Abstract
The efficacy of PPV-23 vaccine on outcomes of pneumococcal disease in adults still remains controversial due mainly to the lack of consistency between the results obtained from observational studies(OSs) and those obtained from randomized controlled trials(RCTs). As a consequence, the complexity in the structure of evidence available, in turn, generates a challenge for combining disparate pieces of evidence quantitatively. In this regard, we used a hierarchical Bayesian inference-based evidence synthesis of RCTs and observational data using a two-stage approach (in addition to a traditional random-effects meta-analysis) to examine the effectiveness of PPV-23 in adults. To this end, 21 studies were included involving 826109 adult participants. By a two-stage Bayesian meta-analysis, which was directly used for combining studies of different designs, the overall log OR (95% credible interval) for IPDs was −0.1048 (−0.3920,−0.0250), indicating a significant protective effect of the vaccination against IPDs. No significant effect of PPV-23 was found on all-cause pneumonia, pneumococcal pneumonia, and death from pneumonia, which confirmed the results obtained by a traditional method followed by stratified and sensitivity analyses. The estimated overall log OR (95% credible interval) was −0.0002 (−0.0241,0.0142), −0.0002 (−0.0110,0.0122), and −6.3912 × 10−5 (−0.0219,0.0131), respectively. The PPV-23 vaccine might be effective in preventing the most severe invasive forms of pneumococcal diseases, but not effective in preventing other clinical outcomes, in the adult population of 18 years and older.
Similar content being viewed by others
Introduction
Approximately, 1.6 million deaths annually occur due to pneumococcal diseases worldwide, with the highest incidence rate in children under 2 years and adults over 65 years of age1,2. Therefore, pneumococcal invasive (such as bacteremia/sepsis and meningitis) and non-invasive infections (such as sinusitis and otitis media) still remain major public health problems with a high morbidity and mortality. In the US, for example, the mortality rate of pneumococcal pneumonia is 13–23% in the elderly, compared to 5–7% in the general population3.
There are two available pneumococcal preventive vaccines: the older Polysaccharide Vaccines (PPVs) that contain a purified capsular polysaccharide and the newer Conjugate Vaccines (PCVs) that include a conjugation of a carrier protein to capsular polysaccharide. The PPVs, which have been developed gradually from 2- to 23-valent vaccines, have been available since the early 1980s4. They are widely recommended for all individuals aged 65 years and older and adults who are at high risk for invasive pneumococcal diseases (IPDs), especially those with a history of a chronic lung disease, heart failure, chronic renal failure, chronic liver disease, diabetes mellitus, and asplenia/sickle cell disease. Furthermore, the immunocompromised people including HIV/AIDS or blood borne dyscrasias patients with cochlear implants or chronic cerebrospinal fluid leak, residents of nursing homes, and patients in long-term care facilities are high-risk populations who are recommended to receive the polysaccharide vaccine. However, the protection level, either in the elderly or at high-risk populations, remains a controversial issue5.
The 23-valent pneumococcal polysaccharide vaccine (PPV-23) includes 23 capsular serotypes (1, 2, 3, 4, 5, 6B, 7 F, 8, 9 N, 9 V, 10 A, 11 A, 12 F, 14, 15B, 17 F, 18 C, 19 A, 19 F, 20, 22 F, 23 F, and 33 F) which account for 72%6 to 95%7 of IPDs, depending on the geographic location8. Typically, IPDs affect sterile body sites including cerebrospinal fluid, pleural fluid, and blood stream. This is while vaccination strategies are aimed to give appropriate protection. On the other hand, the adult pneumococcal vaccination policy is very complex and cannot be fixed; it needs to be adaptive and flexible9,10. Vaccination with PPV-23 has some limitations, including (1) inability to induce immunological memory at any age—it activates only the T cell-independent antibody responses; (2) being poorly immunogenic in infants; (3) no effect on pneumococcal carriage; (4) decline in the proportion of vaccine-covered serotypes since the introduction of PCVs in children; (5) the unproven efficacy of combination use of PPV-23 and PCV-13; and (6) being costly if all eligible individuals are vaccinated10,11.
Although some evidence support protective efficacy of PPVs against IPDs8,12, their effectiveness in preventing pneumonias, mortality, and other pneumococcal infections are still controversial13. Our knowledge about the efficacy of PPV-23 in reducing the risk of IPDs in immunocompetent older adults comes from a number of case-control and cohort studies14,15,16,17,18,19,20,21,22,23. One reason why the efficacy of the vaccine remains controversial may be the lack of consistency between the results obtained from the observational studies (OSs) and those obtained from controlled trials. Empirical studies have shown that insufficient quality of clinical trials including inadequate allocation concealment or failure to blind patients, caregivers or those assessing outcomes, can lead to biases that may threaten the validity of the results and exaggerate treatment effects24. If multiple evidence from different sources is combined into a single pooled effect estimate, it may improve the assessment of clinical effectiveness25. However, the complexity in the structure of evidence available, in turn, generates a challenge for combining disparate pieces of evidence quantitatively. The Cochrane Collaboration advices to consider randomized controlled trials (RCTs) and OSs separately and not to pool the different study types into a single pooled effect estimate26. However, HTA agencies, such as the National Institute for Health and Care Excellence (NICE) in United Kingdom, do not restrict evidence synthesis to RCTs and often demand the identification of all the relevant sources of evidence27. Unique statistical approaches to combine randomized and non-randomized studies in clinical research are increasingly being published in recent years25. Of these, Bayesian hierarchical models (BHMs) have been the most popular method for combining disparate sources of evidence25.
In the present study, first data from all types of study design were combined into a single random-effects meta-analysis, and then stratified the analysis to explore the source of heterogeneity between the studies. Second, a powerful strategy called fully Bayesian hierarchical modelling in two stages28 was used to incorporate data from various study designs into a single meta-analysis. It contrasts with common BHM where a single step is applied to estimate all parameters simultaneously. Therefore, a full Bayesian analysis was presented at the level of each study and then the results were summarized by the posteriors resulting from Markov Chain Monte Carlo Simulation, MCMC (a powerful method when the study-specific data structures are complex)28. The aim was to obtain a more precise estimation of the efficacy of PPV-23 in protecting against different pneumococcal outcomes, including all-cause pneumonia, pneumococcal pneumonia, death from pneumonia, and bacteremia, septicemia or invasive pneumococcal diseases (IPDs) in the adult population aged 18 years and older, and thus enabling clinicians to draw firmer conclusions.
Results
Selected studies
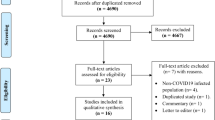
The process of identifying eligible studies is clarified in Fig. 1. In the current study, 128 out of 615 references were considered to be potentially eligible. All the full texts of 128 publications were assessed. Among them, 107 were excluded from the final analysis; 2 described the use of vaccine as only a booster, 9 included simultaneous injection of PPV and PCV vaccines, 11 included simultaneous injection of PPV and influenza vaccines, and 85 only examined the antibody response. Finally, 21 studies were included in the meta-analysis involving 826109 adult participants, of which 6 (28.6%) were RCTs, 10 (47.6%) were cohort studies and 5 (23.8%) were case-control studies (Supplementary Tables S1, S2, and S3).
Among the six RCTs, five (83%) were described as double-blind, but placebo was used as a control. One (17%) was described as open and no intervention was used as a control. In this group, the follow-up time ranged from 1 to 6.7 years (mean 4.1 years); one trial (17%) did not provide information on the duration of follow-up. Two trials (33.3%) were performed in Uganda—a low income country—, one (16.7%) in Spain, one (16.7%) in Japan, one (16.7%) in Sweden and one (16.7%) in America. The study characteristics are shown in Supplementary Table S1.
In the cohort group, the follow-up time ranged from 3 to 10 years (mean 6 years); one study (10%) did not provide information on the duration of the follow-up. All studies in this group were performed in high income countries; six (60%) in Spain, two (20%) in Canada, one (10%) in Sweden, and one (10%) in China, Taiwan (Province of China). Supplementary Table S2 determines the features of cohort studies which were included in the meta- analyses.
In the case-control group, two studies (40%) were performed in the US, two (40%) in Spain, and one (20%) in Brazil. Supplementary Table S3 shows the characteristics of case-control studies that were included in the meta- analyses.
Meta-analyses
Depending on the outcomes, 3 to 6 RCTs and 3 to 13 cohort/case-control studies were included in the meta-analyses. Each analysis included 2293 to 156812 precipitants for RCTs, and 173532 to 655106 precipitants for cohort/case-control studies.
The effect of the PPV-23 vaccine on the relative risks of clinical outcomes according to the random-effects meta-analysis of combined study designs (RCTs and observational studies)
The pooled RR (95% CI) for all-cause pneumonia was 0.870 (0.692–1.092; P = 0.230). The quantity I2 of 74.335% (P = 0) was obtained, indicating that the degree of variability between studies was inconsistent with what would be expected to occur by chance alone. No evidence of publication bias was found; the P-value for Egger’s test was 0.972 (Table 1).
The pooled RR (95% CI) for pneumococcal pneumonia was 0.952 (0.687–1.319; P = 0.767). The Cochran’s Q test had a P-value of 0.006, which corresponded to the quantity I2 of 60.896%, indicating an evidence of heterogeneity between studies. No evidence of publication bias was found (Egger’s P = 0.869; Table 1). After the removal of studies conducted by Christenson et al., Lopez-Palomo et al., Siemieniuk et al., and Alfageme et al.29,30,31,32 with the largest variances (Fig. 2), the RR altered from 0.952 to 0.968 (95% CI; 0.727–1.287; P = 0.821), indicating the high stability of the results. However, there was still strong evidence of heterogeneity (Cochran’s Q test P = 0.035, I2 = 58.372%) but no publication bias was found (Egger’s P = 0.952).
Summary plots of the random-effects meta-analyses of all studies (RCTs and observational studies) of the 23-valent pneumococcal polysaccharide vaccine for four clinical outcomes. The relative risk (squares, proportional to weights used in meta-analysis), with the summary measure and associated confidence intervals were determined for each defined group. (A) all-cause pneumonia, (B) pneumococcal pneumonia, (C) death from pneumonia, and (D); invasive pneumococcal diseases. Values less than 1 indicate a decreased risk of the outcomes, while values greater than 1 indicate an increased risk of the outcomes.
The pooled RR (95% CI) for death from pneumonia was 0.538 (0.162–1.790; P = 0.312). The Cochran’s Q test had a P-value of 0, which corresponded to the quantity I2 of 99.185%, indicating strong evidence of heterogeneity between studies. No evidence of publication bias was found; the P-value for Egger’s test was 0.564 (Table 1). When the two studies of Imaz et al. and Ortqvist et al.33,34 with the largest variances (Fig. 2) were excluded, the results did not change substantially (RR = 0.567, 95% CI; 0.151–2.135, P = 0.402). However, there was still strong evidence of heterogeneity (Cochran’s Q test P = 0, I2 = 99.389%) but no publication bias was found (Egger’s P = 0.581).
The pooled RR (95% CI) in a random-effects model for bacteremia, septicemia or invasive pneumococcal diseases was 0.738 (0.595–0.916; P = 0.006). However, there was strong evidence of both the heterogeneity (Cochran’s Q test P = 0, I2 = 70.182%) and publication bias (Egger’s P = 0.030; Table 1), which might inflate summary relative risk estimates. When the four studies by Christenson et al., Lopez-Palomo et al., Ortqvist et al., and Maruyama et al.30,31,34,35 with the largest variances (Fig. 2) were excluded, the results did not change substantially (RR = 0.762, 95% CI; 0.615–0.944, P = 0.013). Significant heterogeneity (Cochran’s Q test P = 0, I2 = 74.426%) and publication bias (Egger’s P = 0.061) were still detectable across studies. The RRs varied between 0.697 (95% CI; 0.553–0.879, P = 0.002) and 0.804 (95% CI; 0.664–0.973, P = 0.025), by a leave-one-out sensitivity analysis (Table 2). The estimated effect size was statistically significant in all of the analyses in the presence of significant heterogeneity and publication bias. The I2 ranged from 60.446% to 72.018%. Only after the removal of Imaz et al.’s study, the test for bias was no longer statistically significant (Egger’s P = 0.106). Although there was still strong evidence of heterogeneity (Cochran’s Q test P = 0, I2 = 62.557%), the RR altered from 0.738 to 0.697 (95% CI; 553–879; P = 0.002; Table 2).
The effect of the PPV-23 vaccine on the relative risks of clinical outcomes according to the random-effects meta-analysis of RCTs
Among clinical trials, the pooled RR from a random-effects meta-analysis indicated no significant effect of PPV-23 on any of the clinical outcomes including all-cause pneumonia (RR = 1.009, 95% CI; 0.761–1.340; P = 0.948), pneumococcal pneumonia (RR = 0.837, 95% CI; 0.466–1.502; P = 0.550), death from pneumonia (RR = 0.606, 95% CI; 0.354–1.039; P = 0.068), and bacteremia, septicemia or invasive pneumococcal diseases (RR = 0.969, 95% CI; 0.496–1.891; P = 0.926; Table 3). There was strong evidence of heterogeneity for all-cause pneumonia (Cochran’s Q test P = 0.001, I2 = 75.451%) and pneumococcal pneumonia (Cochran’s Q test P = 0.010, I2 = 70.023%), while it was not statistically significant for death from pneumonia (Cochran’s Q test P = 0.575, I2 = 0%) and bacteremia, septicemia or invasive pneumococcal diseases (Cochran’s Q test P = 0.197, I2 = 35.797%). There was no evidence of publication bias for all the clinical outcomes; the P-values for the Egger’s and Begg’s tests were more than 0.1 (Table 3).
In the analysis for pneumococcal pneumonia, after removing Alfageme et al.’s study29 which had the largest variance, the RR altered from 0.837 to 0.908 (95% CI; 0.680–1.214; P = 0.515), indicating the high stability of the results. However, there was still strong evidence of heterogeneity between studies (Cochran’s Q test P = 0.012, I2 = 72.559%). When the two studies by Ortqvist et al., and Maruyama et al.34,35 with the largest variances were excluded from the group of IPDs, the result was still not statistically significant (P = 0.381)
The effect of the PPV-23 vaccine on the relative risks of clinical outcomes according to the random-effects meta-analysis of the cohort/case-control studies
Among the cohort/case-control studies that compared the 23-valent pneumococcal vaccinated with unvaccinated events, the pooled RR from a random-effects meta-analysis indicated a significant effect of PPV-23 on all-cause pneumonia (RR = 0.598, 95% CI; 0.386–0.927; P = 0.022), with no evidence of heterogeneity between studies (Cochran’s Q test P > 0.1, I2 = 54.397%). The P-value for the Begg’s test was 0.296, while Egger’s test had a P-value of 0.033, suggesting a possible bias (Table 4). After removing Ochoa-Gondar et al.’s study36 using a leave-one-out sensitivity analysis, the RR altered from 0.598 to 0.445 (95% CI; 0.270–0.733, P = 0.001).
PPV-23 showed no significant effect on pneumococcal pneumonia (RR = 1.074; 95% CI; 0.636–1.815; P = 0.789; Table 4). There was an evidence of heterogeneity (Cochran’s Q test P = 0.053, I2 = 57.295%) but no evidence of publication bias was found (Egger’s P = 0.690). Even after removing Christenson et al.’s study30 which had the largest variance, the estimated effect size failed to reach statistical significance (RR = 0.967, 95% CI; 0.872–1.073; P = 0.532; Cochran’s Q test P = 0.025, I2 = 67.907%).
No significant effect of PPV-23 was found on death from pneumonia (RR = 0.474, 95% CI; 0.104–2.152; P = 0.333; Table 4). There was strong evidence of heterogeneity (Cochran’s Q test P = 0, I2 = 99.490%) but no evidence of publication bias was found (Egger’s P = 0.589). Even after removing Imaz et al.’s study33 which had the largest variance, the estimated effect size failed to reach statistical significance (RR = 0.523, 95% CI; 0.105–2.614; P = 0.430; Cochran’s Q test P = 0, I2 = 99.592%).
The pooled RR also showed a significant effect of PPV-23 on bacteremia, septicemia or invasive pneumococcal diseases (RR = 0.702, 95% CI; 0.555–0.887; P = 0.003), although evidence of pronounced heterogeneity was found between studies (Cochran’s Q test P = 0, I2 = 75.072%; Table 4). The P-value for the Begg’s test was 0.669, while the Egger’s test had a P-value of 0.028, suggesting a possible bias. When two studies by Christenson et al.30 and López-Palomo et al.31 with the largest variances (wide intervals) were excluded, the results did not change substantially (RR = 0.709, 95% CI; 0.562–0.896, P = 0.004), indicating the stability of the results. However, there was still a strong evidence of heterogeneity between studies (Cochran’s Q test P = 0, I2 = 77.624%). Moreover, the P-value for the Begg’s test was 0.275, while it was 0.015 for the Egger’s test, suggesting a possible bias. The RRs varied between 0.652 (95% CI; 0.507–0.838, P = 0.001) and 0.774 (95% CI; 0.631–0.951, P = 0.015), by a leave-one-out sensitivity analysis (Supplementary Table S4). Then, we conducted a stratified analysis by the study design. In group A, which included 5 case-control studies, the odds ratio (OR) of 0.620 (95% CI; 0.413–0.932; P = 0.021) was obtained. There was still evidence of heterogeneity among studies (Cochran’s Q test P = 0.044, I2 = 59.164%), but no evidence of publication bias (Begg’s P = 0.806; Egger’s P = 0.624) was found. In group B, which included 8 cohort studies, the RR was 0.581 (95% CI; 0.356–0.948; P = 0.030). There was evidence of heterogeneity among studies (Cochran’s Q test P = 0.006, I2 = 64.986%). The test for publication bias was no longer statistically significant (Begg’s P = 0.710; Egger’s P = 0.488).
Estimation of overall log odds ratios of the clinical outcomes by combining different study types in a two-stage Bayesian hierarchical meta-analysis
The overall results were obtained as the median and 2.5th and 97.5th percentiles of the posterior distribution of the parameter mu. We did a random-effects meta-analysis where mu was the mean of the distribution of study treatment effects (the tau_i), which was assumed normally distributed. Estimates were expressed as posterior medians and 95% credible intervals for logarithm of odds ratio. No significant protective effect of the PPV-23 vaccine was found on all-cause pneumonia, where the estimated overall log OR (95% credible interval) was −0.0002 (−0.0241, 0.0142). The vaccination had a negative effect in the Ortqvist et al.’s study. In the other studies, the vaccination did not have any effect on the outcome (Table 5).
The overall log OR (95% credible interval) for pneumococcal pneumonia was −0.0002 (−0.0110, 0.0122), indicating no significant effect of the vaccination on the outcome. The vaccination had a significant effect in Maruyama et al.’s study. In the other studies, the vaccination did not have any effect on the outcome (Table 5).
No significant protective effect of the PPV-23 vaccine was found for death from pneumonia, where the estimated overall log OR (95% credible interval) was −6.3912 × 10−5 (−0.0219, 0.0131). The vaccination had a negative effect in the Johnstone et al.’s study. In the other studies, the vaccination did not have any effect on the outcome (Table 5).
The overall log OR (95% credible interval) on invasive pneumococcal diseases was −0.1048 (−0.3920, −0.0250), indicating a significant protective effect of the vaccination against IPDs. This effect was seen in studies by Breiman et al., Amelia S. M. Veras et al., Vila-Corcoles et al., Tsai et al., Mykietiuk et al., and Maruyama et al. In the other studies, the vaccination did not have any effect on the outcome (Table 5).
Discussion
There is a serious debate concerning the efficacy of the PPV-23 vaccine on clinical outcomes in adults. In this regard, a detailed meta-analysis was conducted including 826109 adult participants distributed into 21 studies with different types of study designs. Each study was involved according to the predefined eligibility criteria. In order to avoid manipulation of these criteria, we did not straightforwardly exclude outliers based on a statistical test of homogeneity. The analyses of either the combined study designs (RCTs and OSs) or observational studies showed that PPV-23 significantly reduced the incidence of IPDs, but not pneumococcal pneumonia and death from pneumonia. The RR ranged from 0.581 to 0.738. The only meta-analysis of cohort studies showed a significant protective effect of PPV-23 against all-cause pneumonia (RR = 0.598). No significant effect of PPV-23 on any of the clinical outcomes was found in the RCTs. When the analysis was restricted to high income countries, for all-cause pneumonia and pneumococcal pneumonia, although the RRs altered, the difference failed to reach significance (RR = 0.852, 95% CI; 0.644–1.128 for ACP and RR = 0.540, 95% CI; 0.178–1.641 for PP). What was remarkable in most analyses, whether all studies were combined or stratified by study designs, was the high levels of heterogeneity, ranged from 57.295% to 99.490%, that might undermine the credibility of the results. Although I2 does not depend on the number of studies or the effect measure used37, it depends on the size of the individual studies in the meta-analysis38. Given the large sample size of the individual studies (i.e., the within-study variation is small) I2 could then be large (like >75%), even if the between-study variation is small. This was therefore a problem particularly when the data of cohort studies were being analysed (having typically large sample sizes). Though any amount of heterogeneity was acceptable as the predefined eligibility criteria were sound and met39,40, we used a random-effects model, assuming a particular form to heterogeneity (often, but not necessarily, a normal distribution), to overcome it. In addition, controlling the outliers on the forest plot and performing sensitivity analyses41 which indicated the stability of the pooled RR estimates and a close correlation between I2 and τ2 (among-study variance)—that means excluding studies to reduce I2 resulted in reducing τ2 39,40—reaffirmed the accuracy and power of the results. The next problem was dealing with the positive publication bias, which is often tricky42,43. The analysis of the PPV-23 vaccine effectiveness on all-cause pneumonia among cohort studies revealed potential sources of bias (P < 0.1 for Egger’s test). It can be misleading if we ignore between-study heterogeneity when assessing publication bias43. As the meta-analysis was not large (i.e., the number of studies was small (<10) for all-cause pneumonia), methods to test or adjust for publication bias in the presence of heterogeneity might not be powerful. In contrast, it might be a serious threat to the validity of meta-analysis for IPDs (P = 0.028 for Egger’s test) as the analysis was done on 13 eligible cohort/case-control studies. However, after stratification by the study design, the potential bias was no longer statistically significant (Egger’s P = 0.624 and 0.488 for case-control and cohort studies, respectively) and was effectively ruled out, further strengthening the validity and robustness of the results.
Supplementary Tablesncies between the meta-analyses of RCTs and observational studies, largely influenced by the study design. The meta-analyses of RCTs failed to identify a significant overall protective effect of PPV-23 on clinical outcomes, whereas the others (observational studies) indicated a protective effect of PPV-23 against all-cause pneumonia and IPDs. Both RCT and observational study estimations of the PPV-23 vaccine effectiveness are significantly affected by the quality of the study designs and both classes of the study have strengths and weaknesses44. Residual confounding, which is a fundamental criticism of observational studies, is always a potential source of discrepancy between RCT and observational studies. Although randomization removes the chances of confounding, there are other biases inherent to RCTs, and the external validity of RCTs may be also limited45. In the present meta-analysis, however, the 5 out of 6 RCTs were described as double–blinding. This might be the most acceptable method to minimize biases caused by the placebo effect. However, it could not always provide us with the unbiased estimations of the effect. High quality scores (3–5 points on the Jadad scale) were assigned for all the included RCTs (Supplementary Table S5). Nine cohort studies had high quality scores (7 or 8 points) on the 9-star NOS, and one had a low score (6 points) (Supplementary Table S6). High quality scores (8 or 9 points on the 9-star NOS) were assigned for all the included case-control studies (Supplementary Table S7).
Most of the previous studies did not evaluate PPV-23 independently; they considered all valencies and were inconsistent due to several methodological flaws8,46,47. We not only evaluated PPV-23 independently, but also excluded studies which included simultaneous injection of PPV and PCV or PPV and IVs, and those in which the other valencies of the PPV vaccine or any other specific vaccine (i.e., PCV or IVs) were used as a control. Otherwise, it could influence the net impact of the 23-valent PPV vaccine on different outcomes and the assumption of the synergistic and additive effects of two vaccines on the outcomes could not be ruled out. However, Diao et al.’s study48 included different interventions in a final meta-analysis of RCTs, which caused the real effectiveness of the 23-valent PPV vaccine to be controversial.
The potential adverse effects of serotype replacement are still a big challenge when implementing routine childhood PCV programs. In particular, where the lost vaccine serotype carriage is almost consistently replaced by non-vaccine serotype carriage, the net effectiveness of a vaccine is less than expected, in general. Moreover, the degree of replacement in disease, which is resulted from replacement in carriage, depends on the invasive potential of the serotypes involved49,50. In the present study, based on the pre-defined exclusion criteria, a limited number of included studies were conducted following childhood PCV vaccination programs. When these studies were excluded, the results did not change substantially (data are not shown), which indicated the stability of the results. In contrast, most of the studies included in Kraicer-Melamed et al.’s meta-analysis of PPV-23 vaccine effectiveness on CAP and IPDs were conducted following PCV-7- or PCV-13-based childhood programs that have decreased the prevalence of PCV-7- or PCV-13-type pneumococcal serotypes in adults, respectively51.
By the use of two-stage Bayesian hierarchical meta-analysis, which was directly used for combining studies of different designs, the overall log OR (95% credible interval) for IPDs was −0.1048 (−0.3920, −0.0250), indicating a significant protective effect of the vaccination against IPDs. No significant effect of PPV-23 was found on all-cause pneumonia, pneumococcal pneumonia, and death from pneumonia, which confirmed the results obtained by a traditional method. The estimated overall log OR values (95% credible interval) were −0.0002 (−0.0241, 0.0142), −0.0002 (−0.0110, 0.0122), and −6.3912 × 10−5 (−0.0219, 0.0131), respectively. As mentioned by Lunn et al.28, two limitations exist in the two-stage Bayesian hierarchical modelling employed in this article. One is the assumption of marginal independence between studies in stage 1 and another is dealing with sparse data. It is worthwhile to note that both of these limitations did not occur here since our Metropolis sampler was non-degenerate in stage 1 and the data was not sparse.
In conclusion, the PPV-23 vaccine might be effective in preventing the most severe invasive forms of pneumococcal diseases, but not effective in preventing other clinical outcomes, in the adult population of 18 years and older. It was validated by a fully Bayesian hierarchical modelling in two stages, which provides a high-performance approach to more complex evidence syntheses, such as multi-parameter evidence synthesis or mixed treatment comparisons. Although the meta-analysis of cohort studies, but not RCTs, indicated a significant protective effect against all-cause pneumonia, the number was not large and thus the results should be interpreted with caution. The high-quality large RCTs are required to more confidently validate the efficacy of the PPV-23 vaccine in protecting against all-cause pneumonia.
Determining the most common circulating pneumococcal serotypes in the community, designing more RCT and analytical studies to evaluate the effectiveness of pneumococcal vaccines, and finally evaluating the effectiveness of PCVs administration along with PPV23 can help to effectively measure the protective effect of PPV23. Regarding the cost benefits of vaccination against IPD compared to the antibiotic therapy strategies, and due to the increasing rate of IPD worldwide, the vaccination of the elderly is recommended.
Methods
Data sources
We searched MEDLINE, EMBASE, LILACS (Latin American and Caribbean Health Sciences Literature), AIM (African Index Medicus), and IndMed (Indian Medlars Centre) databases by using the keywords “23-valent pneumococcal vaccine”, “PPSV-23”, or “PPV-23” in combination with “outcomes”, “clinical outcomes”, or “clinical effectiveness”. These keywords were combined with terms describing the study design; “randomized-controlled trial”, “controlled clinical trial”, “clinical trial”, “cohort study”, and “case-control study”. We attempted to include all randomized comparisons of PPV-23 with a placebo in any type of population.
Inclusion and exclusion criteria
We included RCTs that compared the PPV-23 vaccine with a placebo or no intervention, and cohort studies and case-control studies that compared outcomes between pneumococcal polysaccharide vaccinated and unvaccinated groups. Only English language articles were applied in this study. We excluded uncontrolled and observational intervention studies; animal and laboratory studies; studies examining antibody responses only; studies in which the PPV-23 vaccine was used as a booster after vaccination with conjugate pneumococcal vaccine; studies in which the other valencies of the PPV vaccine or any other specific vaccine (i.e., PCV or influenza vaccines & IVs) were used as a control; studies that included simultaneous injection of PPV and PCV vaccines, and those including the simultaneous injection of PPV and IVs; studies performed in child populations; the results coming from abstracts only; and review articles. Data from each potentially relevant article were extracted independently by two investigators (H.L.-N. and S.L.-N.) and discussed to solve any disagreement. Moreover, the information on the methodologic quality of the studies was extracted. The observed interrater agreement was measured using the kappa statistic. Finally, 21 original papers were left for analyses, which were divided into three groups according to their study design: RCTs29,34,35,52,53,54, cohort studies20,30,31,32,36,55,56,57,58,59, and case-control studies14,15,33,60,61 (Supplementary Tables S1, S2 and S3; Fig. 1). More detailed information about how pneumonia is diagnosed (for example by clinical features such as cough, fever, and pleuritic chest pain, or by chest X-ray or another method) and how pneumococcal pneumonia is confirmed (for example by cultivation or PCR-based methods on different clinical samples such as bronchoalveolar lavage, naso-pharyngeal-throat swabs and nasal swabs) is summarized in Supplementary Table S8. Quality assessment of RCTs and observational studies was determined on the basis of Jadad62 and Newcastle-Ottawa (NOS)63 quality assessment scales, respectively.
Meta-analysis Outcomes
The following four outcomes were considered: (a) all-cause pneumonia; (b) pneumococcal pneumonia; (c) death from pneumonia; and (d) bacteremia, septicemia or invasive pneumococcal diseases (defined as isolation of S. pneumoniae from sterile body fluids).
Classical meta-analysis method
Combined relative risk (RR) and 95% confidence intervals (CIs) were estimated for each study. The significance of pooled RR was tested by Z-test, and a P-value less than 0.05 was considered as statistically significant. The Cochran’s Q statistic was used to test heterogeneity among the included studies. The DerSimonian and Laird random-effects64 model was used to conduct the meta-analyses. Besides, we examined the I2 (I-squared) statistic describing the amount of variations due to true differences (heterogeneity) rather than random errors. The I2 values of 25%, 50%, and 75% corresponded to the low, moderate, and high levels of heterogeneity40, respectively. To explore the source of heterogeneity, we performed subgroup analyses according to the types of study design. Furthermore, the studies which had both the largest variance (wide intervals) and the extreme outlier weight in each clinical outcome group were identified. Then, a leave-one-out sensitivity analysis was conducted to assess the impact of individual studies, and thus the average RR was estimated in the absence of each study and heterogeneity was quantified using both the I2 and τ2 statistics40. The Egger’s regression asymmetry test and the Begg-Mazumdar adjusted rank correlation test were used to statistically assess the potential publication bias65,66. P-values less than 0.10 indicated the presence of bias. All the analyses were performed using a Comprehensive Meta-Analysis software (version 2, Biostat, Englewood, NJ), followed by PRISMA guidelines67, and 2-tailed P-values were calculated in all the tests.
Specifications for a two-stage Bayesian hierarchical model
Let \({y}_{{C}_{i}}\) and \({y}_{{T}_{i}}\) denote the number of control and treatment groups of study \(i,\) respectively. In addition, let \({\pi }_{{C}_{i}}\) and \({\pi }_{{T}_{i}}\) represent the corresponding underlying probabilities (the probability of observing the disease again) of these groups. The total number of individuals in these groups are \({n}_{{C}_{i}}\) and \({n}_{{T}_{i}}\). We have
Consider the model
where \({\xi }_{i}s\) are nuisance parameters and \({\theta }_{i}\) is the treatment effect for study \(i\). Note that \({\theta }_{i}\) is equal to logarithm of the odds ratio for treatment compared with control. So in our cases, the negative values of \({\theta }_{i}\) represent the positive effect of the vaccination while the zero values indicate no effect.
We used the two-stage Bayesian hierarchical modeling proposed in Lunn et al.28. In the first stage, it is assumed that \({\xi }_{i}\,\)s are independent from \(Normal(0,{100}^{2})\) and \({\theta }_{i}\,\)s are independently distributed as \(Normal(0,{100}^{2})\). Also, in the second stage the underlying distributions are
Based on the above distributions for \({\boldsymbol{\theta }}=({\theta }_{1},\ldots ,{\theta }_{N})\), the conditional distribution of \(\mu \) given \(p({\sigma }^{2}|\mu ,{\boldsymbol{\theta }},y)\)\(({\sigma }^{2},{\boldsymbol{\theta }},y)\), that is \(p(\mu |{\sigma }^{2},{\boldsymbol{\theta }},y)\), and the conditional distribution of \(\sigma \) given \((\mu ,{\boldsymbol{\theta }},y)\), that is, are obtained as follows
This implies that the conditional distribution of \(\mu \) given \(({\sigma }^{2},{\boldsymbol{\theta }},y)\) is normal with mean \(\frac{{\sum }_{i=1}^{N}{\theta }_{i}}{{10}^{-4}+N{\sigma }^{2}}\) and variance \({({10}^{-4}+N{\sigma }^{2})}^{-1}\). Also
Therefore the conditional distribution of \({\sigma }^{2}\) given (\(\mu ,{\boldsymbol{\theta }},y)\) is a truncated gamma distribution on the interval \((0,100)\).
References
Ortqvist, A., Hedlund, J. & Kalin, M. Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med 26, 563–574, https://doi.org/10.1055/s-2005-925523 (2005).
Prato, R., Tafuri, S., Fortunato, F. & Martinelli, D. Why it is still important that countries know the burden of pneumococcal disease. Hum Vaccin 6, 918–921 (2010).
Atkinson, W., Hamborsky, J. & McIntyre, L. (Washington, DC: Public Health Foundation, 2007).
Lipsky, B. A. & Hirschmann, J. V. Pneumococcal polysaccharide vaccines do not protect the elderly from pneumococcal infections. Neth J Med 62, 33–35 (2004).
Pitsiou, G. G. & Kioumis, I. P. Pneumococcal vaccination in adults: does it really work? Respir Med 105, 1776–1783, https://doi.org/10.1016/j.rmed.2011.07.008 (2011).
Rohani, M. Y. et al. Epidemiology of Streptococcus pneumoniae infection in Malaysia. Epidemiol Infect 122, 77–82 (1999).
Kyaw, M. H., Clarke, S., Edwards, G. F., Jones, I. G. & Campbell, H. Serotypes/groups distribution and antimicrobial resistance of invasive pneumococcal isolates: implications for vaccine strategies. Epidemiol Infect 125, 561–572 (2000).
Huss, A., Scott, P., Stuck, A. E., Trotter, C. & Egger, M. Efficacy of pneumococcal vaccination in adults: a meta-analysis. CMAJ 180, 48–58, https://doi.org/10.1503/cmaj.080734 (2009).
Carey, G., Crammond, B., Malbon, E. & Carey, N. Adaptive policies for reducing inequalities in the social determinants of health. Int J Health Policy Manag 4, 763–767, https://doi.org/10.15171/IJHPM.2015.170 (2015).
Suzuki, M., Dhoubhadel, B. G., Katoh, S., Ariyoshi, K. & Morimoto, K. 23-valent pneumococcal polysaccharide vaccine against pneumococcal pneumonia. The Lancet Infectious Diseases 17, 803–804, https://doi.org/10.1016/S1473-3099(17)30411-5 (2017).
Principi, N. & Esposito, S. Development of pneumococcal vaccines over the last 10 years. Expert Opin Biol Ther 18, 7–17, https://doi.org/10.1080/14712598.2018.1384462 (2018).
Jackson, L. A. & Janoff, E. N. Pneumococcal vaccination of elderly adults: new paradigms for protection. Clin Infect Dis 47, 1328–1338, https://doi.org/10.1086/592691 (2008).
Moberley, S., Holden, J., Tatham, D. P. & Andrews, R. M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 1, CD000422, https://doi.org/10.1002/14651858.CD000422.pub3 (2013).
Vila-Corcoles, A. et al. Effectiveness of the 23-valent polysaccharide pneumococcal vaccine against invasive pneumococcal disease in people 60 years or older. BMC Infect Dis 10, 73, https://doi.org/10.1186/1471-2334-10-73 (2010).
Benin, A. L. et al. Effectiveness of the 23-valent polysaccharide vaccine against invasive pneumococcal disease in Navajo adults. J Infect Dis 188, 81–89, https://doi.org/10.1086/375782 (2003).
Shapiro, E. D. et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. N Engl J Med 325, 1453–1460, https://doi.org/10.1056/NEJM199111213252101 (1991).
Sims, R. V. et al. The clinical effectiveness of pneumococcal vaccine in the elderly. Ann Intern Med 108, 653–657 (1988).
Farr, B. M. et al. Preventing pneumococcal bacteremia in patients at risk. Results of a matched case-control study. Arch Intern Med 155, 2336–2340 (1995).
Dominguez, A. et al. Effectiveness of pneumococcal vaccination for elderly people in Catalonia, Spain: a case-control study. Clin Infect Dis 40, 1250–1257, https://doi.org/10.1086/429236 (2005).
Vila-Corcoles, A. et al. Protective effects of the 23-valent pneumococcal polysaccharide vaccine in the elderly population: the EVAN-65 study. Clin Infect Dis 43, 860–868, https://doi.org/10.1086/507340 (2006).
Singleton, R. J. et al. Invasive pneumococcal disease epidemiology and effectiveness of 23-valent pneumococcal polysaccharide vaccine in Alaska native adults. Vaccine 25, 2288–2295, https://doi.org/10.1016/j.vaccine.2006.11.065 (2007).
Mooney, J. D. et al. The impact and effectiveness of pneumococcal vaccination in Scotland for those aged 65 and over during winter 2003/2004. BMC Infect Dis 8, 53, https://doi.org/10.1186/1471-2334-8-53 (2008).
Centers for Disease, C., Prevention & Advisory Committee on Immunization, P. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep 59, 1102–1106 (2010).
Juni, P., Altman, D. G. & Egger, M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 323, 42–46 (2001).
Verde, P. E. & Ohmann, C. Combining randomized and non-randomized evidence in clinical research: a review of methods and applications. Res Synth Methods 6, 45–62, https://doi.org/10.1002/jrsm.1122 (2015).
Higgins, J. & Green, S. Cochrane handbook for systematic reviews of interventions version 5.1.0 [Online]. (Available: www.cochrane-handbook.org, accessed on 21 January 2015). (2011).
National Institute for Health and Care Excellence (Nice). Guide to the methods of technology appraisal 2013. National Institute for Health and Care Excellence (NICE). (2013).
Lunn, D., Barrett, J., Sweeting, M. & Thompson, S. Fully Bayesian hierarchical modelling in two stages, with application to meta-analysis. J R Stat Soc Ser C Appl Stat 62, 551–572, https://doi.org/10.1111/rssc.12007 (2013).
Alfageme, I. et al. Clinical efficacy of anti-pneumococcal vaccination in patients with COPD. Thorax 61, 189–195, https://doi.org/10.1136/thx.2005.043323 (2006).
Christenson, B., Lundbergh, P., Hedlund, J. & Ortqvist, A. Effects of a large-scale intervention with influenza and 23-valent pneumococcal vaccines in adults aged 65 years or older: a prospective study. Lancet 357, 1008–1011, https://doi.org/10.1016/S0140-6736(00)04237-9 (2001).
Lopez-Palomo, C. et al. Pneumonia in HIV-infected patients in the HAART era: incidence, risk, and impact of the pneumococcal vaccination. J Med Virol 72, 517–524, https://doi.org/10.1002/jmv.20045 (2004).
Siemieniuk, R. A., Gregson, D. B. & Gill, M. J. The persisting burden of invasive pneumococcal disease in HIV patients: an observational cohort study. BMC Infect Dis 11, 314, https://doi.org/10.1186/1471-2334-11-314 (2011).
Imaz, A. et al. Impact of prior pneumococcal vaccination on clinical outcomes in HIV-infected adult patients hospitalized with invasive pneumococcal disease. HIV Med 10, 356–363, https://doi.org/10.1111/j.1468-1293.2009.00695.x (2009).
Ortqvist, A. et al. Randomised trial of 23-valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle-aged and elderly people. Swedish Pneumococcal Vaccination Study Group. Lancet 351, 399–403 (1998).
Maruyama, T. et al. Efficacy of 23-valent pneumococcal vaccine in preventing pneumonia and improving survival in nursing home residents: double blind, randomised and placebo controlled trial. BMJ 340, c1004, https://doi.org/10.1136/bmj.c1004 (2010).
Ochoa-Gondar, O. et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against community-acquired pneumonia in the general population aged >/=60 years: 3 years of follow-up in the CAPAMIS study. Clin Infect Dis 58, 909–917, https://doi.org/10.1093/cid/ciu002 (2014).
Higgins, J. P., Thompson, S. G., Deeks, J. J. & Altman, D. G. Measuring inconsistency in meta-analyses. BMJ 327, 557–560, https://doi.org/10.1136/bmj.327.7414.557 (2003).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558, https://doi.org/10.1002/sim.1186 (2002).
Coory, M. D. Comment on: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 39 932 author reply 933 https://doi.org/10.1093/ije/dyp157 (2010).
Higgins, J. P. Commentary: Heterogeneity in meta-analysis should be expected and appropriately quantified. Int J Epidemiol 37, 1158–1160, https://doi.org/10.1093/ije/dyn204 (2008).
Patsopoulos, N. A., Evangelou, E. & Ioannidis, J. P. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol 37, 1148–1157, https://doi.org/10.1093/ije/dyn065 (2008).
Dwan, K., Gamble, C., Williamson, P. R. & Kirkham, J. J. & Reporting Bias, G. Systematic review of the empirical evidence of study publication bias and outcome reporting bias - an updated review. PLoS One 8, e66844, https://doi.org/10.1371/journal.pone.0066844 (2013).
Peters, J. et al. Assessing publication bias in meta-analyses in the presence of between-study heterogeneity. Journal of the Royal Statistical Society. Series A (Statistics in Society) 173, 575–591 (2010).
Benson, K. & Hartz, A. J. A comparison of observational studies and randomized, controlled trials. N Engl J Med 342, 1878–1886, https://doi.org/10.1056/NEJM200006223422506 (2000).
Booth, C. M. & Tannock, I. F. Randomised controlled trials and population-based observational research: partners in the evolution of medical evidence. Br J Cancer 110, 551–555, https://doi.org/10.1038/bjc.2013.725 (2014).
Fedson, D. S. Preventing non bacteremic pneumococcal pneumonia in older adults: historical background and considerations for choosing between PCV13 and PPV23. Hum Vaccin Immunother 10, 1322–1330, https://doi.org/10.4161/hv.28797 (2014).
Moberley, S. A., Holden, J., Tatham, D. P. & Andrews, R. M. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev CD000422, https://doi.org/10.1002/14651858.CD000422.pub2 (2008).
Diao, W. Q., Shen, N., Yu, P. X., Liu, B. B. & He, B. Efficacy of 23-valent pneumococcal polysaccharide vaccine in preventing community-acquired pneumonia among immunocompetent adults: A systematic review and meta-analysis of randomized trials. Vaccine 34, 1496–1503, https://doi.org/10.1016/j.vaccine.2016.02.023 (2016).
Flasche, S. et al. Effect of pneumococcal conjugate vaccination on serotype-specific carriage and invasive disease in England: a cross-sectional study. PLoS Med 8, e1001017, https://doi.org/10.1371/journal.pmed.1001017 (2011).
Hanage, W. P. et al. Evidence that pneumococcal serotype replacement in Massachusetts following conjugate vaccination is now complete. Epidemics 2, 80–84, https://doi.org/10.1016/j.epidem.2010.03.005 (2010).
Kraicer-Melamed, H., O’Donnell, S. & Quach, C. The effectiveness of pneumococcal polysaccharide vaccine 23 (PPV23) in the general population of 50 years of age and older: A systematic review and meta-analysis. Vaccine 34, 1540–1550, https://doi.org/10.1016/j.vaccine.2016.02.024 (2016).
French, N. et al. 23-valent pneumococcal polysaccharide vaccine in HIV-1-infected Ugandan adults: double-blind, randomised and placebo controlled trial. Lancet 355, 2106–2111 (2000).
Watera, C. et al. 23-Valent pneumococcal polysaccharide vaccine in HIV-infected Ugandan adults: 6-year follow-up of a clinical trial cohort. AIDS 18, 1210–1213 (2004).
Russell, K. L. et al. Lack of effectiveness of the 23-valent polysaccharide pneumococcal vaccine in reducing all-cause pneumonias among healthy young military recruits: a randomized, double-blind, placebo-controlled trial. Vaccine 33, 1182–1187, https://doi.org/10.1016/j.vaccine.2014.12.058 (2015).
Vila-Corcoles, A. et al. Protective effect of pneumococcal vaccine against death by pneumonia in elderly subjects. Eur Respir J 26, 1086–1091, https://doi.org/10.1183/09031936.05.00030205 (2005).
Mykietiuk, A. et al. Effect of prior pneumococcal vaccination on clinical outcome of hospitalized adults with community-acquired pneumococcal pneumonia. Eur J Clin Microbiol Infect Dis 25, 457–462, https://doi.org/10.1007/s10096-006-0161-8 (2006).
Johnstone, J., Eurich, D. T., Minhas, J. K., Marrie, T. J. & Majumdar, S. R. Impact of the pneumococcal vaccine on long-term morbidity and mortality of adults at high risk for pneumonia. Clin Infect Dis 51, 15–22, https://doi.org/10.1086/653114 (2010).
Gutierrez Rodriguez, M. A. et al. Effectiveness of 23-valent pneumococcal polysaccharide vaccine in adults aged 60 years and over in the Region of Madrid, Spain, 2008–2011. Euro Surveill 19, 20922 (2014).
Tsai, Y. H. et al. The 23-valent pneumococcal polysaccharide vaccine is effective in elderly adults over 75 years old–Taiwan’s PPV vaccination program. Vaccine 33, 2897–2902, https://doi.org/10.1016/j.vaccine.2015.04.068 (2015).
Breiman, R. F. et al. Evaluation of effectiveness of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Arch Intern Med 160, 2633–2638 (2000).
Veras, M. A., Enanoria, W. T., Castilho, E. A. & Reingold, A. L. Effectiveness of the polysaccharide pneumococcal vaccine among HIV-infected persons in Brazil: a case control study. BMC Infect Dis 7, 119, https://doi.org/10.1186/1471-2334-7-119 (2007).
Jadad, A. R. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 17, 1–12 (1996).
Higgins, J. P. T. & Green, S. Section 13.5.2.3. Tools for assessing methodological quality or risk of bias in non-randomized studies. In: Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. London: The Cochrane Collaboration (2011).
DerSimonian, R. & Laird, N. Meta-analysis in clinical trials. Control Clin Trials 7, 177–188 (1986).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & Group, P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Medicine 6, e1000097 (2009).
Acknowledgements
The authors would like to express their gratitude to Dr. Michael Borenstein (Biostat, Inc, New Jersey, USA), a member of the statistical advisory groups of the Cochrane and Campbell Collaborations for his constructive assistance. We are very thankful for Prof. Simon Thompson, Dr. Robert Goudie, and Dr. Jessica Barrett, Medical Research Council (MRC) Biostatistics Unit, University of Cambridge, UK, for providing us with generic codes for fully Bayesian hierarchical modelling in two stages. This study was supported by the Research Council of the Baqiyatallah University of Medical Sciences. There was no additional external funding received for this study. The supporter had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: A.A. S.L.-N. Performed the experiments: H.L.-N. analyzed the data: H.L.-N. S.L.-N. B.M. S.A.J. Contributed reagents/materials/analysis tools: A.A. S.L.-N. Wrote the paper: A.A. H.L.-N. S.L.-N. Performed meta-analysis: H.L.-N. S.L.-N. B.M. S.A.J.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Latifi-Navid, H., Latifi-Navid, S., Mostafaiy, B. et al. Pneumococcal Disease and the Effectiveness of the PPV23 Vaccine in Adults: A Two-Stage Bayesian Meta-Analysis of Observational and RCT Reports. Sci Rep 8, 11051 (2018). https://doi.org/10.1038/s41598-018-29280-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29280-2
This article is cited by
-
Cost-effectiveness of 20-valent pneumococcal conjugate vaccine compared with 23-valent pneumococcal polysaccharide vaccine among adults in a Norwegian setting
Cost Effectiveness and Resource Allocation (2023)
-
Pharmacological and non-pharmacological management of COPD; limitations and future prospects: a review of current literature
Journal of Public Health (2020)
-
Effectiveness of pneumococcal conjugate vaccination against invasive pneumococcal disease among children with and those without HIV infection: a systematic review and meta-analysis
BMC Infectious Diseases (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.