzoros, C. S. Non-alcoholic fatty liver disease and dyslipidemia: An update. Metabolism 65, 1109–1123 (2016).
Puri, R. et al. Impact of statins on serial coronary calcification during atheroma progression and regression. J. Am. Coll. Cardiol. 65, 1273–1282 (2015).
Berry, J. D. et al. Prevalence and progression of subclinical atherosclerosis in younger adults with low short-term but high lifetime estimated risk for cardiovascular disease: the coronary artery risk development in young adults study and multi-ethnic study of atherosclerosis. Circulation 119, 382–389 (2009).
Ozkok, A. et al. Osteoprotegerin/RANKL axis and progression of coronary artery calcification in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 7, 965–973 (2012).
Jung, C. H. et al. The risk of incident type 2 diabetes in a Korean metabolically healthy obese population: the role of systemic inflammation. J. Clin. Endocrinol. Metab. 100, 934–941 (2015).
American Diabetes Association. Standards of medical care in diabetes-2015 abridged for primary care providers. Clin. Diabetes 33, 97–111 (2015).
Furusyo, N. et al. Utility of glycated albumin for the diagnosis of diabetes mellitus in a Japanese population study: results from the Kyushu and Okinawa Population Study (KOPS). Diabetologia 54, 3028–3036 (2011).
National Cholesterol Education Program Expert Panel on Detection Evaluation & Treatment of High Blood Cholesterol in Adults. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) final report. Circulation 106, 3143–3421 (2002).
Stone, N. J. et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American college of cardiology/American heart association task force on practice guidelines. Circulation 129, S1–45 (2014).
Alberti, K. G. et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation 120, 1640–1645 (2009).
Kim, M. K. et al. Clinical practice guidelines for overweight and obesity in Korea. Endocrinol Metab (Seoul) 29, 405–409 (2014).
Moon, J. S., Yoon, J. S., Won, K. C., Cho, I. H. & Lee, H. W. Diagnostic accuracy of 64-slice MDCT coronary angiography for the assessment of coronary artery disease in Korean patients with type 2 diabetes. Diabetes Metab. J. 37, 54–62 (2013).
Jung, C. H. et al. Association of metabolically healthy. obesity with subclinical coronary atherosclerosis in a Korean population. Obesity (Silver Spring) 22, 2613–2620 (2014).
Agatston, A. S. et al. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 15, 827–832 (1990).
Greenland, P., LaBree, L., Azen, S. P., Doherty, T. M. & Detrano, R. C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 291, 210–215 (2004).
DeFilippis, A. P. et al. The association of Framingham and Reynolds risk scores with incidence and progression of coronary artery calcification in MESA (Multi-Ethnic Study of Atherosclerosis). J. Am. Coll. Cardiol. 58, 2076–2083 (2011).
Carson, A. P. et al. Hemoglobin a1c and the progression of coronary artery calcification among adults without diabetes. Diabetes Care 38, 66–71 (2015).
Kronmal, R. A. et al. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 115, 2722–2730 (2007).
Hokanson, J. E. et al. Evaluating changes in coronary artery calcium: an analytic method that accounts for interscan variability. AJR Am. J. Roentgenol. 182, 1327–1332 (2004).
Author information
Authors and Affiliations
Contributions
W.J.L. conceived this study. Y.K.C. and C.H.J. contributed to the design of the study. Y.M.K., J.H.Y., J.L. and S.E.L. conducted data collection. D.H.Y. and J.W.K. conducted the analysis. J.Y.P., C.H.J. and H.K.K. interpreted the results. Y.K.C. wrote the initial draft of the manuscript, with revisions by all authors. The final manuscript was approved by all authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cho, Y.K., Kang, Y.M., Yoo, J.H. et al. The impact of non-alcoholic fatty liver disease and metabolic syndrome on the progression of coronary artery calcification. Sci Rep 8, 12004 (2018). https://doi.org/10.1038/s41598-018-30465-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30465-y
This article is cited by
-
Adipose tissue-derived metabolite risk scores and risk for type 2 diabetes in South Asians
International Journal of Obesity (2024)
-
Association between noninvasive assessment of liver fibrosis and coronary artery calcification progression in patients with nonalcoholic fatty liver disease
Scientific Reports (2020)
-
Non-alcoholic Fatty Liver Disease and Its Links with Inflammation and Atherosclerosis
Current Atherosclerosis Reports (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.