Abstract
NMR spectroscopy is a technology that is widely used in metabolomic studies. The information that these studies most commonly use from NMR spectra is the metabolite concentration. However, as well as concentration, pH and ionic strength information are also made available by the chemical shift of metabolite signals. This information is typically not used even though it can enhance sample discrimination, since many conditions show pH or ionic imbalance. Here, we demonstrate how chemical shift information can be used to improve the quality of the discrimination between case and control samples in three public datasets of different human matrices. In two of these datasets, chemical shift information helped to provide an AUROC value higher than 0.9 during sample classification. In the other dataset, the chemical shift also showed discriminant potential (AUROC 0.831). These results are consistent with the pH imbalance characteristic of the condition studied in the datasets. In addition, we show that this signal misalignment dependent on sample class can alter the results of fingerprinting approaches in the three datasets. Our results show that it is possible to use chemical shift information to enhance the diagnostic and predictive properties of NMR.
Similar content being viewed by others
Introduction
Metabolomics (or metabonomics) is the study of the metabolome in biofluids, cells or tissues extracted from animals and plants by characterizing the metabolic fingerprint or phenotype (or their underlying mechanisms) in a biological system1,2. 1H-NMR spectroscopy is a high-throughput technique that quantifies metabolite concentrations in a reliable and reproducible manner3. 1H-NMR data can be used to classify samples, so it is a powerful means for capturing diagnostic and predictive properties and has promising potential for personalized medicine4.
A metabolite can be characterized in an 1H-NMR spectrum by its characteristic pattern of signals. The metabolite concentration can be measured by estimating the area below any one of these signals. Likewise, each signal has a specific location determined by its chemical shift (the resonant frequency of its nucleus in a magnetic field). For example, lactate concentration can be quantified from a signal with a chemical shift located at 1.33 ppm or from another signal with a chemical shift located at 4.11 ppm5. The chemical shift (that is to say, the location in a spectrum) of signals is influenced by the pH and the ionic strength (mostly mediated by Ca2+ or Mg2 concentration)+ of the sample6. The information about pH and ionic strength given by the chemical shifts has already been proved to be beneficial for the quality control of fruit juice7. A recent article showed that the pH and ionic strength of human urine samples can be extrapolated from chemical shift information8. A wide range of diseases (e.g., tumours9) are characterized by metabolic alkalosis/acidosis10 or ionic imbalance8: these diseases could be better identified in the NMR data with the help of chemical shift information. In addition, theoretical proof of the potential of chemical shift information to separate samples is already available11. Even so, chemical shift information is still not used to characterize these sample properties and possible differences between classes because the pH and ionic strength can be masked by phosphate buffering and the dilution of matrices varies considerably. These factors hinder the interpretability of the pH information provided by DFTMP12 or Chenomx-based pH calibration.
To date, several tools have been developed to automatically quantify metabolite concentrations in 1D 1H-NMR spectra datasets13,14,15, making it easier to collect additional information, including signal chemical shifts. For example, a recent redesign of the Dolphin NMR tool rDolphin using open-source R language provided more flexible and reproducible automatic metabolite profiling in 1D 1H-NMR datasets16. One additional feature of rDolphin is its ability to capture and output additional information (such as the signal parameter values –including chemical shift– from every quantified signal) for further evaluation. The collection of multiple chemical shifts and the open-source availability of complex algorithms able to combine their information make it possible to use chemical shift information to discriminate samples despite the drawbacks of pH masking and dilution mentioned above. In this study, we report an approach to combine the binomial of metabolite concentration and signal chemical shift information in NMR data from metabolomic studies to maximize NMR discriminant potential. To do so, we quantified the metabolite concentrations and signal chemical shifts of three public NMR metabolomic study datasets. We found that chemical shift information can be used to separate samples more effectively than just metabolite concentration information.
Materials and Methods
Datasets
Three NMR datasets from different human matrices from MetaboLights17 (a public repository of metabolomic studies) were analysed and profiled:
-
MTBLS1 Metabolights dataset: fingerprint NMR data (with adaptive binning) was used to analyse metabolomic changes mediated by type 2 diabetes in mouse, rat, and human urine18. The Metabolights dataset provides human urine data of 84 samples from nondiabetics and 48 samples from diabetics.
-
MTBLS237 Metabolights dataset: in human faecal extract samples, fingerprint NMR data was used to determine the metabolic profiling of control subjects and patients with active or inactive ulcerative colitis (UC) and Crohn’s disease (CD)19. The spectra dataset analysed consisted of: 20 control samples, 14 active CD samples, 31 inactive CD samples, 19 active UC samples and 28 inactive UC samples.
-
MTBLS374 Metabolights dataset: the metabolic serum profiles of smokers and nonsmokers were compared in order to study functional alterations caused by smoking through fingerprint data20. The original study analysed 1H-NMR fingerprint data, with the help of 2D spectrum information, to identify metabolites. According to the information available on the repository, the spectra dataset analysed in our study consisted of 56 samples from smokers and 57 samples from nonsmokers.
Details about sample preparation, spectrum acquisition and main results are available in the original manuscripts. Information about the buffer and dietary restrictions in the original studies is available in Supplementary Information. Information about chemical shift variability in metabolite signals after sample preparation is available in Supplementary Fig. 1. The ethical issues regarding the studies associated with the used datasets are described in detail in their original articles18,19,20.
Spectra preprocessing and profiling
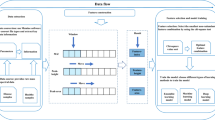
The spectrum preprocessing parameters available in the manuscripts of the studies associated with the datasets used were evaluated to generate 1H-NMR spectra similar to the ones of the original studies. All datasets were normalised using Probabilistic Quotient Normalisation (PQN) as it is the recommended normalisation method in recent reviews21. This method analyses the distribution of quotients of the amplitudes of each spectrum with those of a reference spectrum, and then normalises the spectrum by the median of the distribution of quotients22. Then, data binning (0.0006 ppm) was applied to the spectra before they were profiled by rDolphin. Unreliable relative metabolite concentrations and signal chemical shifts were filtered using a variety of quality indicators (additional information is available in Supplementary Information). Then, univariate outliers for each feature (controlling for sample class) were set as missing values and imputed.
For metabolite concentration information, the final dataset consisted of: MTBLS1, 39 features; MTBLS237, 35 features, MTBLS374, 30 features. For chemical shift information, the features were highly correlated. Consequently, in each dataset, dimensionality was reduced by principal components analysis (PCA) and the dozens of correlated chemical shifts were grouped into 5 independent principal components (enabling the factors influencing signal chemical shifts to be accurately evaluated).
Multivariate analysis
First, an exploratory visualization was performed in both metabolite concentration and chemical shift information datasets to compare their discriminant potential. The visualization was based on the results of a PCA performed to each set of information. During this exploratory visualization, it was also checked that no batch effects exerted an effect on the observed differences.
Next, sample classification was performed using the random forest algorithm, a decision tree-based algorithm which combines predictions and uses bootstrapping to maximize the optimization of bias and variance23,24. The modelling workflow provided by the ‘caret’ R package was used to perform sample classification. The models were trained with an average number of 500 trees, automatic hyperparameter tuning to best adapt to data properties, 500-iteration 0.632 bootstrap resampling to avoid overfitting25, upsampling to maximize the robustness of the models against the class imbalance problem in datasets26, and recursive feature elimination to minimize the influence of non-informative features. Classification was performed in three different variable subsets: 1- Only relative metabolite concentrations, 2- Only signal chemical shifts and 3- Using both relative metabolite concentrations and signal chemical shifts. Results were evaluated using classification accuracy, Cohen’s kappa (a more robust indicator against chance classification and class imbalance) and the area under the receiver operating characteristic (AUROC). In addition, to further evaluate the trained models, the sensitivity, specificity, positive predicted value and negative predicted value are available in Supplementary Information. Lastly, the variable importance in the models generated with both sets of variables was measured.
Reproducibility of study workflow
To validate and reproduce the results, the profiling output, the data analysis workflow and the links for downloading the datasets analysed are available on github.com/danielcanueto/chemical_shift_classification.
Data availability
All the data and the study workflow are available on github.com/danielcanueto/chemical_shift_classification to ensure reproducibility.
Results
Exploratory visualization of PCA information
Visualization of the first two principal components (PCs) of the PCAs of metabolite concentrations and signal chemical shifts suggested higher discriminant power in chemical shift information (Fig. 1). In chemical shift figures, less ellipse overlap (or at least more separated centres) was observed. Although more discriminative power in concentration information might be present in later PCs, the noise-related variance might be able to mask this power more intensely. Also, no batch effects were visible on any dataset.
MTBLS1 dataset
Chemical shift information showed potential for discriminating between diabetic and non-diabetic samples during random forest classification (AUROC 0.831) (Table 1). However, adding chemical shift information did not improve the excellent results obtained with only metabolite concentrations (AUROC 0.979).
MTBLS237 dataset
Chemical shift information, alone or combined with metabolite concentration information, significantly improved sample discrimination in 6 of the 8 subgroup comparisons: Active UC vs Inactive UC (0.917 vs 0.811 in AUROC), Active UC vs Active CD (0.768 vs 0.743 in AUROC), Inactive UC vs Inactive CD (0.870 vs 0.810 in AUROC), Control vs Active UC (0.948 vs 0.914 in AUROC), Control vs Inactive UC (0.943 vs 0.823 in AUROC) and Control vs Inactive CD (0.854 vs 0.825 in AUROC) (Table 2).
MTBLS374 dataset
Random forest classification on smoker and nonsmoker samples showed much higher AUROC values with chemical shift information than with metabolite concentration (0.937 vs 0.856 in AUROC) (Table 3). The combination of both sources of information gave slightly better values than when only chemical shift information was used (AUROC 0.950; Table 3, left).
Discussion
The results of our studies showed that 1D 1H-NMR spectra chemical shift information can give greater insight into sample properties and improve sample classification. In the three datasets analysed, chemical shift information led to good sample classification. In addition, in two of them, chemical shift information helped gave AUROC values higher than 0.9 and improved the classification with only metabolite concentration information.
Relationship between chemical shift and metabolic alkalosis/acidosis
The high classification performance observed in the three study datasets seems to be consistent with what has been previously reported about the alkalosis or acidosis characteristics of the conditions in the associated studies.
The MTBLS1 dataset is associated with the study of the changes in human urine caused by type 2 diabetes. Type 2 diabetes mediates lower pH in urine as a result of greater net acid excretion and fewer ammonia buffers27. A lower pH increases the chemical shift of signals (i.e., the signal moves to the left in a spectrum)28. Accordingly, most signals show a higher chemical shift in the diabetes samples than in the control samples (Supplementary Fig. 2, top). Several signal chemical shifts (such as one of indoxyl sulfate in Supplementary Fig. 2) show an inverse trend to the other signals. This inverse trend may be mediated by the influence of ionic strength. However, it may also be an artefact of the TSP signal used to reference spectra. The pKa of TSP is approximately 5, which makes its signal chemical shift sensitive to pH variation and causes signals with lower sensitivity (like the ones in the phenolic region29) to seem to move in the opposite direction to other signals.
In the case of the MTBLS237 dataset, alkalosis/acidosis in inflammatory bowel disease (the subtypes of which are UC and CD) has been reported elsewhere in the literature30. The relationship between faecal pH and the disease could be influenced by the location of lesions and/or the complex acid-base balances. The pH disturbance could have manifested as acidic pH in the UC samples represented by a higher chemical shift (Fig. 2, right; Supplementary Fig. 2, middle), and has been reported in the literature31. As in the MTBLS1 dataset, several signal chemical shifts show an inverse trend that may be mediated by the use of the TSP signal to reference spectra (Supplementary Fig. 2, middle).
Signals can be misaligned in some sample classes. Low pH mediated by the condition studied increases the chemical shift of the signals. The resulting class-dependent signal misalignment can distort the results of the analysis of fingerprint data: features can show significant differences caused by differences in chemical shift (mediated by pH or ionic strength) rather than by differences in metabolite concentration.
As for the MTBLS374 dataset, respiratory acidosis is typically seen in lung disease developed by smokers32 and in cigarette smoke that contains oxidants with acidic properties33. Signals in the spectra from the smokers group showed a higher chemical shift than the equivalent signals in the non-smokers (Fig. 2, left; Supplementary Fig. 2, bottom). This effect might be mediated by a more acidic pH in smokers’ samples as a consequence of smoking, which would be mostly captured by the second principal component of the PCA of signal chemical shifts (Supplementary Table 1). Unlike the other two datasets, this dataset does not contain any signal chemical shift with an inverse trend. This is consistent with the reference signal being glucose, a metabolite with a pKa (approx. 12) that is quite different from the pH of biological samples and thus much more resilient to pH variability.
Effect of class-dependent signal misalignment on fingerprinting approaches
All the datasets evaluated were processed using fingerprinting approaches in the original studies, in contrast to the profiling approach used here. Fingerprinting approaches perform the classification by looking for significant spectral differences between groups and identifying the metabolites involved in the second stage. On the other hand, profiling approaches start by characterizing the metabolites in the samples and then performing statistical analysis in the second stage. Their different workflows imply variations in how metabolites are identified and how their concentrations are quantified34.
Profiling is deemed to provide more resistance against signal overlap or baseline appearance through the deconvolution of signals in the spectrum lineshape35. However, one factor not evaluated in the differences between fingerprinting and profiling approaches is class-dependent signal misalignment (i.e., the differences in signal chemical shifts between spectra from different sample classes). Fingerprinting reliability is based on the premise that signals are reasonably well-aligned throughout the spectra dataset and, consequently, the differences are caused by differences in metabolite concentrations. It has been theoretically demonstrated that classification in fingerprint data can be influenced by class-dependent signal misalignment (i.e, that the differences found between classes are actually caused by having the metabolite signals located in different bins). However, approaches to minimize this problem (like the use of signal alignment algorithms36) are still not prevalent in the metabolomics field and were not applied in any of the datasets analysed.
In the three datasets analysed, the results of the univariate analysis in fingerprint data were compared before and after signal alignment using the CluPA algorithm37 (the analysis workflow is available in Supplementary Information). Signal alignment decreased the number of significant bins in all datasets (MTBLS374, −42%; MTBLS1, −7%; MTBLS237, −5%). This decrease means an improvement in the quality of classification models, as it can be ensured that the differences between classes are caused by potential biomarkers and not by signal misalignment.
Results confirmed the effect that class-dependent signal misalignment can exert on the results of fingerprinting data. Therefore, they further recommend the adoption of profiling approaches enabled by recent open-source profiling tools to minimize the generation of non-reproducible results. If the fingerprinting approach is still preferred, the implementation of signal alignment algorithms can minimise non-reproducible results; nonetheless, this alignment will involve losing the information given by chemical shift information.
Future directions and challenges
Our study workflow uses publicly available datasets and performs data preprocessing, profiling and statistical analysis with open-source tools following community recommendations38. By sharing this workflow we hope to make the use of chemical shift information in NMR studies more straightforward and more widespread. In addition, we hope the resulting reproducibility helps assess some aspects that need to be taken into account to take maximum advantage of chemical shift information:
-
Some matrices present considerable variations in dilution, which can greatly influence their pH and ionic strength (and, therefore, chemical shift). In addition, chemical shift variability is reduced by adding phosphate buffers (sometimes with added chelators such as EDTA) to the sample39. Both dilution variability and the use of buffers may mask the effects on the chemical shift produced by the condition studied. Consequently, the fact that the discriminative potential observed in MTBLS1 and MTBLS237 datasets was lower than the potential of the MTBLS374 dataset may be due to the higher dilution variability in the matrices studied (human urine and faecal extracts). The use of buffers or chelators should be minimized and sample dilution variability should be reduced if maximum advantage is to be taken of the properties of chemical shift information.
-
It has been suggested that chemical shift information could also be translated to sample pHs and ionic concentrations, hence maximizing the information extracted from a dataset8. Nonetheless, the limitations mentioned above raise concerns about the correct use of this information in several commonly studied matrices. In addition, the fact that these matrices commonly use a signal to reference spectra that is not resilient to pH (such as the TSP signal) may further distort the translation of chemical shifts to pH and ionic concentration values. There are several affordable techniques (e.g., pH meter or potentiometer) for directly measuring pH and ion concentrations that make this challenging translation unnecessary.
-
Studies aiming to take advantage of chemical shift information should ensure consistent sample preparation and spectra acquisition in all samples in order to prevent the discrimination between sample classes being mediated by differences in the preparation or acquisition protocol.
-
Further improvements in the quality of the classification models generated may be made by extracting more chemical shifts from NMR datasets and filtering noise in the chemical shift information (caused by low resolution with the consequent signal overlap in 1H-NMR) prior to model training. High-resolution spectra (e.g., 2D NMR) could help isolate more signals (with their associated chemical shifts) from different nuclei and prevent noise.
References
Lindon, J. C., Nicholson, J. K., Holmes, E. & Everett, J. R. Metabonomics: Metabolic processes studied by NMR spectroscopy of biofluids. Concepts Magn. Reson. 12, 289–320 (2000).
Fiehn, O. Metabolomics–the link between genotypes and phenotypes. Plant Mol. Biol. 48, 155–171 (2002).
Bharti, S. K. & Roy, R. Quantitative 1H NMR spectroscopy. Trends Analyt. Chem. 35, 5–26 (2012).
Beger, R. D. et al. Metabolomics enables precision medicine: ‘A White Paper, Community Perspective’. Metabolomics 12 (2016).
1H NMR Spectrum (HMDB0000190). Human Metabolome Database: 1H NMR Spectrum (HMDB0000190) Available at: http://www.hmdb.ca/spectra/nmr_one_d/1162. (Accessed: 17th February 2018).
Dona, A. C. et al. A guide to the identification of metabolites in NMR-based metabonomics/metabolomics experiments. Comput. Struct. Biotechnol. J. 14, 135–153 (2016).
Spraul, M. et al. Mixture analysis by NMR as applied to fruit juice quality control. Magn. Reson. Chem. 47(Suppl 1), S130–7 (2009).
Takis, P. G., Schäfer, H., Spraul, M. & Luchinat, C. Deconvoluting interrelationships between concentrations and chemical shifts in urine provides a powerful analysis tool. Nat. Commun. 8, 1662 (2017).
Corbet, C. & Feron, O. Tumour acidosis: from the passenger to the driver’s seat. Nat. Rev. Cancer 17, 577–593 (2017).
Galla, J. H. Metabolic alkalosis. J. Am. Soc. Nephrol. 11, 369–375 (2000).
Cloarec, O. et al. Evaluation of the Orthogonal Projection on Latent Structure Model Limitations Caused by Chemical Shift Variability and Improved Visualization of Biomarker Changes in 1H NMR Spectroscopic Metabonomic Studies. Anal. Chem. 77, 517–526 (2005).
Reily, M. D. et al. DFTMP, an NMR reagent for assessing the near-neutral pH of biological samples. J. Am. Chem. Soc. 128, 12360–12361 (2006).
Hao, J., Astle, W., De Iorio, M. & Ebbels, T. M. D. BATMAN–an R package for the automated quantification of metabolites from nuclear magnetic resonance spectra using a Bayesian model. Bioinformatics 28, 2088–2090 (2012).
Gómez, J. et al. Dolphin: a tool for automatic targeted metabolite profiling using 1D and 2D (1)H-NMR data. Anal. Bioanal. Chem. 406, 7967–7976 (2014).
Ravanbakhsh, S. et al. Accurate, fully-automated NMR spectral profiling for metabolomics. PLoS One 10, e0124219 (2015).
Cañueto, D., Gómez, J., Salek, R. M., Correig, X. & Cañellas, N. rDolphin: a GUI R package for proficient automatic profiling of 1D 1H-NMR spectra of study datasets. Metabolomics 14 (2018).
Haug, K. et al. MetaboLights–an open-access general-purpose repository for metabolomics studies and associated meta-data. Nucleic Acids Res. 41, D781–6 (2013).
Salek, R. M. et al. A metabolomic comparison of urinary changes in type 2 diabetes in mouse, rat, and human. Physiol. Genomics 29, 99–108 (2007).
Bjerrum, J. T. et al. Metabonomics of human fecal extracts characterize ulcerative colitis, Crohn’s disease and healthy individuals. Metabolomics 11, 122–133 (2014).
Kaluarachchi, M. R., Boulangé, C. L., Garcia-Perez, I., Lindon, J. C. & Minet, E. F. Multiplatform serum metabolic phenotyping combined with pathway mapping to identify biochemical differences in smokers. Bioanalysis 8, 2023–2043 (2016).
Emwas, A.-H. et al. Recommendations and Standardization of Biomarker Quantification Using NMR-Based Metabolomics with Particular Focus on Urinary Analysis. J. Proteome Res. 15, 360–373 (2016).
Dieterle, F., Ross, A., Schlotterbeck, G. & Senn, H. Probabilistic quotient normalization as robust method to account for dilution of complex biological mixtures. Application in 1H NMR metabonomics. Anal. Chem. 78, 4281–4290 (2006).
Efron, B. & Hastie, T. Computer Age Statistical Inference (2016).
Gromski, P. S. et al. A tutorial review: Metabolomics and partial least squares-discriminant analysis–a marriage of convenience or a shotgun wedding. Anal. Chim. Acta 879, 10–23 (2015).
Efron, B. & Tibshirani, R. Improvements on Cross-Validation: The .632 Bootstrap Method. J. Am. Stat. Assoc. 92, 548 (1997).
Kuhn, M. & Johnson, K. Applied Predictive Modeling (2013).
Maalouf, N. M., Cameron, M. A., Moe, O. W. & Sakhaee, K. Metabolic basis for low urine pH in type 2 diabetes. Clin. J. Am. Soc. Nephrol. 5, 1277–1281 (2010).
Xiao, C., Hao, F., Qin, X., Wang, Y. & Tang, H. An optimized buffer system for NMR-based urinary metabonomics with effective pH control, chemical shift consistency and dilution minimization. Analyst 134, 916–925 (2009).
Tredwell, G. D., Bundy, J. G., De Iorio, M. & Ebbels, T. M. D. Modelling the acid/baseH NMR chemical shift limits of metabolites in human urine. Metabolomics 12, 152 (2016).
Barkas, F., Liberopoulos, E., Kei, A. & Elisaf, M. Electrolyte and acid-base disorders in inflammatory bowel disease. Ann. Gastroenterol. Hepatol. 26, 23–28 (2013).
Vernia, P. et al. Fecal Lactate and Ulcerative Colitis. Gastroenterology 95, 1564–1568 (1988).
Broaddus, V. C. et al. Murray & Nadel’s Textbook of Respiratory Medicine. (Elsevier Health Sciences, 2015).
Pryor, W. A. & Stone, K. Oxidants in cigarette smoke. Radicals, hydrogen peroxide, peroxynitrate, and peroxynitrite. Ann. N. Y. Acad. Sci. 686, 12–27, discussion 27–8 (1993).
Viant, M. R., Ludwig, C. & Günther, U. L. Chapter 2. 1D and 2D NMR Spectroscopy: From Metabolic Fingerprinting to Profiling. Metabolomics, Metabonomics and Metabolite Profiling 44–70 (2007).
Weljie, A. M., Newton, J., Mercier, P., Carlson, E. & Slupsky, C. M. Targeted profiling: quantitative analysis of 1H NMR metabolomics data. Anal. Chem. 78, 4430–4442 (2006).
Vu, T. N. & Laukens, K. Getting your peaks in line: a review of alignment methods for NMR spectral data. Metabolites 3, 259–276 (2013).
Vu, T. N. et al. An integrated workflow for robust alignment and simplified quantitative analysis of NMR spectrometry data. BMC Bioinformatics 12, 405 (2011).
Rocca-Serra, P. et al. Data standards can boost metabolomics research, and if there is a will, there is a way. Metabolomics 12, 14 (2016).
Li, N., Song, Y. P., Tang, H. & Wang, Y. Recent developments in sample preparation and data pre-treatment in metabonomics research. Arch. Biochem. Biophys. 589, 4–9 (2016).
Acknowledgements
D.C. is grateful to the Universitat Rovira i Virgili for providing the Ph.D scholarship. X.C. and N.C. acknowledge the project TEC2015-69076-P financed by the Ministerio de Economía y Competitividad.
Author information
Authors and Affiliations
Contributions
D.C. designed and implemented the study workflow and wrote the manuscript. N.C. proposed the initial idea, made suggestions to improve the study workflow and reviewed the manuscript. R.S. provided feedback on the method and reviewed the manuscript. X.C. provided feedback on the method and reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cañueto, D., Salek, R.M., Correig, X. et al. Improving sample classification by harnessing the potential of 1H-NMR signal chemical shifts. Sci Rep 8, 11886 (2018). https://doi.org/10.1038/s41598-018-30351-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30351-7
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.