Abstract
The organic-inorganic perovskite CH3NH3PbI3 has attracted much attention due to their power conversion efficiency as a potential photovoltaic material, but the role of an external electric field has not been well understood. Based on first-principles calculations, the effects of an external electric field (E) applied along the [111] direction of the orthorhombic perovskite, CH3NH3PbI3, on its electronic structure and optical properties are investigated. Our results indicate that the electric field strength affects the band gap (Eg) of CH3NH3PbI3 (MAPbI3, MA = CH3NH3). The energy difference between the two peaks closest to the Fermi level in the density of states diagram decreases with increasing applied electric field strength along the [111] direction, indicating that the covalent character increases between A-sites cations and I-sites anions. Both the cell volume and the final energy show the same increasing trend. The absorption peaks move toward the visible-frequency range, with the optimal band gap of 1.1–1.45 eV and E = 0.04–0.06 eV/Å/e. In particular, the non-linear change of the second-order Stark effect causes a non-linear change in the band gap.
Similar content being viewed by others
Introduction
The organic-inorganic hybrid perovskite CH3NH3PbI3 has attracted much attention for its use as a photon absorber in thin-film solar cells. In addition, its synthesis is relatively straightforward and can be achieved by simple layer deposition1,2,3,4,5,6. Although great success has been achieved in improving the photovoltaic energy conversion efficiency of this perovskite2,7, perovskites are ideal because they absorb most of the solar spectrum, from the ultraviolet region to the near-infrared region, generate more excitons as more photons absorbed, and bring the energy levels closer to that of the electron transport layer (ETL) or hole transport layer (HTL). The optimal band gap for a single junction solar cell is between 1.1 and 1.4 eV8,9. However, CH3NH3PbI3 has a narrow direct band gap energy (Eg) of 1.51 eV, which is not optimal and hinders its application.
Using first-principles calculations, Amat et al.10 investigated tetragonal APbI3 perovskites with A = Cs+, (CH3NH3)+, and (NH2)2CH+ and found that CH3NH3PbI3 and (NH2)2CHPbI3 have the same band gap. Hao et al.11 studied the performance of CH3NH3Sn1−xPbxI3 perovskite solar cells. Their results indicated that the band gaps of mixed Pb/Sn hybrid perovskites have two extremes, 1.55 and 1.35 eV, depending on the ratio of Pb to Sn, but the band gaps were narrow (<1.3 eV). Furthermore, a band gap of 1.60 eV for CH3NH3PbI312, 2.39 eV for CH3NH3PbBr3, and 3.17 eV for CH3NH3PbCl313,14 was obtained. Moreover, it was shown that the optical band gap can be tuned from a direct band gap of 1.52 eV to an indirect band gap of 2.64 eV by varying the CH3NH3I concentration15. In addition, the band gap of the tetragonal phase of CH3NH3PbI3 decreased with decreasing temperature16, following the relationship Eg (T) = Eg (T0) − b.kB.(T0 − T)2. Thus, finding the optimal band structure remains an important physical problem.
In many cases, the electric field effect can change the geometric, electronic, magnetic, and band structures of materials, and thus regulate their physical properties. Zhao et al.17 reported the influence of external electric fields on the electronic structure and optical properties of TiO2. They found that the band gap of TiO2 becomes narrower with increasing electric field strength, decreasing to 0 eV when the electric field is 0.25 eV. Varignon et al.18 used an electric field to control the Jahn-Teller distortions in bulk perovskites such as SrTiO3, BaMnO3, YMnO3, and BiFeO3. Bellaiche et al. reported that an external electric field could induce polarization paths in PbZr1−xTixO3 perovskites and lead to the expected sequence of tetragonal, A-type monoclinic, and rhombohedral structures19. Xu et al.20 demonstrated that electric fields could induce a change from a ferroelectric phase to an antiferroelectric phase in a lead-free NaNbO3-based polycrystalline ceramic. Therefore, it is very important and valuable to investigate the relationship between external electric fields and the physical properties of the organic-inorganic hybrid perovskite CH3NH3PbI3. CH3NH3PbI3 undergoes two phase transitions, one at 160 K (orthorhombic to tetragonal) and the other at 330 K (tetragonal to cubic)21. Using Density functional theory (DFT) calculations, Leppert et al. investigated the Rashba effect induced by the electric field and strained in the hybrid halide perovskite CH3NH3PbI3 with a tetragonal and cubic structure22. Although the orthorhombic phase is realized with rotations about the C–N axis that freeze out when T ≤ 162 K, temperature is not the only factor affecting these properties. An external electric field or magnetic field could also be an important factor, so we chose the orthorhombic CH3NH3PbI3 as our subject to investigate these effects.
In this article, we investigate the effects of an external electric field applied along the [111] direction on the geometry structure, electronic energy band structure, total density of states, and optical properties of CH3NH3PbI3. This study provides a method for obtaining the optimal band gap of CH3NH3PbI3 and expands the scope of its applications.
Results and Discussion
The effects of the external electric field (E) direction, including the [001], [010], [100], [110], and [111] directions, on the band structure of CH3NH3PbI3 were studied. The application of an electric field along the [111] direction in CH3NH3PbI3 decreases the band gap, while fields aligned along the other directions increase the band gap. Therefore, we only investigated the physical properties of CH3NH3PbI3 under an electric field aligned along the [111] direction to obtain the optimal band gap of 1.1–1.4 eV.
Band structure of CH3NH3PbI3 under the external electric field
The band structure of CH3NH3PbI3 in the absence of an external electric field is shown in Fig. 1 (a,b). When the external electric field is equal to zero, the valence band maximum (VBM) and the conduction band minimum (CBM) are located at the same Γ-point, which indicates that CH3NH3PbI3 possesses direct semiconductor characteristics. The calculated band is 1.726 eV and 1.675 eV, corresponding to the generalized gradient approximation (GGA) functional developed by Perdew, Burke, and Ernzerhof PBE23 and PBEsol24, which are similar to the results25 reported by Menéndez-Proupin et al. The detailed calculation methods were shown in the Method section. It should be noted that the number of conduction bands calculated using GGA + PBEsol is greater than that calculated using GGA + PBE. In the presence of an applied external electric field with a strength ranging from 0.01 to 0.06 eV/Å/e, the band structures of the orthorhombic CH3NH3PbI3 are similar. Figure 1 (c,d) show the band structure when E = 0.06 eV/Å/e. The conduction band shifts downward to the Fermi level (0 eV) and the energy band near the CBM is more dispersed, resulting in a change in the band gaps. All the configurations indicate an indirect band gap semiconductor, and the VBM and CBM are located at the centre of the Γ-point in k space. Because of the narrower indirect band gap of the CH3NH3PbI3 semiconductor, only a small amount of energy is required for the formation of excitons. However, the GGA + PBE and GGA + PBEsol methods underestimate the band gap because of self-interaction errors, suggesting that the actual band gap of CH3NH3PbI3 is slightly smaller than the calculated values.
Band structure of CH3NH3PbI3, calculated by GGA + PBE without the external electric field (a), calculated by GGA + PBEsol without the external electric field (b), calculated by GGA + PBE with the external electric field 0.06 eV/Å/e (c), and calculated by GGA + PBEsol with the external electric field 0.06 eV/Å/e (d).
The band gaps of CH3NH3PbI3 are strongly affected by the applied electrical field strength. As the external electric field strength increases, the band gaps calculated by GGA + PBE first decrease linearly, then increase slightly, and finally, decrease linearly. The band gaps calculated by GGA + PBEsol indicated the optimal value between 1.1 and 1.4 eV for all the structures under an external electric field, as shown in Fig. 2. Therefore, both the results indicated that the external electric field contributes to a decrease in the band gap.
Density of states of CH3NH3PbI3 under an external electric field
Figure 3 (a) calculated by GGA + PBE and (b) calculated by GGA + PBEsol show the total density of states (TDOS) of CH3NH3PbI3 in the presence of an external electric field (E = 0.00–0.06 eV/Å/e). As the external electric field increases, (i) the two peaks corresponding to orbital energies from −22.5 to −15 eV gradually broaden and then become narrow, reaching their maximum widths when E = 0.03 eV/Å/e. (ii) The five peaks corresponding to orbital energies between −15 and −5.5 eV gradually broaden. (iii) Parts of the TDOS of the orbitals crossing the Fermi level increase with increasing external electric field, suggesting that the band gap of CH3NH3PbI3 decreases. (iv) The CBM gradually narrows and shifts to the Fermi level, which also decreases the band gap, suggesting a pseudo-energy gap decrease, while the strength of the covalent character increases between the MA-cations and I anions.
The electron densities of CH3NH3PbI3 when E = 0.00 and 0.06 eV/Å/e were shown in Fig. 4 (a,b), calculated by GGA + PBEsol. It can be seen that: (i) the lost electronic ions are H and Pb cations, and the gain electronic ions are C, N and I anions. (ii) With increasing external electric field, the electron density of C-N increases, thus increasing the strength of the associated covalent bond. (iii) The exact value of gain or lost electrons can be obtained based on Milliken Charge Analysis Method of Wave Function. For the E = 0.00, the lost average H cations have three kinds including 0.34, 0.22, and 0.21 electrons, the lost average Pb cations have one kind with 0.88 electrons, the gain anions with C and N have 0.61 and 0.70 electrons, and the gain I anion have two kinds including 0.36 and 0.52 electrons. For the E = 0.06 eV/Å/e, the lost average H cations have seven kinds including 0.15, 0.16, 0.29, 0.30, 0.34, 0.35 and 0.44 electrons, the lost average Pb cations have one kind with 0.54 electrons, the gain C anions have 0.65 electrons, the gain N anions have two kinds including 0.77 and 0.76 electrons, and the gain I anion have six kinds including 0.29, 0.37, 0.38, 0.39, 0.40, and 0.41 electrons.
Figure 5 shows the partial density of states (PDOS) of CH3NH3PbI3 (GGA + PBEsol) under applied external electric field strengths of E = 0.00 and 0.06 eV/Å/e. The electronic orbitals 5d6s6p, 5s5p, 2s2p, 2s2p, and 1s are modeled as the valence orbitals for Pb, I, C, N, and H, respectively. The s-p hybrid level increases with external electric field increasing, and the effect on the conduction band is much more pronounced than that on the valance band. The peak near the orbital energy level at −5.0 eV splits into two peaks, which is attributed to the Stark effect of the s and p electrons, and one peak position shifts to the Fermi level. The external electric field causes the d electrons of Pb to shift to higher energies, but the d electrons do not affect the s and p electrons.
The Stark effect can induce the splitting of degenerate energy levels. The degree of the band splitting increases with the electric field, and a smaller band gap can be obtained. All peaks of the orbital energy level in the DOS curves broaden and shift to lower energies as E increases. The physical mechanisms underlying the change in the band structure of CH3NH3PbI3 is ascribed to the C-N and Pb-I bond lengths, changes in the lattice structure, and charge transfer between the Pb and I atoms. The lattice distortion caused by the mutation of lattice parameters may lead to change in the microscopic electric structure, band structure, and density of states, similar to the results reported in ref.17.
Optimized structure of CH3NH3PbI3 under an external electric field
The crystal structure determines the physical properties of the material; the lattice parameters (a, b, and c), cell volume (V), bond length (d), and final energies (EFinal) are listed in Table 1. The external electric field applied along the [111] direction stretches the lattice parameters. Figure 6 shows that both the final energies and the cell volumes increase monotonically, and the final energies obtained by GGA + PBE are lower than those obtained by GGA + PBEsol. The GGA + PBE results follow the fitted relationships EFinal (E) = 1591.989E – 12504.565 and V (E) = 1035.804E + 983.666. In addition, the bond distances, including the d(C-N) and d(I-Pb) distances, increase monotonously with increasing E, which leads to weak interactions between C and N as well as between I and Pb. To illustrate the extent of lattice distortion, two parameters (\({n}_{1}=\frac{b\,/\,\sqrt{2}-a}{a}\), and \({n}_{2}=\frac{b\,/\,\sqrt{2}-c}{c}\)) can be used for describing the degree of deviation from the cubic structure26. The curves for these parameters versus the external electric field are shown in Fig. 7. For the results based on the GGA + PBE or GGA + PBEsol method, both n1 and n2 are positive, suggesting that the orthogonal structures are of the O type, where \(\frac{c}{\sqrt{2}} > b\), \(\frac{a}{\sqrt{2}} > b\), and the deviations range from 2.31% to 27.86%, which are much greater than those for single inorganic perovskites27.
In addition, the orientation of the CH3NH3+ ion can be seen in the orthogonal CH3NH3PbI3. Table 1 shows the Cartesian coordinates of the C and N atoms are displaced by 0.01 Å along the [111] direction, which is similar to a past report that Pb and apical I atoms are displaced by 0.1 Å and 0.01 Å along the [001] direction in P4mm CH3NH3PbI3 (ref.22). The Pb-N-C angle decreases with increasing external electric field, which consistents with the increase in the lattice parameters and cell volume.
Optical properties of CH3NH3PbI3 under an external electric field
CH3NH3PbI3 may show different preferential growth directions with different substrates, so obtaining the optical performance in this growth direction is an important physical problem. Let us take the [100] direction as the preferred growth direction as an example to illustrate this problem. Figure 8 (a) calculated by GGA + PBE and (b) calculated by GGA + PBEsol show the optical absorption spectrum with polarized light, where the polarization is along the [100] direction of CH3NH3PbI3 in the presence of an external electric field. It is seen that the electric field significantly influences the optical absorption characteristics of CH3NH3PbI3. We assume the highest intensity absorption peak as the main absorption peak. The values 5.5 eV and 3.68 eV in the range of 0~5 eV for CH3NH3PbI3 calculated by the GGA + PBE and GGA + PBEsol methods are the positions of the main absorption peak without the external electric field, which corresponds to absorbed light with the highest frequency. As the external electric field increases, the peak positions shift to lower frequencies, approaching the visible light region, and full width at half maximum (FWHM) of the absorption peaks decreases, which makes light absorption more effective and thus improves the photoelectric conversion. In addition, there are some absorption peaks at higher energies beyond the visible range, which do not play a major role in photoelectric conversion, but can become a candidate as optical detection device such as ultraviolet band.
As the first-order approximation, the band gap Eg and wavelength should satisfy the following relation:
We can obtain the related data based on eq. 1, as shown in Table 2. The following can be deduced:
-
(i)
The band gap decreases non-linearly with increasing external electric field. When E ≤ 0.03 eV/Å/e, ν and ν100 decrease with an increase in the external electric field; when E ≥ 0.04 eV/Å/e, ν and ν100 increase with external electric field increasing, except ν with 0.04 eV/Å/e based on the GGA + PBE. Here, ν is the frequency calculated by the band gap, ν100 is the frequency along the [100] direction based on the Fig. 8.
-
(ii)
The band gap (Eg) based on the band structure is similar to Eg’ based on the optical properties. Based on adiabatic approximation and single electron approximation, the relationship between absorption coefficient and photon energy can be expressed as,
where a is the absorption coefficient, B is a fitting parameter, h is Planck’s constant, v is frequency, and Eg′ is the band gap. According to the data in Fig. 8, Eg′ values based on the optic properties are shown in Table 2.
Moreover, although the preferred growth direction may be not [100] in practice, we provided a method to obtain the optical properties of CH3NH3PbI3 under an external electric field.
Why does the band gap decrease non-linearly?
For CH3NH3PbI3 under an external electric field, the Hamiltonian can be written as,
Here, \({\hat{H}}_{0}=-\,\frac{{\hslash }^{2}}{2m}{\nabla }^{2}+V(r)\); \(\hat{H}^{\prime} =e\overrightarrow{E}\cdot \overrightarrow{r}=eEr\,\cos \,\theta \); \(e=1.6\times {10}^{-19}{\rm{C}}\); E is the external electric field; and θ is the angle between the direction of the electric field and the radius vector direction \(\overrightarrow{r}\). \(\hat{H}^{\prime} \) is the perturbation. Based on the perturbation theory, the energy levels (Ei) will change to the initial values (E(0)i) by an amount ∆Ei.
∆Ei can be expanded with the additional energy of the electric field, which are Stark effect orders denoted as first order ∆E(1)i or second order ∆E(2)i.
The energy changes owing to the electric field, where <ψi | is the initial state of the system, then we have,
For second-order energy changes, the summation is over all possible states of the system, so,
If E can be considered a constant over the perturbation volume,
From Table 1, it can be seen that the movements of the I− anions are coupled to the movements of the monovalent MA+ cations and the rotation of the MA dipoles. This change in polarizability in the domains can influence the second-order Stark effect through the change in the dielectric constant owing to the change in the optical absorption spectrum28. Then, ΔE(2) may reflect in two possible first-order Stark effects, as shown in Fig. 9. If the value of ΔE(2) is positive, the band gap will widen; if the value of ΔE(2) is negative, the band gap will shrink. However, the second-order Stark effect cannot be larger than the first-order Stark effect, so CH3NH3PbI3 without an external electric field has the maximum band gap. The non-linear extent of the second-order Stark effect cause a non-linear change in the band gap.
The device required that: (i) The Highest Occupied Molecular Orbital (HOMO) of TiO2 as ETL layer must be lower than CBM of perovskite active layer. (ii) The Lowest Unoccupied Molecular Orbital (LUMO) of spiro-omeTAD as HTL layer must be higher than VBM of perovskite active layer. The external electric field induced the Stark effect, splitting energy levels for TiO2 and spiro-omeTAD (shown in Fig. 10), which decreases the HOMO of TiO2 and increases the LUMO of spiro-omeTAD.
How to obtain the external electric field in practice?
As a preliminary exploration, we believe that this study is valuable. Indeed, the calculated results are aimed for the orthorhombic structure and not the tetragonal structure at room temperature, but the orthorhombic MAPbI3 structure can be applied to solar panels in space with lower temperature. Due to cosmic microwave background with 3 K29, the perovskite solar cells with orthorhombic phase can accomplish as Power generation device candidate in space such as International Space Station, satellite, space shuttle, spacecraft, lunar rover vehicle, etc. Moreover, this may provide a new idea to control the properties of the tetragonal structure at room temperature.
The main reason for the orthorhombic to tetragonal transition in CH3NH3PbI3 is temperature, and the external electric field causes the Stark effect splitting energy level of C, N, H, Pb and I, and the two structures with the same element and the similar chemical bond characters. Thus, we inferred that the Stark effect for CH3NH3PbI3 with a tetragonal structure may be observed at room temperature.
The device for realizing the external electric field is shown in Fig. 11, which is similar to the ref. reported by Li et al.30. It noted that the device in this study only provide the external electric field, do not provide electrons. The large DC voltage near 100 V can be obtained by DC boost circuit, which can resolve the electric field strength. The positive electrode is connected with the transparent ITO for visible light, and the negative electrode is connected with Au; the directions of the two electrodes can control the direction of the electric field, including the [111] direction. Moreover, the distance between the positive and negative electrodes, as well as the insulation thickness and dielectric constant, influenced the electric field strength.
Device for realizing an external electric field. Here, VCC is input voltage obtained by the storage battery charged by the solar battery, L is inductance, ue is rectangular square wave pulse signal which control the duty cycle and regulate magnification, IGBT is crystal oscillator, D is diode, R is resistance, and C is capacitance.
It noted that the external electric field of about 0.06 eV/Å/e is large, which is equal to 0.6 V/nm, but this external electric field can be carried out in practice. For example, Hsu et al. used an external magnetic field to adjust the relative energy levels between a skyrmion and a ferromagnet globally, and obtained an electric field of 1 V/nm, which corresponds to a magnetic field of about 40 mT for their system31. Qin et al. applied an external electric field to drive the ultra-low thermal conductivity of silicene. Using an electric field (Ez = 5 V/nm), the lattice thermal conductivity of silicene can be reduce a record low value of 0.091 Wm−1 K−1, which is comparable to that of the best thermal insulation materials32. The main problem caused by the huge electric field may be the device breakdown, which is attributed to the self-sustainable discharge for uniform electric field. The more uniform the electric field is, the higher the self-sustainable discharge voltage is. So the transparent ITO as the positive electrode and the Au film as the negative electrode should possess the rule shapes and flat surfaces, which would decrease the degree of dielectric polarization, and reduce the possibility of the tip discharge.
Conclusion
In conclusion, we have used first-principles calculations to calculate the geometries, band structure, electronic properties, and optical absorption properties of perovskite CH3NH3PbI3 under an external electric field aligned along the [111] direction. The external electric field increases the lattice parameters and the cell volume, stretching the c-axis and influencing the degree of lattice distortion. The external electric field controls the band gap from 1.70 to 1.073 eV, corresponding to external electric field strengths of 0.00 to 0.06 eV/Å/e. The rotation of the MA dipoles induced a second-order Stark effect, and the non-linear change in the second-order Stark effect causes a non-linear change in the band gap. In addition, the absorption peaks and the peak locations are affected by the external electric field. Thus, this study provides a possible method to improve the photoelectric conversion of perovskite solar cells.
Methods
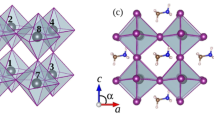
CH3NH3PbI3 perovskite undergoes two phase transitions, one at 160 K (orthorhombic to tetragonal) and the other at 330 K (tetragonal to cubic). The orthorhombic CH3NH3PbI3 structure could be closer to that at the 0 K, so we chose this as the research object given the DFT calculations are performed at 0 K. Based on the structure reported by Menéndez-Proupin et al.25, CH3NH3PbI3 perovskite has an orthorhombic crystal structure in the space group Pnma (no. 62), with lattice parameters of a = 8.8273 Å, b = 12.6793 Å, and c = 8.5099 Å, as shown in Table 3 and Fig. 12. CH3NH3PbI3 has a typical AMX3 perovskite structure with the unit cell consisting of a central lead atom octahedrally coordinated to six iodide atoms. The PbI6 octahedron is located inside a cube with each iodide at the centre of a cubic face, and the CH3NH3 cations are positioned at the corners of the cube. To study the effect of the electric field on the physical properties of CH3NH3PbI3, the external electric field along different directions (x, y, and z) was investigated.
We calculated the physical properties including the energy band, the density of states (DOS), and optical absorption in the orthorhombic perovskite CH3NH3PbI3 exposed to different external electric fields using the Cambridge Serial Total Energy Package (CASTEP)33,34 program. The generalized gradient approximation (GGA) functional developed by Perdew, Burke, and Ernzerhof (1996, 2008, PBE, and PBEsol)23,24 was used. These exchange-correlation functionals employed were same as those in the study of the self-regulation mechanism for charged point defects in hybrid halide perovskites reported by Walsh et al.35. The spin-orbit coupling (SOC) effect is reported to have little influence on the geometric structures36. Ultra-soft pseudopotentials with a cutoff energy of 310 eV (based on test results) were used to describe the interactions between the valence electrons and the ionic core, and including relativistic effects for Pb and I atoms. A 3 × 2 × 3 Monkhorst-Pack k-point scheme was used to calculate the absorption spectra. We performed the convergence test, and found the results with 500 eV cutoff energy and 5 × 5 × 5 Monkhorst-Pack k-point scheme to be similar to those with the above parameters. The convergence tolerances for geometry optimization calculations were set to a maximum displacement of 5.0 × 10−4Å, maximum force of 0.01 eV/Å, maximum energy change of 5.0 × 10−6 eV/atom, maximum stress of 0.02 GPa.
According to the refs24,35, PBEsol as exchange–correlation functional is a revision of the PBE functional, which improves equilibrium properties of densely-packed solids and their surfaces, specifically tailored for solids. It has been shown to yield structural data in accordance with experiment reported by F. Brivio et al.37. This functional predicts the structure of common London-dispersion corrected functions without the addition of an empirical potential.
References
Xing, G., Mathews, N. & Sun, S. et al. Long-range balanced electron-and hole-transport lengths in organic-inorganic CH3NH3PbI3. Science 342, 344 (2013).
Im, J. H., Jang, I. H. & Pellet, N. et al. Growth of CH3NH3PbI3 cuboids with controlled size for high-efficiency perovskite solar cells. Nature nanotechnology 9, 927 (2014).
Kim, H. S. & Park, N. G. Parameters affecting I-V hysteresis of CH3NH3PbI3 perovskite solar cells: effects of perovskite crystal size and mesoporous TiO2 layer. The journal of physical chemistry letters 5, 2927 (2014).
Kim, H. S., Lee, J. W. & Yantara, N. et al. High efficiency solid-state sensitized solar cell-based on submicrometer rutile TiO2 nanorod and CH3NH3PbI3 perovskite sensitizer. Nano letters 13, 2412 (2013).
Lindblad, R., Bi, D. & Park, B. et al. Electronic structure of TiO2/CH3NH3PbI3 perovskite solar cell interfaces[J]. The journal of physical chemistry letters, 2014 5, 648 (2014).
Xie, F. X., Zhang, D. & Su, H. et al. Vacuum-assisted thermal annealing of CH3NH3PbI3 for highly stable and efficient perovskite solar cells. ACS nano 9, 639 (2015).
Yang, Z., Surrente, A. & Galkowski, K. et al. Unraveling the Exciton Binding Energy and the Dielectric Constant in Single-Crystal Methylammonium Lead Triiodide Perovskite. The Journal of Physical Chemistry Letters 8, 1851 (2017).
Albero, J., Asiri, A. M. & García, H. Influence of the composition of hybrid perovskites on their performance in solar cells. Journal of Materials Chemistry A 4, 4353 (2016).
Tress W. Maximum Efficiency and Open-Circuit Voltage of Perovskite Solar Cells[M]//Organic-Inorganic Halide Perovskite Photovoltaics. Springer International Publishing, 53–77 (2016).
Amat, A., Mosconi, E. & Ronca, E. et al. Cation-induced band-gap tuning in organohalide perovskites: interplay of spin–orbit coupling and octahedra tilting. Nano letters 14, 3608 (2014).
Hao, F., Stoumpos, C. C. & Chang, R. P. H. et al. Anomalous band gap behavior in mixed Sn and Pb perovskites enables broadening of absorption spectrum in solar cells. Journal of the American Chemical Society 136, 8094 (2014).
Wehrenfennig, C., Liu, M. & Snaith, H. J. et al. Homogeneous Emission Line Broadening in the Organo Lead Halide Perovskite CH3NH3PbI3–xClx. The journal of physical chemistry letters 5, 1300 (2014).
Li, C., Wei, J. & Sato, M. et al. Halide-substituted electronic properties of organometal halide perovskite films: direct and inverse photoemission studies. ACS applied materials & interfaces 8, 11526 (2016).
Comin, R., Walters, G. & Thibau, E. S. et al. Structural, optical, and electronic studies of wide-bandgap lead halide perovskites. Journal of Materials Chemistry C 3, 8839 (2015).
Ke, X., Yan, J. & Zhang, A. et al. Optical band gap transition from direct to indirect induced by organic content of CH3NH3PbI3 perovskite films. Applied Physics Letters 107, 091904 (2015).
Dittrich, T., Awino, C. & Prajongtat, P. et al. Temperature dependence of the band gap of CH3NH3PbI3 stabilized with PMMA: a modulated surface photovoltage study. The Journal of Physical Chemistry C 119, 23968 (2015).
Zhao, C., Huang, D. & Chen, J. et al. First-principle study for influence of an external electric field on the electronic structure and optical properties of TiO2. RSC Advances 6, 98908 (2016).
Varignon, J., Bristowe, N. C. & Ghosez, P. Electric field control of Jahn-Teller distortions in bulk perovskites. Physical review letters 116, 057602 (2016).
Bellaiche, L., García, A. & Vanderbilt, D. Electric-field induced polarization paths in Pb(Zr1− xTix)O3 alloys. Physical Review B 64, 060103 (2001).
Xu, Y., Hong, W. & Feng, Y. et al. Antiferroelectricity induced by electric field in NaNbO3-based lead-free ceramics. Applied physics letters 104, 052903 (2014).
Poglitsch, A. & Weber, D. Dynamic disorder in methylammoniumtrihalogenoplumbates (II) observed by millimeter-wave spectroscopy. The Journal of chemical physics 87, 6373 (1987).
Leppert, L., Reyes-Lillo, S. E. & Neaton, J. B. Electric Field-and Strain-Induced Rashba Effect in Hybrid Halide Perovskites. The journal of physical chemistry letters 7, 3683 (2016).
Perdew, J. P., Burke, K. & Ernzerhof, M. Generalized gradient approximation made simple. Physical review letters 77, 3865 (1996).
Perdew, J. P., Ruzsinszky, A. & Csonka, G. I. et al. Restoring the density-gradient expansion for exchange in solids and surfaces. Physical Review Letters 100, 136406 (2008).
Menéndez-Proupin, E., Palacios, P. & Wahnón, P. et al. Self-consistent relativistic band structure of the CH3NH3PbI3 perovskite. Physical Review B 90, 045207 (2014).
Ji, D. H., Xiao, X. J. & Zhang, C. M. et al. Regulatory band gap of vacancy at the B sites in CH3NH3Pb1−xI3 perovskite. Modern Physics Letters B 30, 1650294 (2016).
Guo, Y., Roy, S. & Ali, N. et al. Magnetic and electronic transport properties of YbxCa1−xMnO3 compounds. Journal of applied physics 91, 7394 (2002).
Pazoki, M., Jacobsson, T. J. & Kullgren, J. et al. Photoinduced stark effects and mechanism of ion displacement in perovskite solar cell materials. ACS nano 11, 2823 (2017).
Partridge, B. & Page, L. 3K: The Cosmic Microwave Background Radiation. Annales Henri Poincaré 49, 62–63 (1996).
Li, S. D., Wang, X. Y. & Zhang, W. J. et al. The effect of external electric field on the performance of perovskite solar cells. Organic Electronics 18, 107 (2015).
Hsu, P. J., Kubetzka, A. & Finco, A. et al. Electric-field-driven switching of individual magnetic skyrmions. Nature nanotechnology 12, 123 (2017).
Qin, G., Qin, Z. & Yue, S. Y. et al. External electric field driving the ultra-low thermal conductivity of silicene. Nanoscale 9, 7227 (2017).
Milman, V., Winkler, B. & White, J. A. et al. Electronic structure, properties, and phase stability of inorganic crystals: A pseudopotential plane-wave study. International Journal of Quantum Chemistry 77, 895 (2000).
Ong, K. P., Goh, T. W. & Xu, Q. et al. Mechanical origin of the structural phase transition in methylammonium lead iodide CH3NH3PbI3. The journal of physical chemistry letters 6, 681 (2015).
Walsh, A., Scanlon, D. O. & Chen, S. et al. Self-Regulation Mechanism for Charged Point Defects in Hybrid Halide Perovskites. Angewandte Chemie International Edition 54, 1791 (2015).
Umari, P., Mosconi, E. & De Angelis, F. Relativistic GW calculations on CH3NH3PbI3 and CH3NH3SnI3 perovskites for solar cell applications. Scientific reports 4, 4467 (2014).
Brivio, F., Walker, A. B. & Walsh, A. Structural and electronic properties of hybrid perovskites for high-efficiency thin-film photovoltaics from first-principles. APL Materials 1, 042111 (2013).
Acknowledgements
This work was supported by Joint Funds of Department of Science and Technology of Guizhou Province, LiuPanshui Administration of Science and Technology and LiuPanshui Normal University under Contract No.LH[2014]7449 and LH[2014]7456, Research Foundation for Advanced Talents of LiuPanshui Normal University (Grant No. LPSSYKYJJ201404), the Key Project of the Education Department of Guizhou Province (No. KY2015379), the National Science Foundation of China under Contract 11504078, the MIT Creative Team of Liupanshui Normal University No. LPSSYKJTD201402, the Physical Electronic Key Discipline of Guizhou Province No. ZDXK201535, the Outstanding Young Scientist Cultivation Program of Guizhou Province No. 201522.
Author information
Authors and Affiliations
Contributions
Denghui Ji designed and conducted all the calculations, and wrote the paper. Mula Na prepared the figures 1, 2 and 3, Shuling Wang, and Hong Zhang prepared the Figures 4, 5, 6, 7, 8, 9, 10, 11 and 12. Kun Zhu and Congmin Zhang analysed the data. Xiuling Li prepared the Tables. All the authors commented on the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ji, D., Na, M., Wang, S. et al. Role of an external electric field on hybrid halide perovskite CH3NH3PbI3 band gaps. Sci Rep 8, 12492 (2018). https://doi.org/10.1038/s41598-018-29935-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29935-0
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.