Abstract
Avian-origin H5/H7 influenza has the potential to cause the next influenza pandemic. Availability of effective vaccines is an essential part of pre-pandemic preparedness. However, avian influenza surface antigens are poorly immunogenic to humans, which necessitates the use of adjuvants to augment the immunogenicity of pre-pandemic influenza vaccines. Aluminum salts are approved, safe, and affordable adjuvants, but their adjuvanticity for influenza vaccines remains unverified. We conducted the first meta-analysis on this issue. A total of nine randomized controlled trials (2006–2013, 22 comparisons, 2,467 participants in total) compared aluminum-adjuvanted H5N1 vaccines versus non-adjuvanted counterparts. The weighted estimate for the ratio of the seroprotection rate after a single dose of H5N1 vaccine is 0.66 (95% CI: 0.53 to 0.83) by hemagglutination-inhibition assay or 0.56 (95% CI: 0.42 to 0.74) by neutralizing titer assay. The weighted estimate for the risk ratio of pain/tenderness at injection sites is 1.85 (95% CI: 1.56 to 2.19). The quality of evidence is low to very low for seroprotection (due to indirectness and potential reporting bias) and moderate for pain/tenderness (due to potential reporting bias), respectively. The significantly lower seroprotection rate after aluminum-adjuvanted H5N1 vaccines and the significantly higher risk of pain at injection sites indicate that aluminum salts decrease immunogenicity but increase local reactogenicity of pre-pandemic H5N1 vaccines in humans.
Similar content being viewed by others
Introduction
Avian-origin H5N1 influenza, which has caused 860 cases of human infection around the world (2003-September 2017, with 454 deaths, data from World Health Organization), has the potential to cause the next influenza pandemic. Availability of effective vaccines against this zoonotic virus is an essential part of pandemic preparedness. However, H5N1 antigens are poorly immunogenic to humans, which necessitates the use of an adjuvant, a substance that augments the immunogenicity of vaccines, in manufacturing pre-pandemic H5N1 vaccines1,2,3.
Until recently, aluminum salts have been the only adjuvants used in human vaccines licensed in the United States4. Unlike newer oil-in-water adjuvants such as MF59 or AS03, aluminum salts are inexpensive and safe and have been successfully used in diphtheria, tetanus, and pertussis vaccines for more than 80 years4,5. Aluminum salts are also used in hepatitis A, hepatitis B, and human papillomavirus vaccines6. Nevertheless, their adjuvanticity for influenza vaccines remains unverified.
In animal models, experiments consistently demonstrate that aluminum salts enhance the immunogenicity of H5N1 influenza vaccines7. In contrast, randomized controlled clinical trials of aluminum salts-adjuvanted H5N1 influenza vaccines in humans are either statistically inconclusive – likely due to the insufficient sample sizes in each of these clinical trials – or not specifically designed to evaluate the adjuvanticity of aluminum salts8.
To maximize the pandemic preparedness, it is necessary to clarify whether aluminum salts, the approved, safe, and affordable adjuvant, are effective in enhancing the immunogenicity of pre-pandemic influenza vaccines. To overcome the limitation of insufficient statistical power in single randomized controlled trials, we conducted the first meta-analysis on this issue.
Results
Literature search
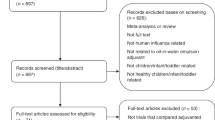
Figure 1 shows the flowchart of the literature search. Trials were eligible if they were randomized controlled trials that compared the immunogenicity of aluminum-adjuvanted H5N1 influenza vaccines versus that of non-adjuvanted counterparts (with the same dose of identical H5 antigen) in healthy individuals. We identified all eligible trials through searching PubMed, EMBASE, Cochrane, CINAHL, Web of Science, Scopus, and Google Scholar, along with reports completed in the clinical trial registry database (ClinicalTrials.gov) before June 30, 2017. We used the following search keywords: “influenza vaccine” AND “aluminum”, filtered by human, clinical trial, and English language. We excluded those trials that did not compare adjuvanted vaccines versus non-adjuvanted counterpart, those that did not involve H5N1 vaccines, and those trials that used different antigen doses across the compared groups.
Of the 2,895 published papers and 28 completed clinical trial reports identified by initial keyword searches, nine non-duplicated randomized trials met the eligibility criteria1,9,10,11,12,13,14,15,16. These nine trials (completed during 2006–2013) included 22 comparisons (some trials consisted of several comparisons that assessed different antigen doses of the same vaccines, see Supplementary Table S1 for the details of each comparison), with a total of 2,467 healthy participants. All included trials used a two-dose schedule (with an interval of one month) for testing pre-pandemic H5N1 vaccines. Of the 22 comparisons, 16 and 12 comparisons reported seroprotection rates (proportions of subjects with titers reaching seroprotection levels, see Methods for the definition) by hemagglutination-inhibition assay and by neutralizing titer assay, respectively, 21–28 days after the first-dose vaccination.
Seroprotection
Compared with non-adjuvanted counterparts, H5N1 vaccines with aluminum salts adjuvant were associated with a significantly lower, rather than higher, seroprotection rate 21–28 days after the first dose. The weighted estimate for the ratio of the seroprotection rate by hemagglutination-inhibition assay was 0.66 (95% confidence interval [CI]: 0.53 to 0.83, I-square: 0.0%) across a range of different antigen doses (Fig. 2). The weighted estimate for the ratio of the seroprotection rate by neutralizing titer assay was 0.56 (95% CI: 0.42 to 0.74, I-square: 0.0%) across a range of different antigen doses (Fig. 3).
After the second dose, aluminum-adjuvanted H5N1 vaccines still did not yield a higher seroprotection rate. The seroprotection rate after the second dose was lower than that conferred by non-adjuvanted counterparts, although the difference was not statistically significant. The weighted estimate for ratios of the seroprotection rate 21–28 days after the second dose of H5N1 vaccine were 0.97 (95% CI: 0.82 to 1.13, I-square: 21.8%) by hemagglutination-inhibition assay (Supplementary Fig. S1) and 0.99 (95% CI: 0.88 to 1.12, I-square: 11.8%) by neutralizing titer assay (Supplementary Fig. S2).
Funnel plot analyses did not show publication biases (Supplementary Fig. S3).
Harm
Compared with non-adjuvanted counterparts, H5N1 vaccines with aluminum salts adjuvant were associated with a significantly higher risk of pain/tenderness at the injection site during the 7 days after the first vaccination, with the weighted risk ratio of 1.85 (95% CI: 1.56 to 2.19, I-square: 30.8%) (Fig. 4). There was no difference in risk of fever after vaccination (weighted risk ratio 1.00, 95% CI: 0.30 to 3.35, I-square: 0.0%) (Supplementary Fig. S4).
After the second dose, aluminum-adjuvanted H5N1 vaccines were still associated with a significantly higher risk of pain/tenderness at the injection site (weighted risk ratio 1.72, 95% CI: 1.20 to 2.46, I-square: 70.0%) (Supplementary Fig. S5). There was no difference in risk of fever after the second vaccination (weighted risk ratio 0.31, 95% CI: 0.06 to 1.52) (Supplementary Fig. S6).
Risk of bias assessment
Three trials, Keitel12, Ehrlich10, and Chichester15, had low risk of bias. Bresson1 was open-label trial. Pan16 was single-blind. Three trials, Bresson1, Bernstein9, and Brady13 did not reported seroprotection data after the first dose vaccination. Two trials, Nolan11 and Keitel14, did not reported adverse events separately after the first vs. after the second dose. The assessment was summarized in Supplementary Figs S7 and S8.
Quality of evidence
We used the GRADE approach to assess the quality of evidence, taking risks of bias, risks of random errors, risks of publication bias, and risks of lack of external validity into consideration. We summarized the findings in Supplementary Table S2 (seroprotection) and Supplementary Table S3 (harm). The certainty of evidence was low to very low for seroprotection rate endpoints due to potential outcome reporting bias (only 16 and 12 of the 22 included comparisons reported seroprotection rates data after the first-dose vaccination) and indirectness (seroprotection is a surrogate for real life protection against infection, disease and death) (Table S2), and moderate for local pain/tenderness at the injection sites (Table S3) due to potential outcome reporting bias (only 15 and 5 of the 22 included comparisons reported pain/tenderness and fever after the first-dose vaccination), respectively. The certainty of evidence was very low for fever due to the wide confidence intervals (0.30 to 3.35 and 0.06 to 1.52) as well as potential outcome reporting bias (Table S3).
Discussion
This is the first meta-analysis of randomized controlled trials on the efficacy of aluminum salts as an adjuvant for pre-pandemic influenza vaccines. Our results showed an inferior seroprotection rate after aluminum-adjuvanted H5N1 vaccines compared with that conferred by non-adjuvanted counterparts. The absence of an increase in seroprotection rates of aluminum salts-adjuvanted vaccines indicates that aluminum salts are not suitable to serve as adjuvants for pre-pandemic H5N1 influenza vaccines for humans.
The observed lack of efficacy might be explained by the Th2 immune response elicited by aluminum salts5; for intracellular pathogens, such as novel influenza virus, a Th1 immune response is required instead17,18,19. The significantly worse seroprotection rate observed in the trial participants received aluminum salts-adjuvanted vaccines suggests that aluminum salts actually interfere with the immunogenicity of pre-pandemic influenza vaccines because the wrong type of T cell response is elicited.
The negative impact of aluminum salts on the immunogenicity of pre-pandemic influenza vaccine in human clinical trials is in sharp contrast with the positive animal experiment results in mice and ferret models. Aluminum salts significantly increase the immunogenicity of H5N1 vaccines, measured by both hemagglutination-inhibition and neutralization titer assays, in both mice20,21,22 and ferrets7,23,24. This discrepancy between animal experiments and human clinical trials highlights an important limitation of animal models as a testing ground for vaccine development: animal models have different toll-like receptor expression patterns compared with humans5. This difference might explain the different effects of aluminum salts in animals and humans. Moreover, there are several well-known differences between species in terms of the pathophysiology and immune responses to influenza virus infection. For example, ferrets are highly susceptible to a wide range of influenza virus isolates, but mice are not. The presence of the mx1 antiviral gene in mice necessitates the use of specifically adapted influenza virus strains, which could markedly differ from the field virus isolates, in mouse models25,26. Each type of animal model has its unique usefulness and limitations in influenza research27,28,29, which makes it impossible to directly generalize animal study results to humans.
With the aim to directly evaluate the effect of adjuvant on immunogenicity, we did not include comparisons of influenza vaccines with different antigen doses in this meta-analysis. Nevertheless, even if multiple-arms comparisons were taken into consideration, the conclusion on the lack of adjuvanticity of aluminum salts for influenza vaccines would be unlikely to change, as shown by a 2009 network meta-analysis study that primarily aimed to identify the best formulation of H5N1 vaccine30. This multiple treatment meta-analysis shows that, unlike non-aluminum adjuvants such as MF59 and AS03, aluminum salts did not significantly enhance the immunogenicity compared with non-adjuvanted vaccines (for comparsons using less than 7.5 mcg H5 antigen the risk differences were 0.01 [95% CI: −0.03 to 0.29] by hemagglutination-inhibition and 0.04 [95% CI: −0.11 to 0.34] by neutralizing titer; for comparisons using 15 mcg H5 antigen dose the risk ratio was 1.05 [95% CI: 0.81 to 1.36]30. Another meta-analysis31 compared the immunogenicity and safety of H5N1 vaccines with different antigen doses but did not present quantitative analysis results on the adjuvanticity of aluminum salts. This 2016 meta-analysis31 included only 8 of the 9 randomized trials enrolled in the present meta-analysis, without the trial (with a total of 545 subjects) reported in 2009 by Brady et al.13, which we included in our meta-analysis.
Currently, eight manufacturers (based in China, Russia, Kazakhstan, Japan, and Australia) provide licensed aluminum-adjuvanted H5N1 vaccines32. While the World Health Organization Strategic Advisory Group of Experts (SAGE) on Immunization stated that “studies using Al(OH)3 in H5 inactivated vaccines have produced variable results that are less than impressive”33, these vaccines are perceived as cost-saving alternatives to the expensive MF59- or AS03-adjuvanted pre-pandemic influenza vaccines. However, our meta-analyses show that, if aluminum salts were not added in the first place, these same vaccines could be more immunogenic against targeted influenza virus strains. The negative impact of aluminum salts on the immunogenicity of H5N1 vaccines might explain the unexpected failure of Emerflu (Sanofi) in 201134. EmerfluTM is a split-virion inactivated pre-pandemic H5N1 influenza vaccine with 30 μg of hemagglutinin and 600 μg of Al(OH)3 and was withdrawn from applications for licenses after pre-marketing trials showed that the seroprotection was below the established criteria34. Our findings that the addition of aluminum salts decreased, rather than increased, the immunogenicity of pre-pandemic influenza vaccines are highly relevant to vaccine manufacturers, which play an important role in pre-pandemic preparedness.
With the newly emerged threat of avian-origin H7N9 influenza from China35,36, H7N9 vaccines have become a priority in research and development for pre-pandemic preparedness. Several teams of researchers are currently testing candidate H7N9 vaccines in animal models37,38,39. One laboratory reported a very good adjuvant effect of aluminum salts for H7N9 vaccines in ferrets37. These promising animal data should be interpreted cautiously40, as illustrated by the negative impact of aluminum salts on the immunogenicity of H5N1 influenza vaccines shown in this meta-analysis.
An important limitation of our study is that only 7 and 5 of the 9 included randomized controlled trials (16 and 12 of the 22 comparisons) reported seroprotection rates data after the first-dose vaccination, the primary outcome of our meta-analysis, by hemagglutination-inhibition assay and by neutralizing titer assay, respectively. We had contacted with the authors of the remaining trials, but was unable to obtain unpublished data. Nevertheless, funnel plot analyses did not detect evidence for publication biases.
Our meta-analysis of all available data reported from randomized controlled trials in human subjects shows that aluminum salts decrease, rather than increase, the immunogenicity of pre-pandemic H5N1 influenza vaccines. Furthermore, aluminum salts increase local reactogenicity, with pain/tenderness at injection sites. Therefore, aluminum salts should not be recommended as adjuvants for these vaccines. This unexpected, but important, finding highlights the limitation of animal models as the testing ground for developing pre-pandemic influenza vaccines for humans.
Methods
Ethical Statement
This is a meta-analysis of published randomized controlled trials reports and is exempted from human subject research review.
Definition of Seroprotection
Seroprotection is defined as a titer of ≥1:40 (or ≥1:32) by hemagglutination-inhibition assay, as pre-specified by the investigators of each trial report; or a titer of ≥1:40 (or ≥1:20) by neutralizing titer assay, as pre-specified by the investigators of each trial report.
Main Outcome
The main outcome is the ratio of the seroprotection rate 21–28 days after receiving the first dose of aluminum-adjuvanted H5N1 influenza vaccines versus that of non-adjuvanted counterparts (with the same antigen). A single dose of aluminum-adjuvanted H5N1 influenza vaccines is immunogenic and safe41 and meets the licensing criteria for interpandemic and pandemic influenza vaccines in the European Union and the United States when the H5 antigen dose is ≥6 mcg41.
Secondary Outcomes
We assessed other potentially important outcomes, including the ratio of the seroprotection rate 21–28 days after receiving the second dose of aluminum-adjuvanted H5N1 influenza vaccines versus that of non-adjuvanted counterparts; and the risk ratios of (a) pain/tenderness at the injection site during the 7 days after the first dose; (b) pain/tenderness at the injection site during the 7 days after the second dose; (c) fever (body temperature higher than 38 °C) during the 7 days after the first dose; and (d) fever (body temperature higher than 38 °C) during the 7 days after the second dose.
Statistical Software
Forest plots were generated for summarizing ratios of seroprotection rates using random effect models. We used a funnel plot to detect publication bias. STATA 9.0 (Stata, Stata Corp LP, College Station, TX, USA) was used for all statistical analyses.
Bias of risk assessment
Risk of bias was assessed using the Cochrane risk of bias tools. It consisted of seven specific domains, including: selection bias (random sequence generation and allocation concealment), performance bias (blinding of participants and personnel and other potential threats to validity), detection bias (blinding of outcome assessment and other potential threats to validity), attrition bias (incomplete outcome data) and reporting bias (selective outcome reporting assessed by comparing outcomes reported in the protocol to those reported in the completed RCT whenever possible)42.
Grade of Evidence
We used the five GRADE considerations to assess the quality of evidence, i.e. risk of bias, inconsistency, indirectness, imprecision, and publication bias42,43. We employed GRADEpro (https://gradepro.org/) to create summary tables of the findings for each outcome. We justified all decisions to downgrade or upgrade the quality of evidence using footnotes and comments.
Data availability
All data analyzed in this study are included in the published article.
References
Bresson, J. L. et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet 367, 1657–1664, https://doi.org/10.1016/s0140-6736(06)68656-x (2006).
Treanor, J. J. et al. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. New Engl J Med 354, 1343–1351, https://doi.org/10.1056/NEJMoa055778 (2006).
Treanor, J. J. et al. Safety and immunogenicity of a recombinant hemagglutinin vaccine for H5 influenza in humans. Vaccine 19, 1732–1737 (2001).
Lee, S. & Nguyen, M. T. Recent advances of vaccine adjuvants for infectious diseases. Immune Network 15, 51–57, https://doi.org/10.4110/in.2015.15.2.51 (2015).
Reed, S. G., Orr, M. T. & Fox, C. B. Key roles of adjuvants in modern vaccines. Nat Med 19, 1597–1608, https://doi.org/10.1038/nm.3409 (2013).
Centers for Disease Control and Prevention. Vaccine Excipient & Media Summary - Excipients Included in U.S (2013).
Heldens, J. G. et al. Feasibility of single-shot H5N1 influenza vaccine in ferrets, macaques and rabbits. Vaccine 28, 8125–8131, https://doi.org/10.1016/j.vaccine.2010.09.097 (2010).
Atmar, R. L. et al. Adjuvants for pandemic influenza vaccines. Curr Top Microbiol Immunol 333, 323–344, https://doi.org/10.1007/978-3-540-92165-3_16 (2009).
Bernstein, D. I. et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis 197, 667–675, https://doi.org/10.1086/527489 (2008).
Ehrlich, H. J. et al. A clinical trial of a whole-virus H5N1 vaccine derived from cell culture. N Engl J Med 358, 2573–2584, https://doi.org/10.1056/NEJMoa073121 (2008).
Nolan, T. M. et al. Phase I and II randomised trials of the safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in healthy adults. Vaccine 26, 4160–4167, https://doi.org/10.1016/j.vaccine.2008.05.077 (2008).
Keitel, W. A. et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J Infect Dis 198, 1309–1316, https://doi.org/10.1086/592172 (2008).
Brady, R. C. et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine 27, 5091–5095, https://doi.org/10.1016/j.vaccine.2009.06.057 (2009).
Keitel, W. A. et al. Safety and immunogenicity of inactivated, Vero cell culture-derived whole virus influenza A/H5N1 vaccine given alone or with aluminum hydroxide adjuvant in healthy adults. Vaccine 27, 6642–6648, https://doi.org/10.1016/j.vaccine.2009.03.015 (2009).
Chichester, J. A. et al. Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: a phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 4, 3227–3244, https://doi.org/10.3390/v4113227 (2012).
Pan, S. C. et al. The Madin-Darby canine kidney cell culture derived influenza A/H5N1 vaccine: a phase I trial in Taiwan. J Microbiol Immunol Infect 46, 448–455, https://doi.org/10.1016/j.jmii.2012.08.002 (2013).
Brewer, J. M. How do aluminium adjuvants work? Immuno Lett 102, 10–15, https://doi.org/10.1016/j.imlet.2005.08.002 (2006).
De Gregorio, E. et al. Alum adjuvanticity: unraveling a century old mystery. Eur J Immunol 38, 2068–2071, https://doi.org/10.1002/eji.200838648 (2008).
Kool, M. et al. Alum adjuvant: some of the tricks of the oldest adjuvant. J Med Microbiol 61, 927–934, https://doi.org/10.1099/jmm.0.038943-0 (2012).
Macleod, M. K. et al. Influenza nucleoprotein delivered with aluminium salts protects mice from an influenza A virus that expresses an altered nucleoprotein sequence. Plos One 8, e61775, https://doi.org/10.1371/journal.pone.0061775 (2013).
Ninomiya, A. et al. Inactivated influenza H5N1 whole-virus vaccine with aluminum adjuvant induces homologous and heterologous protective immunities against lethal challenge with highly pathogenic H5N1 avian influenza viruses in a mouse model. Vaccine 25, 3554–3560, https://doi.org/10.1016/j.vaccine.2007.01.083 (2007).
Yam, K. K. et al. Comparison of AS03 and Alum on immune responses elicited by A/H3N2 split influenza vaccine in young, mature and aged BALB/c mice. Vaccine 34, 1444–1451, https://doi.org/10.1016/j.vaccine.2016.02.012 (2016).
Layton, R. C. et al. Enhanced immunogenicity, mortality protection, and reduced viral brain invasion by alum adjuvant with an H5N1 split-virion vaccine in the ferret. Plos One 6, e20641, https://doi.org/10.1371/journal.pone.0020641 (2011).
Vela, E. M. et al. Efficacy of a heterologous vaccine and adjuvant in ferrets challenged with influenza virus H5N1. Influenza Other Respir Viruses 6, 328–340, https://doi.org/10.1111/j.1750-2659.2011.00321.x (2012).
Staeheli, P. et al. Influenza virus-susceptible mice carry Mx genes with a large deletion or a nonsense mutation. Mol Cell Biol 8, 4518–4523 (1988).
Grimm, D. et al. Replication fitness determines high virulence of influenza A virus in mice carrying functional Mx1 resistance gene. Proc Natl Acad Sci USA 104, 6806–6811, https://doi.org/10.1073/pnas.0701849104 (2007).
Margine, I. et al. Animal models for influenza viruses: implications for universal vaccine development. Pathogens 3, 845–874, https://doi.org/10.3390/pathogens3040845 (2014).
Mak, I. W. et al. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res 6, 114–118 (2014).
Gerdts, V. et al. Use of animal models in the development of human vaccines. Future Microbiol 2, 667–675, https://doi.org/10.2217/17460913.2.6.667 (2007).
Manzoli, L. et al. Immunogenicity and adverse events of avian influenza A H5N1 vaccine in healthy adults: multiple-treatments meta-analysis. Lancet Infect Dis 9, 482–492, https://doi.org/10.1016/s1473-3099(09)70153-7 (2009).
Guo, Q. et al. Immunogenicity and safety of pandemic influenza H5N1 vaccines in healthy adults through meta-analysis. Cell Physiol Biochem 40, 921–932, https://doi.org/10.1159/000453150 (2016).
World Health Organization. Influenza A (H5N1) Vaccine stockpile and inter-pandemic vaccine use background document (2013).
Baz, M. et al. H5N1 vaccines in humans. Virus Res 178, 78–98, https://doi.org/10.1016/j.virusres.2013.05.006 (2013).
European Medicines Agency. Emerflu withdrawal assessment report. Available at, http://www.ema.europa.eu (2011).
Kile, J. C. et al. Update: Increase in Human Infections with Novel Asian Lineage Avian Influenza A(H7N9) Viruses During the Fifth Epidemic - China, October 1, 2016-August 7, 2017. MMWR 66, 928–932, https://doi.org/10.15585/mmwr.mm6635a2 (2017).
Wang, X. et al. Epidemiology of avian influenza A H7N9 virus in human beings across five epidemics in mainland China, 2013–17: an epidemiological study of laboratory-confirmed case series. Lancet Infect Dis 17, 822–832, https://doi.org/10.1016/s1473-3099(17)30323-7 (2017).
Chia, M. Y. et al. Evaluation of MDCK cell-derived influenza H7N9 vaccine candidates in ferrets. Plos One 10, e0120793, https://doi.org/10.1371/journal.pone.0120793 (2015).
Wu, C. Y. et al. Squalene-adjuvanted H7N9 virus vaccine induces robust humoral immune response against H7N9 and H7N7 viruses. Vaccine 32, 4485–4494, https://doi.org/10.1016/j.vaccine.2014.06.043 (2014).
Ou, H. et al. Analysis of the immunogenicity and bioactivities of a split influenza A/H7N9 vaccine mixed with MF59 adjuvant in BALB/c mice. Vaccine 34, 2362–2370, https://doi.org/10.1016/j.vaccine.2016.03.037 (2016).
Wu, U. I. et al. Safety and immunogenicity of an inactivated cell culture-derived H7N9 influenza vaccine in healthy adults: a phase I/II, prospective, randomized, open-label trial. Vaccine 35, 4099–4104, https://doi.org/10.1016/j.vaccine.2017.06.044 (2017).
Vajo, Z. et al. A single-dose influenza A (H5N1) vaccine safe and immunogenic in adult and elderly patients: an approach to pandemic vaccine development. J Virol 84, 1237–1242, https://doi.org/10.1128/JVI.01894-09 (2010).
Higgins, J. P. T. et al. Cochrane handbook for systematic reviews of interventions, version 5.1.0. The Cochrane Collboration. Available at, https://www.handbook.cochrane.org (2011).
Schünemann, H. et al. GRADE handbook for grading quality of evidence and strength of recommendations. Updated: December 04. Available at, http://gdt.guidelinedevelopment.org (2015).
Author information
Authors and Affiliations
Contributions
C.T.F. and Y.J.L. designed the study. Y.J.L. conducted the literature search and meta-analysis of the data. C.T.F. and Y.J.L. drafted the manuscript. Y.J.S. and C.H.C. critically reviewed the draft. All authors reviewed and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lin, YJ., Shih, YJ., Chen, CH. et al. Aluminum salts as an adjuvant for pre-pandemic influenza vaccines: a meta-analysis. Sci Rep 8, 11460 (2018). https://doi.org/10.1038/s41598-018-29858-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29858-w
This article is cited by
-
Long-term exposure to low doses of aluminum affects mineral content and microarchitecture of rats alveolar bone
Environmental Science and Pollution Research (2021)
-
Plant virus particles with various shapes as potential adjuvants
Scientific Reports (2020)
-
Oil-in-water emulsion adjuvants for pediatric influenza vaccines: a systematic review and meta-analysis
Nature Communications (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.