Abstract
The development of mammary gland as a lactogenic tissue is a highly coordinated multistep process. The epithelial cells of lactiferous tubules undergo profound changes during the developmental window of puberty, pregnancy, and lactation. Several hormones including estrogen, progesterone, glucocorticoids and prolactin act in concert, and orchestrate the development of mammary gland. Understanding the gene regulatory networks that coordinate proliferation and differentiation of HC11 Mammary Epithelial stem-like Cells (MEC) under the influence of lactogenic hormones is critical for elucidating the mechanism of lactogenesis in detail. In this study, we analyzed transcriptome profiles of undifferentiated MEC (normal) and compared them with Murine Embryonic Stem Cells (ESC) using next-generation mRNA sequencing. Further, we analyzed the transcriptome output during lactogenic differentiation of MEC following treatment with glucocorticoids (primed state) and both glucocorticoids and prolactin together (prolactin state). We established stage-specific gene regulatory networks in ESC and MEC (normal, priming and prolactin states). We validated the top up-and downregulated genes in each stage of differentiation of MEC by RT-PCR and found that they are comparable with that of RNA-seq data. HC11 MEC display decreased expression of Pou5f1 and Sox2, which is crucial for the differentiation of MEC, which otherwise ensure pluripotency to ESC. Cited4 is induced during priming and is involved in milk secretion. MEC upon exposure to both glucocorticoids and prolactin undergo terminal differentiation, which is associated with the expression of several genes, including Xbp1 and Cbp that are required for cell growth and differentiation. Our study also identified differential expression of transcription factors and epigenetic regulators in each stage of lactogenic differentiation. We also analyzed the transcriptome data for the pathways that are selectively activated during lactogenic differentiation. Further, we found that selective expression of chromatin modulators (Dnmt3l, Chd9) in response to glucocorticoids suggests a highly coordinated stage-specific lactogenic differentiation of MEC.
Similar content being viewed by others
Introduction
The events of cellular differentiation, which lead the transition of a primary cell into lineage-restricted phenotype, are accompanied by activation of specific gene regulatory networks instead of activation of a single or a few genes1,2. The cell fate transitions experience a global transcriptional activation and repression and are regulated by spatiotemporal expression of both transcription factors3,4,5 (TFs) and epigenetic regulators6 (ERs). The transition of mammary epithelial cells (MEC) to lactogenic cells is considered as a model system to comprehensively map the temporal dynamics of gene expression in the context of cellular differentiation.
The mammary gland is an apocrine gland, and its key function is to produce and secrete milk. The basic components of the adult mammary gland are alveoli, which are lined by milk-secreting epithelial cells. Myoepithelial cells and a stromal compartment that are derived from embryonic ectoderm and mesoderm respectively surround the epithelial cells. Several alveoli join to form lobules that have a lactiferous duct, which drains milk into an opening of the nipple7. The development of murine mammary gland initiates during E10-11.5 of embryonic state with the formation of bilateral stripes on either side of the abdomen of a developing embryo, which further organizes into placodes. During puberty, these placodes differentiate into multiple mammary trees and terminal end buds under the influence of estrogen and insulin-like growth factor (IGF)8. Further development of mammary gland is triggered at a virgin stage and continues throughout the pregnancy till the end of parturition, under the influence of various hormones including epidermal growth factor (EGF), glucocorticoids (GC) and prolactin (PRL).
The levels of EGF get elevated during early pregnancy9, and it is thought to play a critical role in the proliferation of normal epithelium, that is required for the growth of terminal buds of the mammary gland10. GC induces genes involved in the maintenance of tight junction complexes and apical junction remodelers such as Adherins, ZO-1 and β-Catenin11,12,13. Furthermore, GC signaling act as a prerequisite for PRL to act on chromatin prepared by GC14. However, the specific role of GC in the context of lactogenic differentiation needs to be understood in detail. GC receptor (GR), upon activated by GC, translocate to the nucleus and gets recruited to the glucocorticoid response elements (GRE) to facilitate transactivation of target genes. In general, GR binding sites are located at >2.5 kb upstream of transcription start sites (TSS) of the corresponding genes14. Even though GR was shown to interact with GRE, it was not always associated with the expression of corresponding genes. These observations suggest the possibility of involvement in long-range chromatin interactions to elicit GCR/GRE mediated gene expression. On the other hand, PRL was shown to elicit its actions on mammary gland during late pregnancy and lactation. Transcription factors, Stat5a and Stat5b, are the primary inducers of lactogenic gene expression under the stimulus of PRL. Conditional deletion of STAT5 before and during pregnancy prevents lactogenic differentiation of epithelial cells and also elicits premature cell death, suggesting a critical role of STAT5 in proliferation, differentiation, and survival of MEC15.
Our understanding of the regulation of gene expression during lactogenesis by various hormones has come from the transcriptional regulation studies on a predominant milk protein gene, β-casein. Under the stimulus, β-casein promoter recruits transcription factors and co-activators at the proximal promoter and enhancers located ~6 kb upstream of its TSS16. GC induces the recruitment of p300 at the β-casein promoter and enhancer sites leading to acetylation of Histones H3 and H416, which is required for transcriptional activation. PRL signaling promotes recruitment of Hdac1 to the β-casein promoter, thereby facilitating transcriptional activation by deacetylation of adjacent enhancer binding protein (CEBP)16. Treatment with GC alone did not produce a detectable increase in β-Casein mRNA levels. A 3-fold increase in β-casein mRNA was detected in cells treated with PRL alone whereas 500-fold induction of β-casein mRNA was observed upon treatment with both GC and PRL16. It was also observed that GC treatment alone led to a rapid increase in histone H3 acetylation and treatment with both GC and PRL was required for stable association of p300 and RNA polymerase II at both promoter and enhancer region of β-casein, highlighting to the importance of long-range interactions at stage-specific context16. Gene expression profiles from multiple mammary cell types representing various developmental stages using 15 K and 20 K oligonucleotide microarray were reported earlier17,18,19. However, these studies do not provide state-specific (Normal, Primed and PRL treated HC11 cells) gene expression/regulatory networks in a comprehensive manner. It is known that mammary gland development occurs at various stages and several hormones including GC and PRL orchestrate these synergistic events. A comprehensive understanding of genome-wide transcriptome profile of MEC representing various stages of mammary gland differentiation would provide greater details of regulation by GC and PRL.
In this study, we comprehensively analyzed the gene regulatory networks of HC11 MEC in response to 1) treatment with EGF and Insulin (considered as normal MEC), 2) Primed with GC (considered as P), and 3) treatment with both GC and PRL (considered as PRL). We also cultured murine embryonic stem cells (ESC) and compared its transcriptome with normal HC11 MEC to identify the dynamics of epithelial cell-specific gene regulatory networks. This study provides an in-depth transcriptome analysis of the profound physiological changes brought about by the action of GC and PRL in MEC.
Results
HC11 MEC undergoes G0/G1 arrest during lactogenic differentiation
It is known that a regulated withdrawal from the cell cycle allows cells of any kind to differentiate. Therefore, to assess whether lactogenic differentiation of MEC accompanies cell cycle arrest, we performed lactogenic differentiation of MEC as described earlier20. We confirmed the formation of mammospheres, which were more prominent after the treatment with PRL (Fig. 1A). Cellular differentiation is associated with cell cycle withdrawal; therefore, we analyzed the expression of cell cycle regulators by immunoblotting. Expression of cyclinD1, D3, CDK2, CDK6, and p21 decreased in differentiated MEC compared to proliferating and confluent normal MEC (Fig. 1B,B’). Cell cycle analyses revealed that majority of cells (77–84%) in MEC (normal & differentiated) were accumulated in the G0/G1 stage, while most of the cells in ESC are in S-phase (Fig. 1C). Accumulation of MEC in G0/G1 stage and expression of cell cycle regulators (Fig. 1B,C) suggests the progression to cell cycle arrest, which may facilitate differentiation. Further, we assessed the expression of specific markers pertinent to stage-specific lactogenic differentiation. ESC expressed the putative markers such as Pou5f1, Nanog, and Sox2. HC11 MECs expressedTpx2 and Nek2, whereas, HC11 primed cells expressed Krt15 and Boc and PRL treated HC11 cells expressed Csn2 and Wap (Fig. 1D). We assessed the expression of specific markers pertinent to lactogenic differentiation based on FPKM values obtained from RNA-seq analysis (See below) and found that identical sets of genes are induced during lactogenic differentiation of HC11 MEC (Fig. 1D’).
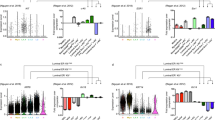
Characterization of HC11 MEC undergoing lactogenic differentiation. (A) Bright field microscopic images of actively growing ESC, undifferentiated HC11 cells in presence of EGF and INS (N) and HC11 cells primed with GC (P) alone and HC11 cells treated with GC and PRL. Note the formation of clear dome-shaped mammospheres under PRL condition. Scale bar represents 100 μM. (B) Immunoblot analysis of cell cycle regulators in actively growing (N*), confluent stage undifferentiated normal (N’) HC11 cells along with HC primed (P) and PRL treated cells showing a gradual reduction in their levels in comparison with Actin-B. Full-length blot ECL images are provided in Supplementary Fig. S2. (B’) Quantitative analysis of cell cycle regulators normalized against β-Actin showing a gradual reduction in their levels during lactogenic differentiation. (C) Table showing the percentage of ESC, N, P and PRL treated HC11 cells at G0/G1, S and G2/M phase of cell cycle showing Predominantly in S phase for ESCs and G0/G1 phage for rest of HC11 cell types. (D) Real-time PCR analysis of cell-type specific gene expression analysis representing ESC, N, P, and PRL treated HC11 cells. (D’) RNA-seq data presentative FPKM values for the respective cell-type-specific genes.
RNA-seq analysis of ESC and differentiated HC11 MEC
To quantify the changes in the expression levels of each transcript during lactogenic differentiation and to comprehensively understand the profile of all the transcripts, we performed RNA-seq and analyzed the data in ESC, normal MEC and MEC treated with GC and PRL. Qualitative analysis of RNA-seq data is provided in the Supplementary Table 1.
We compared the transcriptome profile of HC11 MEC with ESC after aligning them with GRCm38/mm10 mouse reference genome assembly. In case of ESC, 78% of total reads were uniquely mapped to the reference mouse genome, whereas ~88% of the reads were uniquely mapped in case of both normal MEC and treated MEC (Supplementary Table 2). Mixed datasets of highly correlated (>0.9) duplicate samples (Supplementary Table 3) from ESC, normal, primed and PRL treated MEC were used to derive respective ‘fragments per kilobase per million reads’ (FPKM) values using Sub-read version 1.5.0. We considered genes with an FPKM value of minimum 1, as expressed and found a total of 12016 genes expressed in ESC, 11210 in normal MEC, 11418 in GC treated MEC and 10879 with PRL treated MEC (Fig. 2A; Supplementary Tables 4–7). While a large number of genes (9623) express constitutively in ESC and MEC, relatively less number of genes are differentially expressed during lactogenic differentiation (Fig. 2A). 1584 genes were selectively expressed in ESC, 114 in normal MEC, 158 in primed MEC and 68 in PRL treated MEC (Fig. 2A). It is noteworthy that ESC expressed a large number of unique genes compared with both normal and differentiated MEC (Figs 2A and 3A). We further categorized highly expressed genes from ESC, normal, primed and PRL states based on FPKM values. Among them, genes with higher FPKM values were predominantly housekeeping and ribosomal genes. However, some of these genes were cell-specific, indicating that these might be induced selectively during GC and PRL treatment (Fig. 3B).
RNA-seq expression analysis of ESC and HC11 MEC undergoing lactogenic differentiation and its validation by real-time PCR. (A) Venn diagram showing expression of total, unique and overlapping genes (>1 FPKM value) in ESC, Normal (N), GC primed (P) and PRL treated HC11 cells. (B) Venn diagram showing unique and overlapping, upregulated genes between ESC vs N, N vs P and P vs PRL treated HC11 cells (Log2 ≥1). (C) Venn diagram showing unique and overlapping, downregulated genes between ESC vs N, N vs P and P vs PRL treated HC11 cells (Log2 ≤−1). (D) Schematic diagram showing a total number of genes which are differentially upregulated (Up arrow) and downregulated (Down arrow) between ESC vs Normal (N), N vs GC primed (P) and PRL treated HC11 cells. (E) Real-time PCR analysis of the top upregulated and (F) downregulated in Normal (N), GC primed (P) and PRL treated HC11 cells and their respective RNA-seq FPKM values of (E’) upregulated genes and (F’) downregulated genes.
Comparative analysis of unique and differentially expressed genes in ESC and HC11 MEC undergoing lactogenic differentiation. (A) Heat map showing top 20 uniquely expressed genes, (B) highly expressed, (C) up-regulated and (D) downregulated genes in replicates of ESC (ESC 1 & 2), Normal HC11 cells (N1 & 2), GC primed (P1 & P2) and PRL treated HC11 cells (PRL1 & PRL 2).
Lactogenic differentiation induces differential expression of a large number of transcripts
We next sought to understand the differentially expressed genes during various stages of lactogenic differentiation. We analyzed differentially expressed genes using DEseq221. A Log2 fold change of ≥+1 and ≤−1 values were considered as a cutoff to define up and downregulation respectively, with a p-adjusted value of <0.001. This analysis showed that 2725, 1141 and 385 genes were upregulated and 3478, 1026 and 655 genes were downregulated when compared ESCs vs. normal, normal vs. GC and GC vs. PRL treated HC11 cells, respectively (Fig. 2B–D; Supplementary Tables 16, 20, 24, 28, 32, 36). It should be noted that the total number of differentially expressed genes (both up and downregulated) were decreased from ESC vs. normal MEC to normal vs. GC treated MEC to GC vs. PRL treated MEC. Although, both GC and PRL are key factors of lactogenic differentiation, a large number of genes were differentially expressed (upregulated: 1141; downregulated: 1026) upon priming with GC than both GC and prolactin treatment (PRL; upregulated: 385 and downregulated: 655). We selected a list of genes such as Ogn, Cyp2f2, Capn6, Adcy8, Csn2, Ptgds, Klk1b9, Arl4d and Pigr (upregulated), and Etv4, Gzmc, 4732456N10rik, Fam64a, Krt42, Mki67, Cenpf, Cenpe, Shcbp1 and 2810417H13Rik (downregulated) based on RNA-seq data from all three types of MEC and validated their expression by RT-PCR (Fig. 2E,F). Expression of each transcript by RT-PCR is in concurrence with respective FPKM values (Fig. 2E’,F’). Top 20 uniquely expressed genes in ESC, N: Normal P: Primed and Prolactin treated MEC are represented as a heat map (Fig. 3A). Similarly, top 20 highly expressed genes (Fig. 3B), top 20 highly upregulated genes (Fig. 3C) and top 20 highly downregulated (Fig. 3D) genes between ESC, N, P and PRL treated MEC were represented as heat maps.
Lactogenic differentiation induces differential expression of a large number of Transcription factors and epigenetic regulators
It is generally considered that the expression of cell-type-specific genes is under the control of TFs3,4 and ERs5. Therefore, we analyzed TFs and ERs that were differentially transcribed during lactogenic differentiation of MEC (Supplementary Tables 8–15). We observed that 2196, 1990, 2002 and 1998 TFs are expressed in ESC, normal, primed and PRL states of MEC, respectively (Fig. 4A). On the other hand, 837, 797, 800 and 797 ERs were expressed in ESC, normal, primed and PRL states of MEC, respectively (Fig. 4D). It is noteworthy that 1894 TFs and 775 ERs were constitutively expressed in both ESC and MEC (Fig. 4A,D). Next, we compared the expression of these regulatory factors during various stages of lactogenic differentiation. We observed that 315, 74 and 47 TFs (Fig. 4B) and 91, 16 and 7 ERs (Fig. 4E) were upregulated in ESC vs. normal MEC, normal vs. GC primed, and primed vs. PRL treated MEC, respectively. Similarly, we found that 546, 186 and 114 TFs (Fig. 4C), and 229, 107 and 55 ERs (Fig. 4F) were downregulated in ESC vs. normal, normal vs. GC primed and primed vs. PRL treated MEC, respectively. Although a large number of TFs and ERs are differentially expressed between ESC and MEC, priming of MEC with GC resulted in more differentially expressed TFs and ERs than PRL treatment. The total number of up and downregulated TFs and ERs during lactogenic differentiation of MEC are summarized in Fig. 4G. Heatmaps for highly expressed, up-and downregulated TFs are provided in Fig. 5A–C. Heatmaps for highly expressed, up-and downregulated ERs are provided in Fig. 5D–F.
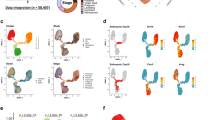
RNA-seq expression analysis of transcription factors (TFs) and epigenetic regulators (ERs) in ESC and HC11 MEC undergoing lactogenic differentiation. (A) Venn diagram showing total, unique and overlapping expression of TFs between ESC vs N, N vs P and P vs PRL treated HC11 cells (>0 FPKM). (B) Venn diagram showing unique and overlapping expression of upregulated (log2 ≥1) and (C) downregulated (≤−1 FPKM) TFs between ESC vs N, N vs P and P vs PRL treated HC11 cells. (D) Venn diagram showing unique and overlapping expression of ERs between ESC vs N, N vs P and P vs PRL treated HC11 cells (>0 FPKM). (E) Venn diagram showing unique and overlapping expression of upregulated (Log2 ≥1) and (F) down-regulated (Log2 ≤−1) ERs between ESC vs N, N vs P and P vs PRL treated HC11 cells. (G) Schematic diagram showing a total number of TFs/ERs which are differentially upregulated (Up arrow) and downregulated (Down arrow) between ESC vs Normal (N), N vs GC primed (P) and PRL treated HC11 cells.
Comparative analysis of unique and differentially expressed TFs and ERs in ESC and HC11 MEC undergoing lactogenic differentiation. (A,B and C) Heat map showing top 20 highly expressed, up-regulated and down-regulated TFs and (D,E and F) ERs respectively in replicates of ESC (ESC 1 & 2), Normal HC11 cells (N1 & N2), GC primed (P1 & P2) and PRL treated HC11 cells (PRL1 & PRL 2).
Analysis of gene expression between ESC vs. normal HC11 MEC
Upregulated genes
A total of 2725 genes were significantly upregulated in normal MEC when compared with ESC (Fig. 2D). Among them, krt5, Igfbp5, Krt14, and Brinp3 were significantly upregulated (Fig. 3C; Supplementary Table 16). These genes encode for keratins, that are involved in extracellular matrix support, and IGF binding protein, which helps in the proliferation and survival of MECs22. We also found that 315 TFs and 91 ERs were significantly upregulated in normal MEC compared to ESC (Fig. 4G; Supplementary Tables 17 and 18). Trpv6, Wfdc3, Barx2, Elf5 and Ehf (TFs), and Dmp1, Ifih1, Sox9, Aim1 and Sox8 (ERs) were significantly upregulated in normal MEC (Fig. 5B,E). We confirmed the expression of top upregulated TFs (Fig. 6A) and ERs (Fig. 6C) by RT-PCR. FPKM data of RNA-seq for these TFs and ERs is comparable to that of RT-PCR data (Fig. 6A’,C’). Among TFs that are upregulated, Elf5 is very specific to normal MEC and shown to regulate mammary stem or progenitor cell population23,24. Based on upregulated genes, we performed KEGG pathway analysis and found several pathways activated in MECs including focal adhesion, proteoglycans and PI3k-Akt signaling pathway (Fig. 7A; and Supplementary Table 19). We also performed different pathway analyses like BioCarta, and Wiki pathways, which are shown in Supplementary Fig. S1 and pathway scores, are shown in Supplementary Tables 40 and 41.
Real-time PCR validation of top up or downregulated TFs and ERs in ESC and HC11 MEC undergoing lactogenic differentiation. (A) Bar chart showing real-time PCR measured relative expression of upregulated and (B) downregulated TFs along with its respective RNA-seq FPKM values (A’ and B’) in ESC, N, P and PRL treated HC11 cells. (C) Bar chart showing real-time PCR measured relative expression of up-regulated and (D) down-regulated ERs along with its respective RNA-seq FPKM values (C’ and D’) in ESC, N, P and PRL treated HC11 cells.
Pathway analysis of genes that are differentially expressed between ESC and HC11 MEC undergoing lactogenic differentiation. (A, C & E) KEGG pathways analysis of genes that are either upregulated and (B,D & F) downregulated between ESC vs normal HC11 cells (N), N vs GC primed (P) and P vs PRL treated HC11 cells respectively.
Downregulated genes
Similarly, we observed that about 3478 genes were downregulated in normal MEC compared to ESC (Fig. 2D; Supplementary Table 20). The downregulated genes include Nanog, Apoe, Akap12, Mybl2, and Gm2373 (Fig. 3D). Most of the genes that were downregulated found to be associated with the maintenance of stem cell state. We identified that 546 and 229 TFs and ERs are downregulated in MEC, respectively (Fig. 4G; Supplementary Tables 21 and 22). Zfp42, Pou5f1, Esrrb, and Dnmt3l are among the top downregulated TFs (Fig. 5C), whereas Sox2, Mtcl1, Ddx3y, and Cdyl2 are the top downregulated ERs (Fig. 5F). Among them, Pou5f1 and Sox2 are very important for the maintenance of ESC pluripotency25,26. The decreased expression of these factors in MEC suggests that these cells are differentiated. We confirmed the expression of top downregulated TFs (Fig. 6B) and ERs (Fig. 6D) by RT-PCR. FPKM data obtained from RNA-seq for these TFs and ERs is comparable to that of RT-PCR data (Fig. 6B’,D’). KEGG pathway analysis showed that genes involved in insulin secretion, dilated cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy (ARVC), cell adhesion molecules and hypertrophic cardiomyopathy (HCM) were downregulated in MEC compared to ESC (Fig. 7B; Supplementary Table 23).
Analysis of gene expression in HC11 MEC during GC priming
Upregulated genes
A total of 1141 genes were differentially expressed in MEC treated with GC compared to normal MEC (Fig. 2D). Some of the upregulated genes include Ogn, Cyp2f2, Capn6, Ibsp, and Adcy8 (Fig. 3C; Supplementary Table 24). Interestingly, we found only 74 TFs and 16 ERs upregulated in GC primed vs normal MEC (Fig. 4G; Supplementary Tables 25 and 26). Upregulated TFs in GC primed MEC include Rhpn2, Hivep3, Ehf and Tsc22d3 whereas upregulated ERs include Cited4, Luzp2, Jade1 and Tox (Fig. 5B,E). We have validated the expression of significantly upregulated TFs and ERs by RT-PCR (Fig. 6A,C). The expression of upregulated TFs and ERs is in concurrence with FPKM data of RNA-seq (Fig. 6A’,C’). The epigenetic regulator and a transcriptional coactivator, Cited4 that was upregulated during priming is known to be involved in milk secretion from mammary gland27. Further, genes shown to play a role in chromatin remodeling and decompaction such as Chd928 and Myc29 are significantly upregulated during priming with GC (Figs 5E, 6C and C’). Most of the upregulated genes are known to be involved in pathways corresponding to protein digestion and absorption, gastric acid secretion, salivary secretion, glutamatergic synapse, melanogenesis and circadian entrainment (Fig. 7C and Supplementary Table 27).
Downregulated genes
We found a total of about 1026 genes that were significantly downregulated in HC11 MEC during priming with GC (Fig. 2D; Supplementary Table 28), which include Etv4, Gzmc, 4732456N10rik, Fam64a and Krt42 (Fig. 3D). Moreover, we found that 186 TFs and 107 ERs were downregulated in GC primed HC11 MEC (Fig. 4G; Supplementary Tables 29 and 30). Etv4, Id3, Hist1h3h, Cenpa, and E2f7 are some of TFs that were downregulated during priming (Fig. 5C), whereas Ncapg, Aurkb, Nek2, Hist1h2ai, and Cenpe are some of the downregulated ERs (Fig. 5F). The TFs Etv4, E2f7, and Aurkb are known to be involved in cellular proliferation30,31,32. Further, we validated the expression of top downregulated TFs (Fig. 6B) and ERs (Fig. 6D) by RT-PCR and decreased expression of these TFs and ERs during priming is comparable with FPKM data obtained from RNA-seq (Fig. 6B’,D’). The significantly downregulated genes were mostly involved in the cellular proliferation, DNA binding, and cellular growth-related functions. Pathway analysis of the downregulated genes showed that pathways related to cell cycle, DNA replication, Fanconi anemia pathway, homologous recombination as well as pyrimidine metabolism are all downregulated (Fig. 7D; Supplementary Table 31).
Analysis of gene expression in PRL treated HC11 MEC
Upregulated genes
To gain further insight into the changes in HC11 MEC during lactogenic differentiation, gene expression profile, pathway analysis and expression of TFs and ERs were analyzed in GC treated vs. prolactin + GC (PRL) treated HC11 MEC. Gene expression analysis revealed a total of 385 genes to be significantly upregulated in MEC treated with PRL vs. MEC treated with GC (Fig. 2D; Supplementary Table 32). Csn2, Wap, Pip, Ptgds, Klk1b9, Arl4d, and Pigr are some of the highly expressed genes in MEC treated with PRL (Fig. 3A,C). It is interesting to note that PRL triggers the expression of major milk proteins such as Csn2, Wap, and Pip, which constitute up to 80% of total milk proteins. We noticed that 47 TFs and 7 ERs were upregulated with PRL treatment (Fig. 4G; Supplementary Tables 33 and 34). Trib3, Ddit3, Nr1d1, Maff, and Atf3 are some of the TFs upregulated with PRL treatment and Rere, Srf and Myc are some of the ERs highly expressed in PRL treated HC11 MECs (Fig. 5B,E). Upon validation of top upregulated TFs (Fig. 6A) and ERs (Fig. 6C) by RT-PCR, it was revealed that the RT-PCR confirms the FPKM data (Fig. 6A’,C’). Trib3, a TF that is associated with various ER stress responses, is upregulated in the lactating period, which requires a high demand for protein synthesis33. Xbp1 is another TF that was upregulated during PRL treatment and is required for proliferation and differentiation of MEC34. KEGG analysis revealed that PRL activates several other pathways that include endocrine and other factor-regulated calcium reabsorption, mineral absorption, renin-angiotensin system and biosynthesis of amino acids (Fig. 7E and Supplementary Table 35).
Downregulated genes
In PRL treated HC11 MEC, 655 genes were downregulated compared with primed MECs (Fig. 2D). Genes such as Mki67, Cenpf, Cenpe, and Shcbp1 were downregulated upon PRL treatment (Fig. 3D; Supplementary Table 36). We found that 114 TFs and 46 ERs, respectively are downregulated in PRL treated MEC (Fig. 4G; Supplementary Tables 37 and 38). Highly downregulated TFs are Mki67, Ccna2, Neil3, Top2a and Erg, and ERs are Cenpf, Cenpe, Smc2, Hells and Aurkb (Fig. 5C,F). We validated the expression of downregulated TFs and ERs by RT-PCR (Fig. 6B,D) and is comparable with FPKM data (Fig. 6B’,D’). KEGG analysis for downregulated genes revealed several pathways that are affected such as cell cycle, p53 signaling, Fanconi anemia pathway, and focal adhesion (Fig. 7F; Supplementary Table 39).
Confirmation of specific factors expression during lactogenic differentiation
In addition to mRNA expression analysis, immunoblotting for selected markers were performed to determine their expression during lactogenic differentiation of HC11 MECs (Fig. 8A,B). Expression of Stat5a and 5b were increased during differentiation and mainly, the expression of Stat saturated during priming. Interestingly, expression of Dnmt3l protein level is increased during the course of differentiation of HC11 MECs in contrast to RT-PCR data and FRKM data (Fig. 6B,B’). Furthermore, our data reveal that Hdac11 expression increased with PRL treatment (Fig. 8A,B).
Discussion
The development of mammary gland and lactogenic differentiation of MEC are highly complex biological processes and mediated by several endocrine factors including glucocorticoids, prolactin, insulin, and EGF. Precise and coordinated control of gene expression is vital for lactogenesis. Recent microarray-based studies in HC11 MECs investigated the role of PRL and its relevance to breast cancer18. However, the mechanism of multistage lactogenic differentiation of MEC remains unclear. To gain insights into the mechanism of lactogenic differentiation, we employed HC11 MEC and compared the profile of transcripts from normal, GC primed, and PRL treated cells. Further, we garnered the clues about key TFs and ERs that drive the lineage-specific differentiation of ESC to MEC. It is interesting to note that priming induces PRL receptor in MEC, which could help in eliciting PRL signaling following treatment with PRL. Our data suggest that lactogenic differentiation of MEC occurs in a hierarchical manner, where priming with GC might alter chromatin accessibility and sets a base required for the PRL to elicit its action during lactation.
HC11 MEC are widely used in lactogenic differentiation system in vitro and form mammospheres during lactogenic differentiation20. The transcriptome profile revealed that only a subset (22%) of genes was differentially expressed in MEC when compared to ESCs. Sox9, Runx2, Gata3, Ehf, Elf5, and Bcl11a are TFs and ERs that are critical for the mammary gland development and are reported to maintain luminal progenitor population in the mammary gland35,36,37,38. We found that these factors are highly expressed in normal HC11, which resembles luminal progenitor cells of the mammary gland. We found up-regulation of Smad3 in MEC when compared to ESC. Smad3 is a downstream molecule of TGF-β pathway and antagonizes STAT5/PRL signaling and promotes cellular proliferation of normal mammary epithelial cells39. We also observed that major regulators of stemness such as Pou5f1, Sox2, and Nanog are downregulated in MEC indicating that cell type-specific gene expression is prerequisite for specialized functions.
We observed that some of the genes (Brca1, Krt6a, Krt6b, Krt5, and Melk), which are indicative of stem cell-like characteristics are highly expressed in normal MEC, and are downregulated during differentiation, are majorly involved in cell proliferation. We speculate that normal HC11 cells become proliferative upon exposure to EGF. Ncapg, Cenpa, Cenpe, Arukb, Kif4, and Kif22 are some of the genes involved in mitotic chromosome condensation and segregation and are majorly required for cellular proliferation. These genes are upregulated in normal MEC whereas, in response to GC treatment, these genes are downregulated. Another class of Ets family of TFs are Etv4 and Etv5 which are majorly involved in cell motility and cell growth are significantly downregulated since they are not required for normal MEC40. During priming with GC, HC11 MEC ceases cell proliferation concomitant with downregulation of above-mentioned genes.
Further, our data suggest GC alone brings about differential expression of a vast number of genes (Fig. 2). Priming of MECs with GC induces various ERs; Cited4, Luzp2, Jade1, Jade2, Chd9, Myc, and Tox. Earlier studies showed that Cited4 is actively involved during lactogenic differentiation27. Cited4 binds to CBP/p300, a transcriptional regulator as well as isoforms of the TFAP2 transcription factor. Cited4 thereby modulates TFAP2 dependent genes, thus might be helpful to access the chromatin by signal transducers and transcriptional activators during PRL treatment27,41,42,43. Jade family proteins serve as transcriptional activators via their histone acetyltransferase activity44 and Id family TFs are diversely expressed during mammary gland development. Id2, which gets induced during GC treatment, is crucial for survival and differentiation of MEC. This suggests that GC play a critical role to ensure differentiation of MEC during lactogenesis45,46. GC activated GCR was shown to remodel chromatin in Brg1 dependent and independent manner47. In this regard, GC induction of Chd9, a remodeling enzyme shown to play a role in loosening chromatin in growing oocyte28 and Myc, a protein shown to play an essential role in decompaction of chromatin29 suggest that there might be a link between these factors in global remodeling of chromatin, however, its relevance in the context of GC signaling remains to be explored further.
Transcriptome profile of HC11 MEC treated with PRL revealed that only 3.5% of genes were differentially expressed compared to GC primed HC11 MEC. Out of the highly expressed TFs and ERs, c-Myc is expressed during lactation. Knockdown studies of c-Myc in mammary gland showed a defect in alveolar cell proliferation and differentiation resulting in decreased milk production48. High expression of E-Cadherin (Cdh1) and down-regulation of Klf4 in HC11 cell-types suggest that HC11 cells are partial primary cells49 as it was known that Klf4 suppresses epithelial to mesenchymal transition in breast cancer cells through induction of E-cadherin. PRL treatment induces milk fatty acid biosynthesis by inducing expression of various TFs such as Fabp4, Lipe, and Srebf1, which are primary regulators of fatty acid homeostasis in lactating mammary gland50,51,52. In this context, we observed PRL mediated actions are preferably localized to milk production and survival of lactating epithelial cells, as GC prepares these cells for PRL by modulating the accessibility of chromatin and survival of epithelial cells.
Previously reported transcriptome data of HC11 cells was obtained using microarray and are of 15 and 20 K gene probe resolution17,19. In this study, we provided RNA-seq of higher resolution with nearly 50–60 million reads, whereas the recommended coverage is 15–20 million read53. The data in our study provides greater insight into gene expression in response to GC and PRL treatment during lactogenic differentiation of HC11 mammary epithelial cells.
Materials and Methods
Cell culture and lactogenic differentiation of HC11 MEC
HC11 MEC were cultured and differentiated essentially as described earlier20 Briefly, murine HC11 MEC stem-like cell line with passage number 6 (from Dr. Nancy E Hynes laboratory, Switzerland) were cultured with RPMI1640-GlutaMAXTM (Gibco) medium supplemented with 10% FBS (US Origin GIBCO), insulin (5 µg/ml, Sigma Catno#I6634), 1X Anti-biotic/Anti-mycotic (Gibco), and EGF (20 ng/ml, Sigma Cat.no#E4127) until they reach confluence under 5% CO2. Lactogenic differentiation of HC11 cells was initiated by withdrawal of EGF containing medium and replacement with fresh priming medium consisting of RPMI1640-GlutaMAXTM complete medium with 5% FBS, insulin (5 µg/ml), hydrocortisone (1 µg/ml, Sigma #H4001) and were grown at 37 °C for 48 hrs under 5% CO2. Alternatively, the primed cells were subjected to PRL treatment by supplementing with fresh RPMI1640-GlutaMAXTM complete medium with 5% FBS, insulin (5 µg/ml), hydrocortisone (1 µg/ml) and prolactin (5 µg/ml, NIH, #NIDDK-oPRL-21) and cells were then incubated at 37 °C for 72 hrs under 5% CO2 to complete lactogenic differentiation. Cells were harvested at 72 hrs and referred to them as PRL state of HC11 MEC.
Culturing of murine embryonic stem cells ESC
The mouse ESC line R1 was maintained on a layer of mitomycin-C treated mouse embryonic fibroblast feeder cells and harvested as described54 with few modifications. Briefly, mouse embryonic fibroblasts were grown at ~60% confluence in a 0.1% gelatin-coated Petri plates with a standard DMEM medium. Fibroblasts were inactivated by replacing standard medium with DMEM containing 5% FBS and 2.5% mitomycin-C for 3 hrs. R1 ESC were revived on inactivated fibroblasts using modified DMEM medium at 37 °C and 7% CO2. The components of modified DMEM include 10% FBS, mouse leukemia inhibitory factor (LIF) (1000 U/ml), inhibitors (MEK 0.5 µg/ml: GSK: 1.5 µg/ml), 1% Penicillin-streptomycin solution, 1X Non-essential amino acids, 2mM L-glutamine, and 50 μM 2-Mercaptoethanol. Grown Ebs of ESC on fibroblast feeder cells were subjected to trypsinization (0.5% Trypsin for 30 sec at 37 °C) and individual ESC that were devoid of fibroblasts were transferred onto fresh Petri plates and cultured in DMEM medium containing 10% FBS. ESC were selectively enriched over fibroblasts owing to the potential of fibroblasts to attach to the Petri plates faster than ESCs. These ESC were harvested for total RNA isolation, RNA-seq library preparation and sequencing.
Cell cycle analysis
Cell cycle analysis was performed for ESC, normal HC11 cells, HC11 cells exposed to priming conditions, and PRL treatment. Respective cells were cultured as detailed above, trypsinized and were washed three times with PBS (0.1 M, pH 7.4). Following washing with PBS, cells were fixed in 70% ethanol at −20 °C for 24 h. After fixation, cells were washed with ice-cold PBS and stained with buffer containing propidium iodide (50 μg/ml) and RNaseA (200 mg/ml), at 37 °C for 30 min in the dark. Cells cycle analysis was performed using the flow cytometer (BD Fortessa).
RNA extraction, library construction, and sequencing
Total RNA from ESC, Normal MEC, GC primed, and PRL treated MEC was isolated using Trizol (Invitrogen#15596026) according to manufacturer instructions. RNA was further purified using a commercially available kit (GCC Biotech#GR1003). 20 μg of purified RNA from each sample was treated with 10 Units of DNase1 (Roche # 04716728001) and were further purified by using G Sure cell culture RNA isolation kit. From each RNA sample, Ribosomal RNA was removed using Ribo-Zero kit (NEB#E6310L), and further mRNAs were enriched using Oligo (dT) beads. Illumina paired-end library was prepared as per the NEBNext® Ultra™ RNA Library Prep Kit (NEB#E7530S). All the libraries were paired-end sequenced using Illumina HiSeq2500 sequencing platform.
RNA-seq reads quality control and mapping
Raw sequence reads in the FASTQ format were further processed to remove Illumina adaptor sequences by using Trimmomatic55. The resultant raw reads were compressed to.gz format and were deposited in GEO repository (GSE107419). After filtering, approximately 55–65 million paired-end reads were processed for each sample. The reads were mapped to mouse reference genome (GRCm38/mm10) using TopHat2.1.045. The mismatch parameter was set to 2 and all other parameters to default.
Quantification and Identification of differentially expressed genes
Quantification of the expression of different genes was done with the tool ‘Sub-read version1.5.0’ using the features count mode method. Briefly, Total read count for each gene was obtained and was then normalized to obtain FPKM (Fragments per Kilobase exon per million reads sequenced) values. An FPKM ≥ 1 was considered as a threshold for determining whether a gene is expressed or not56. For differential gene expression analysis, DESeq2 version 1.24 was used. Differential gene expression analysis involved steps like model dependent p-value estimation using negative binomial distribution and Wald test, and adjusted p-value estimation based on multiple hypothesis testing. A log2 Fold change value of ≥1 is taken as a cutoff to define upregulated and a value of ≤−1 to define downregulated genes. The p-adjusted threshold value was set to 0.001 to maintain statistical significance. gg-plots package (R programming) was used to generate the heat maps representing the data. Venn diagrams representing the data were generated using Ugent web tool (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Identification of differentially expressed TFs and ERs
TFs in mouse were identified using the sequence-specific DNA binding or transcription regulation terms in GO functional annotation57 (Supplementary Table 44). List of ERs and their coding genes were obtained from previous literature58,59,60 (Supplementary Table 45). Corresponding expression (>0 FPKM) and log2 fold change values (≥1 up-regulation; ≤−1 downregulation) of TFs and ERs were extracted from the RNA-seq data. Here, it should be, however, note that some of the genes are represented both under TFs and ERs (Supplementary Table 46). Heat maps and Venn diagrams were generated as described in the previous section.
Pathway analysis of differentially expressed genes
Top upregulated and downregulated genes in ESC vs normal, normal vs Primed and Primed vs PRL were uploaded to DAVID database61,62 to derive the KEGG pathway enrichment and GO annotation data. All differentially expressed gene lists were uploaded in DAVID functional annotation from which KEGG pathway enrichment scores list was derived. Biocarta and Wiki pathways were obtained from existing databases63,64. Gene set enrichment analysis was calculated similarly to the method published previously65,66, and log2 fold change of each gene was calculated by comparing different samples. Mean log2 fold change of a pathway was calculated by summing the scores of individual genes. Gene-set enrichment score was calibrated against the background distribution using randomly sampled n (number of genes in a pathway) scores and calculating mean log2 score. This process was repeated over 10000 times. The mean and standard deviation of the sampling distribution thus obtained was used in correcting the original score.
Real-time RT-PCR analysis
Differentially expressed genes in RNA-seq analysis were validated by quantitative real-time PCR (CFX96#Bio-Rad). DNase treated 1 µg RNA was taken as input from each sample to synthesize cDNA using iScript cDNA synthesis kit (Bio-Rad). cDNA template was diluted, and PCR conditions were followed according to manufacturer protocol (Kappa Biosystems). β-actin was used as a housekeeping internal control gene and controls with no template were also included in PCR plate. All primers used in this experiment were designed to span exon-exon junctions to minimize genomic DNA contamination. Relative gene expression was calculated using the method −2ΔΔCT 67. All the graphs were generated using Graph pad prism software. Significance was calculated using two-way ANOVA by using graph pad prism and represented as [*] on top of error bars. All gene-specific primers used were listed in Supplementary Table 42.
Immunoblotting: Cell lysate from ESC and MEC (normal, Primed and Prolactin treated) was prepared and protein was extracted using RIPA buffer
A total of 30 μg of protein from each sample was resolved on 8% SDS-PAGE and subsequently transferred to nitrocellulose membrane by electroblotting. The nitrocellulose membrane was blocked in 5% non-fat milk for 1 hr and cut as per the respective protein molecular weight range. Subsequently, all the blots were incubated with the respective primary antibody at 4 °C overnight and then incubated with appropriate HRP linked secondary antibodies for 1 hr at room temperature. Blots were developed using the enhanced chemiluminescence (Bio-Rad#1705062) and imaged using Chemidoc (Bio-Rad). The protein band intensities were quantified using Image J software and normalized to β-actin expression. All the raw and contrast adjusted blots are represented in the supplementary information (Supplementary Fig. S2). All the antibodies and dilution factors used for this study were listed in Supplementary Table 43.
Data availability
All the raw data generated and analyzed in this study were deposited in GEO, accession number: GSE107419.
References
Salasznyk, R. M. et al. Focusing of gene expression as the basis of stem cell differentiation. Stem Cells Dev 14, 608–620, https://doi.org/10.1089/scd.2005.14.608 (2005).
Bach, K. et al. Differentiation dynamics of mammary epithelial cells revealed by single-cell RNA sequencing. Nat Commun 8, 2128, https://doi.org/10.1038/s41467-017-02001-5 (2017).
Sharmin, M., Bravo, H. C. & Hannenhalli, S. Heterogeneity of transcription factor binding specificity models within and across cell lines. Genome Res 26, 1110–1123, https://doi.org/10.1101/gr.199166.115 (2016).
Arvey, A., Agius, P., Noble, W. S. & Leslie, C. Sequence and chromatin determinants of cell-type-specific transcription factor binding. Genome Res 22, 1723–1734, https://doi.org/10.1101/gr.127712.111 (2012).
Choukrallah, M. A. & Matthias, P. The Interplay between Chromatin and Transcription Factor Networks during B Cell Development: Who Pulls the Trigger First? Front Immunol 5, 156, https://doi.org/10.3389/fimmu.2014.00156 (2014).
Maruyama, R. et al. Epigenetic regulation of cell type-specific expression patterns in the human mammary epithelium. PLoS Genet 7, e1001369, https://doi.org/10.1371/journal.pgen.1001369 (2011).
Sternlicht, M. D. Key stages in mammary gland development: the cues that regulate ductal branching morphogenesis. Breast Cancer Res 8, 201, https://doi.org/10.1186/bcr1368 (2006).
Macias, H. & Hinck, L. Mammary gland development. Wiley Interdiscip Rev Dev Biol 1, 533–557, https://doi.org/10.1002/wdev.35 (2012).
Fenton, S. E. & Sheffield, L. G. Prolactin inhibits epidermal growth factor (EGF)-stimulated signaling events in mouse mammary epithelial cells by altering EGF receptor function. Mol Biol Cell 4, 773–780 (1993).
Coleman, S., Silberstein, G. B. & Daniel, C. W. Ductal morphogenesis in the mouse mammary gland: evidence supporting a role for epidermal growth factor. Dev Biol 127, 304–315 (1988).
Buse, P., Woo, P. L., Alexander, D. B., Reza, A. & Firestone, G. L. Glucocorticoid-induced functional polarity of growth factor responsiveness regulates tight junction dynamics in transformed mammary epithelial tumor cells. J Biol Chem 270, 28223–28227 (1995).
Murtagh, J. et al. Organization of mammary epithelial cells into 3D acinar structures requires glucocorticoid and JNK signaling. J Cell Biol 166, 133–143, https://doi.org/10.1083/jcb.200403020 (2004).
Woo, P. L., Ching, D., Guan, Y. & Firestone, G. L. Requirement for Ras and phosphatidylinositol 3-kinase signaling uncouples the glucocorticoid-induced junctional organization and transepithelial electrical resistance in mammary tumor cells. J Biol Chem 274, 32818–32828 (1999).
John, S. et al. Chromatin accessibility pre-determines glucocorticoid receptor binding patterns. Nat Genet 43, 264–268, https://doi.org/10.1038/ng.759 (2011).
Cui, Y. et al. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol 24, 8037–8047, https://doi.org/10.1128/MCB.24.18.8037-8047.2004 (2004).
Kabotyanski, E. B., Huetter, M., Xian, W., Rijnkels, M. & Rosen, J. M. Integration of prolactin and glucocorticoid signaling at the beta-casein promoter and enhancer by ordered recruitment of specific transcription factors and chromatin modifiers. Mol Endocrinol 20, 2355–2368, https://doi.org/10.1210/me.2006-0160 (2006).
Wang, W., Jose, C., Kenney, N., Morrison, B. & Cutler, M. L. Global expression profiling reveals regulation of CTGF/CCN2 during lactogenic differentiation. J Cell Commun Signal 3, 43–55, https://doi.org/10.1007/s12079-009-0047-5 (2009).
Williams, C., Helguero, L., Edvardsson, K., Haldosen, L. A. & Gustafsson, J. A. Gene expression in murine mammary epithelial stem cell-like cells shows similarities to human breast cancer gene expression. Breast Cancer Res 11, R26, https://doi.org/10.1186/bcr2256 (2009).
Perotti, C. et al. Characterization of mammary epithelial cell line HC11 using the NIA 15k gene array reveals potential regulators of the undifferentiated and differentiated phenotypes. Differentiation 78, 269–282, https://doi.org/10.1016/j.diff.2009.05.003 (2009).
Morrison, B. & Cutler, M. L. Mouse Mammary Epithelial Cells form Mammospheres During Lactogenic Differentiation. J Vis Exp. https://doi.org/10.3791/1265 (2009).
Anders, S. & Huber, W. Differential expression analysis for sequence count data. Genome Biol 11, R106, https://doi.org/10.1186/gb-2010-11-10-r106 (2010).
Allar, M. A. & Wood, T. L. Expression of the insulin-like growth factor binding proteins during postnatal development of the murine mammary gland. Endocrinology 145, 2467–2477, https://doi.org/10.1210/en.2003-1641 (2004).
Choi, Y. S., Chakrabarti, R., Escamilla-Hernandez, R. & Sinha, S. Elf5 conditional knockout mice reveal its role as a master regulator in mammary alveolar development: failure of Stat5 activation and functional differentiation in the absence of Elf5. Dev Biol 329, 227–241, https://doi.org/10.1016/j.ydbio.2009.02.032 (2009).
Chakrabarti, R. et al. Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat Cell Biol 14, 1212–1222, https://doi.org/10.1038/ncb2607 (2012).
Zhang, S. & Cui, W. Sox2, a key factor in the regulation of pluripotency and neural differentiation. World journal of stem cells 6, 305–311, https://doi.org/10.4252/wjsc.v6.i3.305 (2014).
Hailesellasse Sene, K. et al. Gene function in early mouse embryonic stem cell differentiation. BMC Genomics 8, 85, https://doi.org/10.1186/1471-2164-8-85 (2007).
Yahata, T. et al. Cloning of mouse Cited4, a member of the CITED family p300/CBP-binding transcriptional coactivators: induced expression in mammary epithelial cells. Genomics 80, 601–613 (2002).
Ooga, M. et al. Chd9 mediates highly loosened chromatin structure in growing mouse oocytes. Biochem Biophys Res Commun 500, 583–588, https://doi.org/10.1016/j.bbrc.2018.04.105 (2018).
Kieffer-Kwon, K. R. et al. Myc Regulates Chromatin Decompaction and Nuclear Architecture during B Cell Activation. Mol Cell 67, 566–578 e510, https://doi.org/10.1016/j.molcel.2017.07.013 (2017).
Kurpios, N. A. et al. The Pea3 Ets transcription factor regulates differentiation of multipotent progenitor cells during mammary gland development. Dev Biol 325, 106–121, https://doi.org/10.1016/j.ydbio.2008.09.033 (2009).
Westendorp, B. et al. E2F7 represses a network of oscillating cell cycle genes to control S-phase progression. Nucleic Acids Res 40, 3511–3523, https://doi.org/10.1093/nar/gkr1203 (2012).
Regan, J. L. et al. Aurora A kinase regulates mammary epithelial cell fate by determining mitotic spindle orientation in a Notch-dependent manner. Cell Rep 4, 110–123, https://doi.org/10.1016/j.celrep.2013.05.044 (2013).
Szegezdi, E., Logue, S. E., Gorman, A. M. & Samali, A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep 7, 880–885, https://doi.org/10.1038/sj.embor.7400779 (2006).
Davis, K. R. et al. XBP1 Regulates the Biosynthetic Capacity of the Mammary Gland During Lactation by Controlling Epithelial Expansion and Endoplasmic Reticulum Formation. Endocrinology 157, 417–428, https://doi.org/10.1210/en.2015-1676 (2016).
Malhotra, G. K. et al. The role of Sox9 in mouse mammary gland development and maintenance of mammary stem and luminal progenitor cells. BMC Dev Biol 14, 47, https://doi.org/10.1186/s12861-014-0047-4 (2014).
Ferrari, N., McDonald, L., Morris, J. S., Cameron, E. R. & Blyth, K. RUNX2 in mammary gland development and breast cancer. J Cell Physiol 228, 1137–1142, https://doi.org/10.1002/jcp.24285 (2013).
Kouros-Mehr, H., Slorach, E. M., Sternlicht, M. D. & Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127, 1041–1055, https://doi.org/10.1016/j.cell.2006.09.048 (2006).
Zhou, J. et al. Elf5 is essential for early embryogenesis and mammary gland development during pregnancy and lactation. EMBO J 24, 635–644, https://doi.org/10.1038/sj.emboj.7600538 (2005).
Cocolakis, E. et al. Smad signaling antagonizes STAT5-mediated gene transcription and mammary epithelial cell differentiation. J Biol Chem 283, 1293–1307, https://doi.org/10.1074/jbc.M707492200 (2008).
Hollenhorst, P. C., Paul, L., Ferris, M. W. & Graves, B. J. The ETS gene ETV4 is required for anchorage-independent growth and a cell proliferation gene expression program in PC3 prostate cells. Genes Cancer 1, 1044–1052, https://doi.org/10.1177/1947601910395578 (2011).
Yahata, T. et al. The MSG1 non-DNA-binding transactivator binds to the p300/CBP coactivators, enhancing their functional link to the Smad transcription factors. J Biol Chem 275, 8825–8834 (2000).
Braganca, J. et al. Human CREB-binding protein/p300-interacting transactivator with ED-rich tail (CITED) 4, a new member of the CITED family, functions as a co-activator for transcription factor AP-2. J Biol Chem 277, 8559–8565, https://doi.org/10.1074/jbc.M110850200 (2002).
Shioda, T., Fenner, M. H. & Isselbacher, K. J. MSG1 and its related protein MRG1 share a transcription activating domain. Gene 204, 235–241 (1997).
Panchenko, M. V., Zhou, M. I. & Cohen, H. T. von Hippel-Lindau partner Jade-1 is a transcriptional co-activator associated with histone acetyltransferase activity. J Biol Chem 279, 56032–56041, https://doi.org/10.1074/jbc.M410487200 (2004).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol 14, R36, https://doi.org/10.1186/gb-2013-14-4-r36 (2013).
Roschger, C. & Cabrele, C. The Id-protein family in developmental and cancer-associated pathways. Cell Commun Signal 15, 7, https://doi.org/10.1186/s12964-016-0161-y (2017).
John, S. et al. Interaction of the glucocorticoid receptor with the chromatin landscape. Mol Cell 29, 611–624, https://doi.org/10.1016/j.molcel.2008.02.010 (2008).
Stoelzle, T., Schwarb, P., Trumpp, A. & Hynes, N. E. c-Myc affects mRNA translation, cell proliferation and progenitor cell function in the mammary gland. BMC Biol 7, 63, https://doi.org/10.1186/1741-7007-7-63 (2009).
Yori, J. L., Johnson, E., Zhou, G., Jain, M. K. & Keri, R. A. Kruppel-like factor 4 inhibits epithelial-to-mesenchymal transition through regulation of E-cadherin gene expression. J Biol Chem 285, 16854–16863, https://doi.org/10.1074/jbc.M110.114546 (2010).
Zhou, H. et al. Variation in the bovine FABP4 gene affects milk yield and milk protein content in dairy cows. Sci Rep 5, 10023, https://doi.org/10.1038/srep10023 (2015).
Zidi, A. et al. Genetic variation at the goat hormone-sensitive lipase (LIPE) gene and its association with milk yield and composition. J Dairy Res 77, 190–198, https://doi.org/10.1017/S0022029910000099 (2010).
Rudolph, M. C. et al. Sterol regulatory element binding protein and dietary lipid regulation of fatty acid synthesis in the mammary epithelium. Am J Physiol Endocrinol Metab 299, E918–927, https://doi.org/10.1152/ajpendo.00376.2010 (2010).
Liu, Y., Zhou, J. & White, K. P. RNA-seq differential expression studies: more sequence or more replication? Bioinformatics 30, 301–304, https://doi.org/10.1093/bioinformatics/btt688 (2014).
Bibel, M., Richter, J., Lacroix, E. & Barde, Y. A. Generation of a defined and uniform population of CNS progenitors and neurons from mouse embryonic stem cells. Nat Protoc 2, 1034–1043, https://doi.org/10.1038/nprot.2007.147 (2007).
Bolger, A. M., Lohse, M. & Usadel, B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics 30, 2114–2120, https://doi.org/10.1093/bioinformatics/btu170 (2014).
Love, M. I., Huber, W. & Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15, 550, https://doi.org/10.1186/s13059-014-0550-8 (2014).
Ashburner, M. et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet 25, 25–29, https://doi.org/10.1038/75556 (2000).
Shipra, A., Chetan, K. & Rao, M. R. CREMOFAC–a database of chromatin remodeling factors. Bioinformatics 22, 2940–2944, https://doi.org/10.1093/bioinformatics/btl509 (2006).
Fazzio, T. G., Huff, J. T. & Panning, B. Chromatin regulation Tip(60)s the balance in embryonic stem cell self-renewal. Cell Cycle 7, 3302–3306, https://doi.org/10.4161/cc.7.21.6928 (2008).
Gendler, K., Paulsen, T. & Napoli, C. ChromDB: the chromatin database. Nucleic Acids Res 36, D298–302, https://doi.org/10.1093/nar/gkm768 (2008).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4, 44–57, https://doi.org/10.1038/nprot.2008.211 (2009).
Huang da, W., Sherman, B. T. & Lempicki, R. A. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37, 1–13, https://doi.org/10.1093/nar/gkn923 (2009).
Liberzon, A. et al. Molecular signatures database (MSigDB) 3.0. Bioinformatics 27, 1739–1740, https://doi.org/10.1093/bioinformatics/btr260 (2011).
Pico, A. R. et al. WikiPathways: pathway editing for the people. PLoS Biol 6, e184, https://doi.org/10.1371/journal.pbio.0060184 (2008).
Arun, P. V. et al. Identification and functional analysis of essential, conserved, housekeeping and duplicated genes. FEBS Lett 590, 1428–1437, https://doi.org/10.1002/1873-3468.12192 (2016).
Ideker, T., Ozier, O., Schwikowski, B. & Siegel, A. F. Discovering regulatory and signalling circuits in molecular interaction networks. Bioinformatics 18(Suppl 1), S233–240 (2002).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Acknowledgements
National Institute of Animal Biotechnology, Department of Biotechnology, India (NIAB/CP/111/2012) and Indian Council of Medical Research, India (ICMR/90/9/2012/SCRT(TF)/BMS for funding to SK2. TS and PKG are grateful to UGC-CSIR and RN for RGNF for Junior and senior research fellowships. VU acknowledges DST-Women fellowship (SR/WOS-A/LS-172/2016). SM is grateful to DBT for a junior research fellowship. We thank Dr Nancy E. Hynes for providing HC11 cells and Dr Andras Nagi for R1 mESCs. We also thank Dr Bramanandam Manavathi for his help in the establishment of the HC11 cell differentiation system. FACS facility at the Department of Animal Biology, School of Life Sciences, is acknowledged.
Author information
Authors and Affiliations
Contributions
S.K.2* conceived and designed research; T.S., R.N., S.M. performed experiments; T.S., P.K.G., S.K.1, V.U. analyzed data; A.K.P., S.Y., and S.K.2 interpreted the data; T.S., A.K.P., and S.K.2 wrote the manuscript with the inputs from rest of the authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sornapudi, T.R., Nayak, R., Guthikonda, P.K. et al. Comprehensive profiling of transcriptional networks specific for lactogenic differentiation of HC11 mammary epithelial stem-like cells. Sci Rep 8, 11777 (2018). https://doi.org/10.1038/s41598-018-30122-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-30122-4
This article is cited by
-
Single-Cell Transcription Mapping of Murine and Human Mammary Organoids Responses to Female Hormones
Journal of Mammary Gland Biology and Neoplasia (2024)
-
Hormonal regulation of mammary gland development and lactation
Nature Reviews Endocrinology (2023)
-
Inhibiting β-catenin disables nucleolar functions in triple-negative breast cancer
Cell Death & Disease (2021)
-
Controlled synchronization of prolactin/STAT5 and AKT1/mTOR in bovine mammary epithelial cells
In Vitro Cellular & Developmental Biology - Animal (2020)
-
RNA sequencing of murine mammary epithelial stem-like cells (HC11) undergoing lactogenic differentiation and its comparison with embryonic stem cells
BMC Research Notes (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.