Abstract
Bacteria can adjust their genetic programs via alternative σ factors to face new environmental pressures. Here, we analyzed a unique set of paralogous alternative σ factors, termed σIs, which fine-tune the regulation of one of the most intricate cellulolytic systems in nature, the bacterial cellulosome, that is involved in degradation of environmental polysaccharides. We combined bioinformatics with experiments to decipher the regulatory networks of five σIs in Clostridium thermocellum, the epitome of cellulolytic microorganisms, and one σI in Pseudobacteroides cellulosolvens which produces the cellulosomal system with the greatest known complexity. Despite high homology between different σIs, our data suggest limited cross-talk among them. Remarkably, the major cross-talk occurs within the main cellulosomal genes which harbor the same σI-dependent promoter elements, suggesting a promoter-based mechanism to guarantee the expression of relevant genes. Our findings provide insights into the mechanisms used by σIs to differentiate among their corresponding regulons, representing a comprehensive overview of the regulation of the cellulosome to date. Finally, we show the advantage of using a heterologous host system for analysis of multiple σIs, since information generated by their analysis in their natural host can be misinterpreted owing to a cascade of interactions among the different σIs.
Similar content being viewed by others
Introduction
Bacteria can sense the extracellular environment and transmit information intracellularly by using different types of signal transduction mechanisms1. After sensing the environment, one type of response is regulation of genes at the level of transcription initiation by alternative sigma (σ) factors allowing bacteria to adjust their transcriptional programs to environmental changes2,3. σ factors are the key component of RNA polymerase, since they provide promoter specificity. All bacteria harbor one primary σ factor (known as the housekeeping σ factor, σ70 or σA) that is responsible for basal expression level of most genes. When the environmental conditions change, the housekeeping σ factor is substituted by the alternative σ factors, thereby redirecting the RNA polymerase to alternative promoters of genes that will help the bacterium deal with the new environmental conditions4.
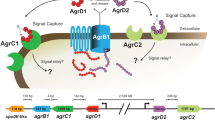
Cellulolytic clostridia are anaerobic bacteria that use plant cell-wall polysaccharides as carbon sources. The structural complexity and composition of their substrate, together with the anoxic conditions of their ecosystem, have generated selective pressures for the evolution of extracellular multi-enzyme nanomachines called cellulosomes for efficient degradation of plant cell-wall polysaccharides5. During the process of cellulosic biomass breakdown, cellulolytic clostridia control the type(s) of enzymatic subunits present in the cellulosome complex to suit the type(s) of polysaccharide(s) that are exposed during the degradation process6,7. In the cellulosome-producing bacteria Clostridium (Ruminiclostridium) thermocellum, the enzymatic composition of the cellulosome is probably regulated by a group of at least 6 paralogous alternative σI factors that are related to the Bacillus subtilis σI factor8,9,10,11,12. However, the regulons of this set of σIs are poorly understood.
The features of the σIs are consistent with almost all of the characteristics of the ECF (extracytoplasmic function) σ factors2. Both σIs and ECF σ factors share the following features. (i) They usually autoregulate their own expression. (ii) They are usually located in an operon with an anti-σ factor gene. In the case of σI factors, the latter gene is termed rsgI (i.e., regulation of sigI) that controls the activity of its cognate σ factor. (iii) The anti-σ factor is composed of three parts: an extracytoplasmic sensory module(s), a transmembrane domain, and an intracellular domain that sequesters the σ factor. (iv) The σ factor is activated by inhibiting the activity of the anti-σ factor.
The main difference between σI and ECF σ factors resides in their protein structure. Alternative σI factors harbor only the σ2 domain of the σ70 family that is involved in the recognition of the −10 promoter element, and the σ4 domain is substituted by a C-terminal domain termed σI-C that is likely involved in the recognition of the −35 promoter element8. Taken together, the above-mentioned characteristics render σI factors unique members of the σ70 family.
Some cellulosome-producing clostridia are characterized by multiple σI factors. The σI factors of C. thermocellum are highly homologous with identities between 36 and 45% among them. This observation raises the question of how a set of highly homologous alternative σI factors can avoid crosstalk among each other. This is of particular interest in a bacterium like C. thermocellum, which contains 8 paralogous σIs, as well as other cellulosome-producing clostridia [notably, Clostridium clariflavum, Clostridium straminosolvens, Clostridium sp. Bc-iso-3, Acetivibrio cellulolyticus, and Bacteroides (Pseudobacteroides) cellulosolvens] which also produce between 8 and 16 highly homologous alternative σI factors that are presumably involved in similar regulatory networks. Moreover, there are reports where a set of ECF σ factors exhibits high crosstalk13,14,15, wherein the latter set shows less conservation compared to that of the C. thermocellum σI factors. For example, the B. subtilis ECF σM, σW and σX factors share identities of only 25 to 32% but present high regulatory overlap14.
It should be noted that there is continued controversy regarding the classification of the cellulosome-producing clostridia16, and, in particular, for those species that produce complex or multiplex cellulosomes, characterized by a multiplicity of scaffoldin genes17,18. In this context, Acetivibrio cellulolyticus and Bacteroides cellulosolvens were clearly misclassified in the original works19,20, and both species were later determined to be members of the greater clostridial assemblage21. These latter discrepancies were not fully resolved by the recent attempt to reclassify of Bacteroides cellulosolvens as Pseudoacteroides cellulosolvens22.
In the present work, we demonstrate how a collection of five alternative σI factors in C. thermocellum, namely σI1, σI2, σI3, σI4 and σI6, regulate the expression of 17 genes encoding different cellulosomal components. This analysis shows for the first time a sophisticated regulatory network of several alternative σ factors, which control the enzymatic composition of the cellulosome. Furthermore, our results show that σI factors from cellulosome-producing bacteria use highly conserved promoter sequences to delimit the genes that are under control of a given σI. Our findings indicate that the −35 promoter element, proposed to be recognized by the novel domain σI-C, is critical for the specificity of each σI factor. This promoter element can be divided into two regions: a highly-conserved homopolymeric A-tract motif in the 3′ region, that we herein propose as a general motif for σI-dependent promoter recognition, and a more divergent region upstream of the A-tract motif that provides specificity to each σI factor. By using this information, we identified the regulons of one σI factor in P. cellulosolvens, a bacterium that produces the most complex cellulosomal system described until now23. Our results provide a better view into the mechanisms used by multiple alternative σIs to differentiate their corresponding regulons. This information is crucial for future efforts to predict regulons of multiple σI factors in cellulolytic clostridia.
Results
Deciphering the regulatory networks of σI factors
In a previous work, we predicted 40 putative σI-dependent promoters in C. thermocellum by bioinformatic analysis10. To overcome the lack of convenient genetic tools to work directly in C. thermocellum24, we analyzed the recognition of the predicted promoters by C. thermocellum σI3 and σI6 in a heterologous B. subtilis host system10. This analysis revealed that the main types of enzymatic genes regulated by C. thermocellum σI3 and σI6 are pectin-degrading enzymes and xylanases, respectively10. In order to confirm the predicted σI-dependent promoters of the spectrum of C. thermocellum σI factors that are proposed to be involved in the regulation of genes coding saccharolytic enzymes (σI1 to σI6)9,10, we herein mapped the transcriptional start sites (TSSs) of the sigI2-rsgI2, sigI3-rsgI3, sigI4-rsgI4 and sigI5-rsgI5 operons by the rapid amplification of cDNA ends (5′-RACE) technique (Supplementary Fig. S1). The TSSs of the sigI1-rsgI1 and sigI6-rsgI6 operons were mapped in a previous work9. During this analysis, however, we failed to identify a TSS of the sigI4-rsgI4 and sigI5-rsgI5 operons using this technique. The σI-dependent promoter sequences associated with the TSSs mapped in the present work are shown in Fig. 1A.
Regulons of C. thermocellum alternative σI factors. (A) Alignment of σI-dependent promoters that were experimentally confirmed. The most conserved nucleotides are highlighted in color. TSSs are indicated by arrowheads and the TSSs identified in the present work (Supplementary Fig. S1) are underlined. The TSS of sigI1, sigI6, cipA, xyn11B and xyn10Z were identified in previous works9,11,43. Distances between the promoter sequences and the first codon of corresponding genes are shown in the column labeled 5′-UTR (5′-untranslated region). GH, glycoside hydrolase; Doc, dockerin; CBM, carbohydrate-binding module; Coh, cohesin; X, X-module (module of unknown function); CotH, spore coat protein H; UNK, unknown sequence; HP, hypothetical protein; Abf, Alpha-L-arabinofuranosidase; Rga, rhamnogalacturan acetylesterase; Rgl, rhamnogalacturonan lyase; SLH, S-layer homology domain; CE, carbohydrate esterase. (B) Recognition of σI-dependent promoters by C. thermocellum σI factors. The respective promoters were fused to a LacZ reporter gene and their recognition by the different C. thermocellum σI factors was tested in a B. subtilis heterologous host, grown in 24-well cell culture plates with Spizizen’s minimal medium and X-gal. The development of blue color indicated the activation of the σI-dependent promoters by a given C. thermocellum σI factor.
Subsequently, in order to decipher the regulatory networks of C. thermocellum σI factors, we tested the recognition of the previously predicted σI-dependent promoters10 with each of the C. thermocellum σI-factors (i.e., σI1 -σ6) in a heterologous B. subtilis host system (Fig. 1B). The members of the promoter library were previously fused to a lacZ reporter gene10. In the present work, the activation of the reporter was tested in 24-well cell culture plates with Spizizen’s minimal medium using 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-gal) as substrate (Fig. 1B). During the analysis, C. thermocellum σI5 did not recognize any of the predicted promoters. As shown in Fig. 1B, with few exceptions, the majority of the σI-dependent promoters are specific for their own σI factor. Interestingly, the major crosstalk between σIs occurs in the two most important cellulosomal genes, cipA and cel48S (Fig. 1B). These genes encode the primary scaffoldin of the cellulosome, CipA11,18,25, and the most abundant enzyme in the cellulosome, Cel48S6,26,27, respectively.
σI-dependent promoters are structured into three motifs
According to the consensus promoter sequence, generated with the σI-dependent promoters that were experimentally confirmed (Fig. 1), we divided the σI-dependent promoters into three regions: (i) a highly conserved CGAA tetrad in the −10 element, (ii) a homopolymeric AAAA tetrad, herein termed the “A-tract motif” at the 3′ end of the −35 element, and (iii) a divergent region upstream of the A-tract motif. The most highly conserved promoter motifs, i.e., the A-tract in the −35 element and the CGAA tetrad in the −10 element, are proposed to be implicated in the “general” recognition of promoters by σIs in cellulosome-producing bacteria (Fig. 1A). We suggest that the least conserved region, upstream of the A-tract motif in the −35 element, is implicated in the specificity of the different σI factors. Hence, we termed this divergent region as “region of specificity” (Fig. 1A). The alignments generated with the promoter sequences of each σI regulon support this observation (Fig. 2). A comparison of the region of specificity shows how each σI factor has different preferences. For example, whereas C. thermocellum σI1 favors promoters with a CTC triad immediately upstream of the A-tract motif, C. thermocellum σI3 prefers a CCC triad two nucleotides upstream of the A-tract motif (Fig. 2).
Dissection of C. thermocellum σI-dependent promoters. The conservation of nucleotides shown in the alignment is divided into σI1-, σI2-, σI3-, σI4-, and σI6-dependent promoters. The promoter sequences of each σI regulon were taken from Fig. 1A. The most conserved nucleotides of each σI regulon are highlighted.
Site-directed mutagenesis analysis confirmed the three motifs of σI promoters
The high stringency of promoter recognition by alternative σI factors was analyzed using C. thermocellum σI3, because it recognizes highly conserve promoters with a characteristic C triad upstream of the A-tract motif in the −35 promoter element (Fig. 2). First, in order to verify the σI3-dependent promoters, we mapped the TSSs of rgl11A and rga12A by the 5′-RACE technique (Supplementary Fig. S1). Next, the crucial nucleotides for recognition of C. thermocellum σI-dependent promoters were analyzed by site-directed mutagenesis experiments using the σI3-dependent promoter of rgl11A. The σI3-dependent promoter of rgl11A was selected for the analysis because previous work showed that this promoter presented the highest activation by the C. thermocellum σI3 factor10.
A promoter library with single transversion mutations (A to T or T to A, and C to G or G to C) in both −35 and −10 promoter elements was created. Additional transversion mutations were created upstream and downstream of both −35 and −10 promoter elements, replacing less conserved nucleotides. Subsequently, the promoter library was fused to a lacZ-gfp reporter operon. The promoter activities were thus studied in the heterologous B. subtilis host system. Quantification of promoter activities was performed by measuring GFP fluorescence, and the fluorescence of the different promoter variants was compared to that of the wild-type rgl11A promoter.
As shown in Fig. 3, mutations in the most conserved nucleotides in both −35 and −10 promoter elements have a negative effect, abolishing the detection of the GFP fluorescence. Mutations in less conserved nucleotides have a negligible effect, showing promoter activities at the same level as that of the wild-type promoter. Interestingly, the mutation from T to A between the highly conserved CCC and AAA triads of the −35 promoter element (rgl11A-Mut8; sequence underlined in CCCCTAAA) increased the fluorescence by 53%. As can be observed in the WebLogo shown in Fig. 3, C. thermocellum σI3 apparently prefers promoter sequences enriched in adenines downstream of the −35 promoter element. This observation can explain the rise in activity identified in the promoter version of rgl11A-Mut8.
Evaluation of the validity of the σI promoter motifs by mutagenesis. The rgl11 A σI3-dependent promoter was selected for this analysis, because it presented the highest activation by the C. thermocellum σI3 factor10. The WebLogo was generated with σI3-dependent promoter sequences (Fig. 1A) and is shown to illustrate the effect of mutations in the most conserved nucleotides of the three motifs: (i) the −10 promoter element, (ii) the A-tract motif and (iii) the region of specificity. Mutations are indicated by highlighted nucleotides, and the effect of each mutation is shown as relative activity, compared to that of the wild-type control promoter rgl11A, defined as 100%. The relative activity shown is the average of three independent experiments. Predicted −35 and −10 promoter elements are indicated by lines above the rgl11A promoter sequences. The nucleotide code Y represents C or T, W represents A or T, and N represents any nucleotide. ND means not detected.
σI factor recognizes highly stringent promoter sequences
To confirm the implication of the region of specificity in the stringency of σI-dependent promoters, we searched for σI-dependent promoters in the genome of P. cellulosolvens that resemble those of the C. thermocellum σI3-dependent promoters. P. cellulosolvens was chosen for further comparison, because it produces the most complex cellulosome known to date23, and a previous report showed that as the cellulosome becomes more elaborate, the bacterium harbors more σI factors11. This observation was confirmed by our analysis which revealed that P. cellulosolvens genome encodes 16 σI paralogues (Supplementary Table S4), making this bacterium an excellent model for the study of multiple σI factors.
For the identification of putative σI-dependent promoters in P. cellulosolvens, we first analyzed the upstream regions of all predicted σI genes (Supplementary Table S4). The search for promoters upstream of P. cellulosolvens σI genes was performed taking into account that the AAA triad and the CG dyad in the −35 and −10 promoter regions, respectively, are the most conserved nucleotides of the σI-dependent promoters of cellulosome-producing bacteria (Fig. 1). The predicted putative σI-dependent promoters of P. cellulosolvens σI genes are shown in Supplementary Table S4.
In order to predict putative σI-dependent promoters, the genome sequence of P. cellulosolvens was analyzed using the same promoter motifs that were employed during the analysis of the upstream region of σI genes (AAA in the −35 region and CG in the −10 region). To delimit the promoter search, we included A, T or C downstream of the CG dyad in the −10 region (CGHH, where H represents A, T or C), because the predicted promoters of σI genes contain these nucleotides (Supplementary Table S4). The spacing between the −10 and −35 promoter elements was allowed to be between 12 to 15 bases. During this analysis, a collection of 140 σI-dependent promoters were predicted (Supplementary Table S5).
Subsequently, in order to find putative P. cellulosolvens σI-dependent promoter sequences resembling those of C. thermocellum σI3-dependent promoters, the collection of predicted σI-dependent promoters of P. cellulosolvens was analyzed by searching for cytosine enrichments in the region of specificity in the −35 promoter element. This analysis allowed the identification of one putative promoter upstream of P. cellulosolvens sigI11 and five putative promoters upstream of genes encoding saccharolytic enzymes (Bccel_3806, Bccel_5179, Bccel_5541, Bccel_5619 and Bccel_5627). The alignment of the P. cellulosolvens predicted promoters is shown in Fig. 4. The highly conserved CCC triad can be observed in the region of specificity immediately upstream of the A-tract motif in the −35 promoter element. Additionally, the highly conserved CGCAT pentad in the −10 promoter element can also be observed. The majority of the P. cellulosolvens predicted promoters correspond to genes encoding catalytic modules which are probably involved in pectin degradation, such as pectate lyase, pectin esterase, Rga and Rgl [Fig. 4; ref.28,29,30].
Alignment of P. cellulosolvens σI11-dependent promoter sequences and quantitative evaluation of their recognition by the P. cellulosolvens σI11 factor. The WebLogo was generated with the sequence shown in the alignment. Distances between the promoter region sequences used for the alignment and the first codon of corresponding genes are shown in the 5′-UTR (5′-untranslated region) column. Promoter activity was measured by quantifying the fluorescence (average of three independent experiments). Fluorescence units (FU) were calculated when cells reached an optical density (600 nm) of 1. ΔFU represents the activity of the induced promoter after subtracting the fluorescence obtained under uninduced conditions. ND (not detected) was assigned where ΔFU was negative, zero or the standard deviation exceeded ΔFU. As a negative control, the predicted σI-dependent promoter of the P. cellulosolvens σI3 gene was used. CBM, carbohydrate-binding module; Doc, dockerin; GH, glycoside hydrolase; Rga, rhamnogalacturan acetylesterase; PT, Pro-Thr repeat (pfam04886); Rgl, rhamnogalacturonan lyase.
Finally, to test the ability of P. cellulosolvens σI11 to recognize the predicted promoters shown in Fig. 4, we fused the predicted promoters to a gfp-lacZ reporter operon. To overcome the lack of genetic tools in P. cellulosolvens, the promoter activities were also studied in a heterologous B. subtilis host system10. The recognition of the predicted promoters by P. cellulosolvens σI11 was analyzed by measuring GFP fluorescence. As shown in Fig. 4, all the predicted promoters were recognized by P. cellulosolvens σI11, suggesting that this alternative σ factor is likely involved in the regulation of genes encoding pectin-degrading enzymes, similar to its orthologous C. thermocellum σI3 (Fig. 1B).
Discussion
Cellulolytic clostridia produce one of the most efficient systems to degrade plant-cell wall polysaccharides — the multi-enzyme cellulosome complex31. The genomes of this group of bacteria contain dozens of genes, which encode different types of carbohydrate-active hydrolyzing enzymes and structural scaffoldin subunits, whose regulation is fine-tuned during the hydrolysis of biomass32. Although the cellulolytic capacities of cellulosome-producing bacteria have been the subject of study for many years33,34,35, the regulatory mechanisms that govern these processes are poorly known. Here, we show how a collection of five alternative σI factors in C. thermocellum, namely σI1, σI2, σI3, σI4 and σI6, regulate the expression of 17 genes encoding different cellulosomal components (Fig. 1). This analysis reveals for the first time a sophisticated regulatory network of several alternative σ factors, which together control the enzymatic composition of the cellulosome (Fig. 1). Our results show how each σI factor has a particular regulon (Fig. 2) that correlates with its corresponding anti-σI factor that selectively senses a target polysaccharide8,36,37. Furthermore, with the results collected in C. thermocellum, we were able to predict the putative regulon of the P. cellulosolvens σI1 factor (Fig. 4) and provide experimental evidence, for the first time in P. cellulosolvens, of the regulation of genes encoding cellulosomal components by an alternative σ factor (Fig. 4).
Alternative σI factors are a subfamily of the σ70 family which are unique, because not all σI factors are involved in the regulation of genes encoding saccharolytic enzymes or cellulosomal components. B. subtilis has only one σI factor that is induced by heat-shock12 and is involved in the maintenance of cell envelope integrity and homeostasis38. These observations show how σIs are malleable alternative σ factors that have been adapted in different bacteria to perform various functions. However, during the course of evolution, when σI genes were extensively duplicated in the genome of cellulolytic clostridia to regulate the enzymatic composition of the cellulosome, their dependent promoter sequences were specialized in order to avoid regulatory overlap.
In the current work, three important observations support the hypothesis that alternative σI factors recognize stringent promoter sequences to discriminate among dozens of promoters that share similar motifs. First, each σI factor involved in the regulation of genes encoding saccharolytic enzymes and cellulosomal components in C. thermocellum has specific regulons with characteristic promoter sequences (Fig. 2). Second, mutagenesis analysis performed with the σI3-dependent promoter of C. thermocellum rgl11A revealed a drastic reduction in promoter activity when the most conserved nucleotides were mutated (Fig. 3). Finally, the C. thermocellum σI3 promoter consensus sequence allowed the identification of genes encoding putative pectin-degrading enzymes in another cellulolytic clostridium, P. cellulosolvens (Fig. 4).
Our results demonstrate that the region located upstream of the A-tract motif in the −35 promoter element has a crucial role for the discrimination of σI-dependent promoters by the different σI factors (Figs 1 and 2). In general, all σI factors recognize promoters with a CGAA tetrad and an A-tract motif in the −10 and −35 promoter elements, respectively (Figs 1 and 2). However, the region upstream of the A-tract motif is characteristic to each σI regulon (Fig. 2). Indeed, this region, herein named region of specificity, was used to identify σI-dependent promoters of genes involved in pectin degradation in the genome of P. cellulosolvens (Fig. 4).
The key role of the −35 promoter element for the recognition of σI factors is also supported by the conservation of homopolymeric A-tract motifs (Fig. 1). Intriguingly, in contrast to the A-tract motifs of σI-dependent promoters, a recent report demonstrated in B. subtilis that a homopolymeric T-tract motif contributes to the activation of several ECF σ factors13. The latter T-tract motif is located downstream of the highly conserved AAC triad in the −35 promoter element of ECF σ factors. Here, the relevance of the A-tract motif was demonstrated during the mutagenesis analysis of the σI3-dependent promoter of C. thermocellum rgl11A. We demonstrated how single mutations in the AAA triad of the −35 promoter element destroyed the recognition of the promoter by C. thermocellum σI3 (Fig. 3). Furthermore, the addition of an extra A, by mutating the T located immediately upstream of the A-tract motif (i.e., rgl11A-Mut8; sequence underlined in CCCCTAAA) increased the strength of the promoter, providing promoter activity 53% higher than that of the wild-type promoter (Fig. 3).
It is also interesting that both T-tract and A-tract motifs are located in the same position in the −35 promoter region of ECF σ- and σI-dependent promoters, respectively [ref.13 and Fig. 1, respectively]. Hence, we propose that this motif may help avoid crosstalk between ECF σs and σIs. In this context, the respective −10 promoter elements of ECF σ- and σI-dependent promoters are highly similar [ref.13 and Fig. 1, respectively]. The discrimination of promoters is critical in cellulolytic clostridia that harbor multiple σI factors, not only to avoid crosstalk between the different σIs, but also to discriminate between the promoters of other types of alternative σ factors, such as the ECF σs. According to the Microbial Signal Transduction database (MiST2.2, http://mistdb.com/), the genome of C. thermocellum encodes 7 ECF σs, and our analysis revealed that the P. cellulosolvens genome encodes at least 22 ECF σs (Supplementary Table S6). Therefore, the possibility of having ECF σ- and σI-dependent promoters with similar −35 regions is very high. Consequently, the A-tract motif of σI-dependent promoters may also serve to avoid their recognition by ECF σ factors. This idea is further supported by the observation that none of the σI-dependent promoters, which have been tested in our laboratory by using the B. subtilis heterologous host cell system (that is devoid of its native σI/RsgI system), were activated by the resident B. subtilis σ factors10,11.
It is worth mentioning that genomic context may also play a defined role in promoter selectivity. It has been reported that some important sequences which reside outside of the classic −35 and −10 promoter elements can be implicated in the recognition of the promoter39,40. For example, most of the promoters that are dependent on the Escherichia coli and Salmonella enterica σE, an ECF σ factor, require a sequence upstream of the −35 promoter element (UP-element) to increase their strength39. Likewise, promoters that are dependent on σI factors may also require UP-elements. The presence of motifs that reside outside of the classic −35 and −10 promoter elements that help to increase the strength of the promoter, or compensate for a poor −35 or −10 promoter element that deviates from consensus, is a phenomenon known as “mix and match”40,41,42. Future identification of σI-dependent promoters may be improved by taking into account mix-and-matching as a promoter recognition mechanism, thereby generating a better understanding of the biological function of each σI factor.
It is also important to note that, although the C. thermocellum σI factors have a defined regulon with little crosstalk, the major regulatory overlap between σIs occurs in the two most important cellulosomal genes, cipA11,18,25 and cel48S6,26,27 (Fig. 1B). Close inspection of the σI-dependent promoter of C. thermocellum cipA and cel48S reveals that both −35 and −10 promoter regions are identical, with a CCCCTCAAA nonad and a CGAA tetrad, respectively (Fig. 1A). Additionally, the σI-dependent promoter of C. thermocellum cipA and cel48S have a conserved AT dyad, three nucleotides downstream of the CGAA tetrad in the −10 promoter element that is present in nearly all of the σI-dependent promoters shown in Fig. 1A. These observations suggest that the σI-dependent promoter of C. thermocellum cipA and cel48S may represent a type of “universal” promoter that is used by the bacterium to assure expression of relevant genes.
Previous works have shown that both CipA and Cel48S are fundamental components of the cellulosome25,26,27. Hence their expression should be assured in the presence of a wide number of polysaccharide substrates and conditions6,27. In this sense, it would seem logical to use more than one alternative σ factor to regulate their expression. However, the recognition of specific promoter sequences by each of the regulators would be the consequence of a long and complex evolutionary process. A more practical approach to avoid this process is the utilization of a single “universal promoter” such as the one presented in this work. From an evolutionary point of view, it is very likely that the common ancestor of these σI factors recognized a similar promoter sequence to that used by cipA and cel48S. Later, each of the duplicated genes evolved to encode specialized versions of the σIs capable of recognizing unique promoters, thus reducing unnecessary crosstalk and limiting their regulons while maintaining regulatory overlap of these critical components.
Interestingly, the σI-dependent promoters of C. thermocellum sigI3 harbor almost the same promoter elements present in cipA and cel48S σI-dependent promoters. In contrast, the σI-dependent promoters of C. thermocellum sigI3 are only recognized by σI3 (Fig. 1B). The difference is present in the −10 promoter element. Whereas the σI-dependent promoter of cipA and cel48S have the CGAA tetrad in the −10 element, the σI-dependent promoter of sigI3 has the CGTA tetrad. Additionally, the conserved AT dyad, downstream of the −10 element that is present in the σI-dependent promoter of cipA and cel48S (Fig. 1A), is less conserved in the promoter of sigI3, since it has a GT dyad in the same position (Fig. 1A). Thus, these small changes in the −10 promoter element can also help to improve specificity and avoid regulatory overlap.
Direct analysis of the multiple σI factors in C. thermocellum represents a serious challenge, owing to the regulatory overlap between the different σI factors with some of the important genes, such as cipA and cel48S (Fig. 1). Moreover, the genome of C. thermocellum may harbor additional as-yet-unidentified σI-dependent promoters that may be activated by more than one σI factor. Therefore, during future studies of a particular σI factor directly in C. thermocellum, we could expect a cascade of interactions among the different σIs. Additionally, cellulosomal component genes can also have σA-dependent promoters11,43 and other regulatory proteins44, making the analysis of σIs directly in C. thermocellum more complex. If these observations are not taken into account during the direct analysis of σIs in C. thermocellum, the results obtained can be misinterpreted. These observations apply also for other cellulolytic clostridia with multiple σIs, such as P. cellulosolvens (Supplementary Table S4). Hence, as shown in the present report, the application of the heterologous B. subtilis host system for analysis of the multiple σI factors of cellulolytic clostridia is advantageous. The predicted σI-dependent promoters can be experimentally tested in B. subtilis and corroborated in vivo by mapping the TSSs of their associated genes (Supplementary Fig. S1).
In conclusion, in the present work, we show how the employment of classical microbiology genetic tools, such as the LacZ reporter system45, together with the well-known Gram-positive bacterium B. subtilis as heterologous host10, enabled us to decipher the regulatory networks of the multiple alternative σI factors, of one of the most efficient and most intricate cellulolytic systems in nature – the bacterial cellulosome.
Methods
Bacterial strains, growth media and culture conditions
C. thermocellum DSM 1313 and P. cellulosolvens DSM2933 were obtained from the DSMZ (German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany). The recognition of predicted σI-dependent promoters by C. thermocellum and P. cellulosolvens σI factors was analyzed in a heterologous B. subtilis host system that was developed in a previous work10. The B. subtilis strains constructed used in this work (Supplementary Table S1) are isogenic derivatives of the B. subtilis strain CO02 that is devoid of its sigI-rsgI operon10.
C. thermocellum and P. cellulosolvens were grown using the media and condition described by the ref.9 and the DSMZ, respectively. B. subtilis and E. coli were cultivated routinely on solid LB Broth (Lennox, Difco, BD Diagnostics, Maryland, USA) or in liquid LB Broth (at 250 rpm) at 37 °C. The expression of genes under the PxylA promoter was induced with D-xylose using a final concentration of 10 g/L (Sigma-Aldrich). When required the following antibiotics (all from Sigma-Aldrich) were added at the indicated final concentration: ampicillin (100 µg/mL, Amp), kanamycin (50 µg/mL, Kan), chloramphenicol (5 µg/mL, Cam) or erythromycin (3 µg/mL, Erm).
DNA manipulation techniques and construction of plasmids
The primers and plasmids used in the present work are listed in Tables S2 and S3, respectively. Plasmids were constructed by standard molecular cloning techniques using restriction enzymes and ligase, or by the ligase-independent cloning technique based on the In-Fusion HD Cloning Kit (Clontech Laboratories, Inc., California, USA).
The pAX01 integration vector was used to express the C. thermocellum and P. cellulosolvens σI factors in B. subtilis46. This plasmid has the xylose-inducible promoter PxylA to control the expression of a gene of interest, integrates at B. subtilis lacA locus and harbors an erm cassette as a selectable marker. First, pAX01 was linearized with the restriction enzyme BamHI. Later, P. cellulosolvens sigI11 was PCR-amplified using primers P1 and P2. Finally, the PCR product was cloned using the In-Fusion HD Cloning Kit into the linearized pAX01 vector, thereby obtaining the pAX01-Bc-SigI11 plasmid. To express the C. thermocellum σI1, σI2, σI3, σI4, σI5 and σI6 factors in B. subtilis we used the pAX01 derived plasmids, pAX01-SigI1, pAX01-SigI2, pAX01-SigI3, pAX01-SigI4, pAX01-SigI5 and pAX01-SigI6, that were constructed in previous works10,11.
The regulons of C. thermocellum σI1, σI2, σI3, σI4, σI5 and σI6 were analyzed by using the 40 putative σI-dependent promoters that were predicted in a previous work10. With the exception of the putative predicted σI-dependent promoters of C. thermocellum cel48S, we used a library of the previously predicted promoters that was fused to the LacZ reporter gene of the pBS1C-LacZ integration vector in a previous work10. pBS1C-LacZ integrates at the B. subtilis amyE locus and carries a cam resistance cassette as a selectable marker47. In the case of the putative predicted σI-dependent promoters of C. thermocellum cel48S, its sequence was PCR-amplified using primers P3 and P4. Subsequently, the PCR product was digested with the restriction enzymes EcoRI and BamHI and fused to the LacZ reporter gene of pBS1C-LacZ that was previously cut with the same restriction enzymes, thereby obtaining the pProm-Ct-Cel48S derived plasmid.
In order to study the important nucleotides for the recognition of σI-dependent promoters, we used the pBS1C-GFP-LacZ integration vector which harbors a promoterless gfp-lacZ reporter operon11. pBS1C-GFP-LacZ integrates at the B. subtilis amyE locus and carries a cam resistance cassette as a selectable marker11. The analysis of promoter recognition was performed with mutant versions of the σI3-dependent promoter of C. thermocellum rgl11A that were created by site-directed mutagenesis and fused to the gfp-lacZ reporter operon of pBS1C-GFP-LacZ. To introduce individual mutations in both −35 and −10 promoter regions, the reverse primers from P5 to P22, which contain the mutated nucleotide, were used with the forward primer P23. In order to compare the mutant versions, a wild type version of the rgl11A σI3-dependent promoter was PCR-amplified using the primer pair P23-P24. After PCR amplification of the promoter mutant versions, the PCR products were digested with the restriction enzymes EcoRI and BamHI. Finally, each digested PCR product was cloned into pBS1C-GFP-LacZ that was digested previously with the same restriction enzymes, thereby obtaining the pBS1C-GFP-LacZ derived plasmids listed in Supplementary Table S3.
The recognition of σI-dependent promoters by P. cellulosolvens σI11 was analyzed with the pBS1C-GFP-LacZ integration vector. The predicted σI-dependent promoters of Bccel_5622 (P. cellulosolvens σI11 gene), Bccel_3856, Bccel_5179, Bccel_5541, Bccel_5619 and Bccel_5627 were PCR-amplified using the primer pairs P25-P26, P27-P28, P29-P30, P31-P32, P33-P34 and P35-P36, respectively. Later, each PCR product was digested with the restriction enzymes EcoRI and BamHI, except the promoter of Bccel_5541 that was PCR-amplified for cloning with the In-Fusion system. Finally, each digested PCR product was cloned into the pBS1C-GFP-LacZ plasmid that was digested previously with the same restriction enzymes, thereby obtaining the pBS1C-GFP-LacZ derived plasmids listed in Supplementary Table S3 (plasmid #26 to #30). In the case of the predicted promoter of Bccel_5541, its DNA sequence was cloned into pBS1C-GFP-LacZ (previously linearized with EcoRI and BamHI) using the In-Fusion HD Cloning Kit (Supplementary Table S3, plasmid #31).
Mapping of the TSSs
In order to map the TSSs we purified total RNA of C. thermocellum following the protocols described in a previous publication11. The mRNA 5′-ends were mapped using the 5′-RACE technique with the SMARTer® RACE 5′/3′ kit (Clontech) according to supplier protocols. Briefly, total RNA was subjected to RT-PCR with random primers and the SMARTer II oligonucleotide. Subsequently the 5′-RACE-Ready cDNA was submitted to a PCR amplification using the Universal Primer A Mix (a combination of Universal Primer Long and Universal Primer Short) and the gene specific primer (P40, P41, P42, P43 or P44 for sigI2, sigI3, sigI4, rgl11A and rga12A, respectively; Supplementary Table S2). Then, this PCR-product was subjected to a second PCR with the Universal Primer Short and the nested gene specific primer (P45, P46, P47, P48 or P49 for sigI2, sigI3, sigI4, rgl11A and rga12A, respectively; Supplementary Table S2). Finally, the PCR-products were gel purified, cloned into pRACE using the In-Fusion® HD Cloning kit and sequenced.
Construction of B. subtilis strains and analysis of promoter activities
B. subtilis was transformed by using the natural competence method48. Chromosomal integration of plasmids by a double-crossover event was confirmed by colony PCR using the primers listed in Tables S2. To analyze the LacZ reporter system, B. subtilis strain samples were taken from the −80 °C glycerol stock, inoculated in LB broth (3 mL) with Cam and grown overnight. The next day, the cells were centrifuged 15 min at 3000 × g and resuspended in 1 mL of Spizizen’s minimal media48 with fructose (1.8 g/L final) as the carbon source. Finally, the cells were incubated in 24-well cell culture plates at 150 rpm. To observe the LacZ activity, the Spizizen’s minimal media was supplemented with X-gal (40 mg/L final). To measure the fluorescence associated to the GFP reporter system, we followed the protocol described elsewhere11. Fluorescence units (FU) were calculated when the cells reached an optical density at 600 nm of 1 and represent the activity of the induced promoter after subtracting the values obtained under uninduced conditions, i.e. ΔFU = FUinduced cells − FUuninduced cells.
Bioinformatics
Promoter motifs searches were carried out with the Pattern Locator program49. The analysis of the promoter motif sequences was performed with the Jalview software50. Multiple sequence alignment (MSA) of promoter sequences was performed using the T-Coffee algorithm51 implemented by Jalview. DNA sequence logos were generated with the program WebLogo52.
Data availability
All data generated or analysed during this study are included in this published article (and its Supplementary Information files).
References
Mascher, T. Signaling diversity and evolution of extracytoplasmic function (ECF) σ factors. Curr. Opin. Microbiol. 16, 148–55 (2013).
Staroń, A. et al. The third pillar of bacterial signal transduction: classification of the extracytoplasmic function (ECF) σ factor protein family. Mol. Microbiol. 74, 557–581 (2009).
Browning, D. F. & Busby, S. J. W. Local and global regulation of transcription initiation in bacteria. Nat. Rev. Microbiol. 14, 638–650 (2016).
Helmann, J. D. The extracytoplasmic function (ECF) sigma factors. Adv. Microb. Physiol. 46, 47–110 (2002).
Bayer, E. A., Belaich, J.-P., Shoham, Y. & Lamed, R. The cellulosomes: multienzyme machines for degradation of plant cell wall polysaccharides. Annu. Rev. Microbiol. 58, 521–554 (2004).
Raman, B. et al. Impact of pretreated Switchgrass and biomass carbohydrates on Clostridium thermocellum ATCC 27405 cellulosome composition: a quantitative proteomic analysis. PLoS One 4, e5271 (2009).
Blouzard, J. C. et al. Modulation of cellulosome composition in Clostridium cellulolyticum: adaptation to the polysaccharide environment revealed by proteomic and carbohydrate-active enzyme analyses. Proteomics 10, 541–554 (2010).
Kahel-Raifer, H. et al. The unique set of putative membrane-associated anti-sigma factors in Clostridium thermocellum suggests a novel extracellular carbohydrate-sensing mechanism involved in gene regulation. FEMS Microbiol. Lett. 308, 84–93 (2010).
Nataf, Y. et al. Clostridium thermocellum cellulosomal genes are regulated by extracytoplasmic polysaccharides via alternative sigma factors. Proc. Natl. Acad. Sci. 107, 18646–18651 (2010).
Muñoz-Gutiérrez, I. et al. Decoding biomass-sensing regulons of Clostridium thermocellum alternative Sigma-I factors in a heterologous Bacillus subtilis host system. PLoS One 11, e0146316 (2016).
Ortiz de Ora, L. et al. Revisiting the regulation of the primary scaffoldin gene in Clostridium thermocellum. Appl. Environ. Microbiol. 83, e03088–16 (2017).
Zuber, U., Drzewiecki, K. & Hecker, M. Putative sigma factor SigI (YkoZ) of Bacillus subtilis is induced by heat shock. J. Bacteriol. 183, 1472–1475 (2001).
Gaballa, A. et al. Modulation of extracytoplasmic function (ECF) sigma factor promoter selectivity by spacer region sequence. Nucleic Acids Res. 46, 134–145 (2018).
Mascher, T., Hachmann, A.-B. & Helmann, J. D. Regulatory overlap and functional redundancy among Bacillus subtilis extracytoplasmic function σ factors. J. Bacteriol. 189, 6919–6927 (2007).
Huang, X., Fredrick, K. L. & Helmann, J. D. Promoter recognition by Bacillus subtilis σW: autoregulation and partial overlap with the σX regulon. J. Bacteriol. 180, 3765–3770 (1998).
Yutin, N. & Galperin, M. Y. A genomic update on clostridial phylogeny: Gram-negative spore formers and other misplaced clostridia. Environ. Microbiol. 15, 2631–2641 (2013).
Bayer, E. A., Shoham, Y. & Lamed, R. In The Prokaryotes: Prokaryotic Physiology and Biochemistry. (ed Rosenberg, E.), 215–266 (Springer-Verlag, Berlin, 2013).
Xu, Q. et al. Dramatic performance of Clostridium thermocellum explained by its wide range of cellulase modalities. Sci. Adv. 2, e1501254 (2016).
Giuliano, C. & Khan, A. W. Cellulase and sugar formation by Bacteroides cellulosolvens, a newly isolated cellulolytic anaerobe. Appl. Environ. Microbiol. 48, 446–448 (1984).
Saddler, J. N. & Khan, A. W. Cellulase production by Acetivibrio cellulolyticus. Can. J. Microbiol. 26, 760–765 (1980).
Lin, C., Urbance, J. W. & Stahl, D. A. Acetivibrio cellulolyticus and Bacteroides cellulosolvens are members of the greater clostridial assemblage. FEMS Microbiol. Lett. 124, 151–155 (1994).
Horino, H., Fujita, T. & Tonouchi, A. Description of Anaerobacterium chartisolvens gen. nov., sp. nov., an obligately anaerobic bacterium from Clostridium rRNA cluster III isolated from soil of a Japanese rice field, and reclassification of Bacteroides cellulosolvens Murray et al. 1984 as Pseudobacteroides cellulosolvens gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 64, 1296–1303 (2014).
Zhivin, O. et al. Unique organization and unprecedented diversity of the Bacteroides (Pseudobacteroides) cellulosolvens cellulosome system. Biotechnol. Biofuels 10, 211 (2017).
Guss, A. M., Olson, D. G., Caiazza, N. C. & Lynd, L. R. Dcm methylation is detrimental to plasmid transformation in Clostridium thermocellum. Biotechnol. Biofuels 5, 30 (2012).
Olson, D. G., Giannone, R. J., Hettich, R. L. & Lynd, L. R. Role of the CipA scaffoldin protein in cellulose solubilization, as determined by targeted gene deletion and complementation in Clostridium thermocellum. J. Bacteriol. 195, 733–739 (2013).
Morag, E., Bayer, E. A., Hazlewood, G. P., Gilbert, H. J. & Lamed, R. Cellulase Ss (CelS) is synonymous with the major cellobiohydrolase (subunit S8) from the cellulosome of Clostridium thermocellum. Appl. Biochem. Biotechnol. 43, 147–151 (1993).
Yoav, S. et al. How does cellulosome composition influence deconstruction of lignocellulosic substrates in Clostridium (Ruminiclostridium) thermocellum DSM 1313? Biotechnol. Biofuels 10, 1–16 (2017).
McDonough, M. A., Kadirvelraj, R., Harris, P., Poulsen, J. C. N. & Larsen, S. Rhamnogalacturonan lyase reveals a unique three-domain modular structure for polysaccharide lyase family 4. FEBS Lett. 565, 188–194 (2004).
Mølgaard, A., Kauppinen, S. & Larsen, S. Rhamnogalacturonan acetylesterase elucidates the structure and function of a new family of hydrolases. Structure 8, 373–383 (2000).
Jayani, R. S., Saxena, S. & Gupta, R. Microbial pectinolytic enzymes: a review. Process Biochem. 40, 2931–2944 (2005).
Artzi, L., Bayer, E. A. & Moraïs, S. Cellulosomes: bacterial nanomachines for dismantling plant polysaccharides. Nat. Rev. Microbiol. 15, 83–95 (2017).
Zverlov, V. V., Kellermann, J. & Schwarz, W. H. Functional subgenomics of Clostridium thermocellum cellulosomal genes: identification of the major catalytic components in the extracellular complex and detection of three new enzymes. Proteomics 5, 3646–3653 (2005).
Lamed, R., Setter, E., Kenig, R. & Bayer, E. A. The cellulosome: a discrete cell surface organelle of Clostridium thermocellum which exhibits separate antigenic, cellulose-binding and various cellulolytic activities. Biotechnol. Bioeng. Symp. 13, 163–181 (1983).
Bayer, E. A., Kenig, R. & Lamed, R. Adherence of Clostridium thermocellum to cellulose. J. Bacteriol. 156, 818–827 (1983).
Lamed, R., Setter, E. & Bayer, E. A. Characterization of a cellulose-binding, cellulose-containing complex in Clostridium thermocellum. J. Bacteriol. 156, 828–836 (1983).
Bahari, L. et al. Glycoside hydrolases as components of putative carbohydrate biosensor proteins in Clostridium thermocellum. J. Ind. Microbiol. Biotechnol. 38, 825–832 (2011).
Yaniv, O. et al. Fine-structural variance of family 3 carbohydrate-binding modules as extracellular biomass-sensing components of Clostridium thermocellum anti-σI factors. Acta Crystallogr. D Biol. Cryst. 70, 522–534 (2014).
Tseng, C. L. & Shaw, G. C. Genetic evidence for the actin homolog gene mreBH and the bacitracin resistance gene bcrC as targets of the alternative sigma factor SigI of Bacillus subtilis. J. Bacteriol. 190, 1561–1567 (2008).
Mutalik, V. K., Nonaka, G., Ades, S. E., Rhodius, V. A. & Gross, C. A. Promoter strength properties of the complete Sigma E regulon of Escherichia coli and Salmonella enterica. J. Bacteriol. 191, 7279–7287 (2009).
Hook-Barnard, I. G. & Hinton, D. M. Transcription initiation by mix and match elements: flexibility for polymerase binding to bacterial promoters. Gene Regul. Syst. Biol. 1, 275–293 (2007).
Guzina, J. & Djordjevic, M. Promoter recognition by extracytoplasmic function σ factors: analyzing DNA and protein interaction motifs. J. Bacteriol. 198, 1927–1938 (2016).
Guzina, J. & Djordjevic, M. Mix-and-matching as a promoter recognition mechanism by ECF σ factors. BMC Evol. Biol. 17, 12 (2017).
Sand, A. et al. Three cellulosomal xylanase genes in Clostridium thermocellum are regulated by both vegetative SigA (σA) and alternative SigI6 (σI6) factors. FEBS Lett. 589, 3133–3140 (2015).
Wilson, C. M. et al. LacI transcriptional regulatory networks in Clostridium thermocellum DSM1313. Appl. Environ. Microbiol. 83, e02751–16 (2017).
Zuber, P. & Losick, R. Use of a lacZ fusion to study the role of the spoO genes of Bacillus subtilis in developmental regulation. Cell 35, 275–283 (1983).
Hartl, B., Wehrl, W., Wiegert, T., Homuth, G. & Schumann, W. Development of a new integration site within the Bacillus subtilis chromosome and construction of compatible expression cassettes. J. Bacteriol. 183, 2696–2699 (2001).
Radeck, J. et al. The Bacillus BioBrick Box: generation and evaluation of essential genetic building blocks for standardized work with Bacillus subtilis. J. Biol. Eng. 7, 29 (2013).
Cutting, S. & Vander Horn, P. In Molecular biological methods for Bacillus (eds Harwood, C. & Cutting, S.) 27–74 (John Wiley and Sons Ltd, 1990).
Mrázek, J. & Xie, S. Pattern locator: A new tool for finding local sequence patterns in genomic DNA sequences. Bioinformatics 22, 3099–3100 (2006).
Waterhouse, A. M., Procter, J. B., Martin, D. M. A., Clamp, M. & Barton, G. J. Jalview Version 2-A multiple sequence alignment editor and analysis workbench. Bioinformatics 25, 1189–1191 (2009).
Notredame, C., Higgins, D. G. & Heringa, J. T-Coffee: a novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 302, 205–217 (2000).
Crooks, G. E., Hon, G., Chandonia, J.-M. & Brenner, S. E. WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 (2004).
Acknowledgements
The authors appreciate Dr. Ilya Borovok (Tel Aviv University) for his helpful advice during the course of this work and Dr. Milana Voronov-Goldman (Tel Aviv University) for technical support. This research was supported by the Israel Science Foundation (24/11 to R.L., 1349/13 to E.A.B. and 177/14 to Y.S.). Additional support was issued to R.L. by The Sidney E. Frank Foundation through the ISF. This research was funded in part by the joint Israel-China ISF-NSFC Research Grant (2566/160 to E.A.B. and Y.F.) – National Natural Science Foundation of China (31661143023 to Y.F.). I.M.-G. is grateful for the award of a Martin Kushner Schnur Post-Doctoral Fellowship at the Weizmann Institute. L.O.O. was supported by the “Consejo Nacional de Ciencia y Tecnología - México” with a PhD scholarship (440354). Y.S. holds the Erwin and Rosl Pollak Chair in Biotechnology at the Technion. E.A.B. is the incumbent of The Maynard I. and Elaine Wishner Chair of Bio-organic Chemistry at the Weizmann Institute of Science.
Author information
Authors and Affiliations
Contributions
L.O.O. and I.M.-G. contributed to conception and design; L.O.O. acquired data; L.O.O., R.L., E.B. and I.M.-G. analyzed and interpreted data; Y.-J.L., J.X., Q.C., Y.F. and Y.S. contributed reagents, materials, analysis and tools; L.O.O., R.L., E.B. and I.M.-G. prepared figures and drafted the article; Y.-J.L., J.X., Q.C., Y.F. and Y.S. critically revised the article; L.O.O., R.L., E.B. and I.M.-G. approved the final version to be published; R.L., E.B. and I.M.-G. directed and supervised the research.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ortiz de Ora, L., Lamed, R., Liu, YJ. et al. Regulation of biomass degradation by alternative σ factors in cellulolytic clostridia. Sci Rep 8, 11036 (2018). https://doi.org/10.1038/s41598-018-29245-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29245-5
This article is cited by
-
Current models in bacterial hemicellulase-encoding gene regulation
Applied Microbiology and Biotechnology (2024)
-
Structure of the transcription open complex of distinct σI factors
Nature Communications (2023)
-
Unifying themes and distinct features of carbon and nitrogen assimilation by polysaccharide-degrading bacteria: a summary of four model systems
Applied Microbiology and Biotechnology (2021)
-
Unraveling essential cellulosomal components of the (Pseudo)Bacteroides cellulosolvens reveals an extensive reservoir of novel catalytic enzymes
Biotechnology for Biofuels (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.