Abstract
The revised criteria of the 8th American Joint Committee on Cancer (AJCC) cancer staging system consider depth of invasion as one of the factors that determine stage in distal bile duct (DBD) cancer, but exclude adjacent organ invasion. The aims were to evaluate the association between adjacent organ invasion and relapse-free survival (RFS) and overall survival (OS) after curative surgical resection of DBD cancer and to propose optimal criteria for predicting clinical outcomes. In this retrospective cohort study, 378 patients with DBD cancer treated in multi-institutions between 1996 and 2013 were investigated. This study evaluated the relationship between clinicopathologic parameters and adjacent organ invasion and used organ invasion to compare the survival times of each group. Among 204 patients with adjacent organ invasion, 152 were in the single-organ invasion group and 52 were in the dual-organ invasion group based on a review of microscopic slides. In univariate and multivariate analyses, patients with dual-organ invasion had a shorter RFS and OS time than those with single-organ invasion. Organ invasion should be included as one of the factors that determine the AJCC stage; this might ultimately help to predict better the survival rate of patients with DBD cancer.
Similar content being viewed by others
Introduction
Beginning in the 7th American Joint Committee on Cancer (AJCC) staging manual, distal bile duct (DBD) cancer and perihilar bile duct (PBD) cancer were staged separately based on biological behaviors. The stages remain separated in the new, 8th AJCC staging manual. The 8th AJCC staging manual adopts different T criteria for PBD and DBD cancer. In the revised criteria, the T stage for DBD cancer is based on depth of invasion (DOI): T1, <5 mm; T2, 5–12 mm; T3, >12 mm1. In addition, N stage is classified according to the number of metastatic lymph nodes: N0, no metastatic regional lymph nodes; N1, 1–3 metastatic regional lymph nodes; N2, >3 metastatic regional lymph nodes2. One of the main changes in the 8th AJCC staging manual is the exclusion of tumour infiltration of adjacent organs such as the pancreas, duodenum, and gallbladder. In DBD, a unique, complex anatomy comprising various organs could provide very important information to classify AJCC stage. To define tumour stage, the previous 7th AJCC staging system considered the association between the tumour and surrounding organs, which have particular, complex anatomic structures. The 8th AJCC staging system referred to the millimeter-based criteria in patients collected from a single institution, but it was modified without considering the anatomical specificity3. Furthermore, cut- off value was determined in patients with both DBD or PBD cancer and did not take into account the different biological characteristics between DBD and PBD cancer. With the exception of adjacent organ invasion, controversy remains regarding other aspects of the 8th AJCC tumour staging as prognostic predictors in patients with DBD cancer.
The AJCC staging system according to adjacent organ invasion is still practically used in various cancers. Even in PBD cancer, criteria based on adjacent structures is still being used to determine the 8th AJCC staging. The adjacent organ invasion might still provide important information for determining advanced-stage DBD cancer with successful tumour removal, although it was excluded from the T criteria, which only consider DOI. In the previous 7th T criteria of the DBD cancer, the T1 and T2 stages were defined as “Tumour confined to the bile duct” and “tumour invading beyond the wall of the bile duct”, respectively, whereas the T3 stage included organ invasion, such as the gallbladder, pancreas, duodenum, or other adjacent organs. Nevertheless, an inadequate number of validation studies was the main reason that organ invasion was excluded from the 8th AJCC staging system. Previously published data were reported based on the results of analyses of both DBD and PBD cancer, because both cancers were categorized as “extrahepatic bile duct cancer” until the 6th edition of the AJCC staging manual1,4,5,6,7,8. Past studies on the relationship between patient survival and organ invasion had inadequate numbers of study participants, which is why they have little prognostic significance9,10.
We validated the prognostic impact of the 8th and 7th AJCC staging system in 374 patients with DBD cancer. We investigated the survival rate according to the number of infiltrating organ to further enhance prognostic accuracy. The aims of this study were to evaluate the association between adjacent organ invasion and relapse-free survival (RFS) and overall survival (OS) after curative surgical resection of DBD cancer and to suggest supplementary criteria for predicting clinical outcomes.
Results
Clinicopathological Characteristics
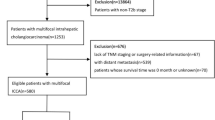
At the time of surgery, the patients had the following T and N criteria: T1, 142 (37.6%); T2, 186 (49.2%); and T3, 50 (13.2%); N0, 196 (51.9%); N1, 154 (40.7%); N2, 28 (7.4%). Two hundred four patients had associated adjacent organ invasion, including invasion of the pancreas, duodenum, and gallbladder. One hundred fifty-two and 52 patients had single- and dual-organ invasion, respectively (Fig. 1). The distribution of AJCC stage was as follows: I, 94 (24.9%); IIA, 136 (36%); IIB, 120 (31.7%); and IIIA 28 (7.4%). Among 378 patients, 262 (69.3%) died during the follow-up period (median survival, 28 months; range, 4–195 months). Two hundred thirty (60.8%) had recurrence: 152 (40.2%) patients with local recurrence and 78 (20.6%) patients with new lymph node metastasis or new distant organ metastasis.
Comparisons of Survival Rate According to Clinicopathological Parameters
Of 378 patients, the OS rates were as follow: 312 patients (82.5%) at 1 year, 197 (52.1%) at 3 years, and 164 (43.4%) at 5 years. The survival rate was lower with higher AJCC stages. However, there was no survival difference between stage I and IIA, between stage IIB and IIIA, between T2 and T3 or between N1 and N2, according to the AJCC stage. According to 7th AJCC stage, there was no survival difference between stage IB and IIA, between T2 and T3 (Fig. 2).
Kaplan–Meier survival curve according to the 8th and 7th AJCC staging system and T and N criteria. According to 8th AJCC (A–C), the survival curve shows no survival difference between AJCC stage I versus IIA, AJCC stage IIB versus III, T2 versus T3 and N1 versus N2 (p = 0.169, 0.426, 0.164 and 0.138 respectively). According to 7th AJCC (D–F), the survival curve shows no survival difference between AJCC stage IB versus IIA and T2 versus T3 (p = 0.942 and 0.834, respectively). There are relative differences of survival among the remaining groups.
Other clinicopathological parameters such as gross type (HR, 1.67; 95% CI, 1.21–2.32; P = 0.002), histological grade (HR, 2; 95% CI, 1.48–2.7; P < 0.001), pancreatic invasion (HR, 1.33; 95% CI, 1.04–1.7; P = 0.021), duodenal invasion (HR, 1.94; 95% CI, 1.41–2.67; P < 0.001) lymphovascular invasion (HR, 1.42; 95% CI, 1.11–1.81; P = 0.005), perineural invasion (HR, 1.72; 95% CI, 1.3–2.27; P < 0.001), and margin status (HR, 1.42; 95% CI, 1.07–1.89; P = 0.015) were significantly related to OS. (Table 1)
Comparisons of Clinicopathological Parameters in Patients with and without Organ Involvement
Of all 378 patients, 204 patients had adjacent organ invasion. Patients with organ invasion had a significantly higher incidence of infiltrative gross type, poorly histological grade, lymphovascular invasion, perineural invasion and margin involvement, compared to those without organ invasion (all P < 0.05) (Table 2).
Comparisons of Clinicopathological Parameters Between Single- and Dual-organ Invasion
Of 204 patients with adjacent organ invasion, 152 patients had adjacent single-organ invasion as follows: pancreas, 146; duodenum, 4; gallbladder, 2. Fifty-two patients had dual-organ invasion as follows: pancreas and duodenum, 51; duodenum and gallbladder, 1. Patients with dual-organ invasion had a significantly higher incidence of infiltrative gross type and advanced N stage, compared to those with single-organ invasion (all P < 0.05) (Table 3).
Survival Difference Between Single- and Dual-Organ Invasion in 204 Patients
The RFS time was as follows: For patients with no organ invasion, the median survival was 27 months (recurrent rate: 51.7%, 90/174), for single-organ invasion, the median survival was 23 months (66.4% 101/152), and for dual-organ invasion, the median survival was 13 months (75%, 39/52). The OS time was as follows: For patients with no organ invasion, the median survival was 35 months (mortality rate: 61.5%, 107/174), for single-organ invasion, the median survival was 29 months (73%, 111/152) and for dual-organ invasion, the median survival was 19 months (84.6%, 44/52).
In univariate analyses of 202 patients with organ invasion, the RFS (HR, 1.65; 95% CI, 1.14–2.4; P = 0.008) and OS (HR, 1.87; 95% CI, 1.31–2.66; P = 0.001) were significantly different between patients with single- and dual-organ invasion (Fig. 3). Other factors including histological grade (RFS: HR, 1.89; 95% CI, 1.28–2.77; P = 0.001; OS: HR, 1.73; 95% CI, 1.19–2.52; P = 0.004) and N criteria (RFS: HR, 1.71; 95% CI, 1.22–2.39; P = 0.002; OS: HR, 1.59; 95% CI, 1.15–2.19; P = 0.005) were also associated with a worse RFS and OS. In multivariate analysis (confounding factors: gross type histological grade, T criteria, N criteria, lymphatic/perineural invasion and margin status), there was a significant difference in RFS (HR, 1.67; 95% CI, 1.12–2.49; P = 0.013) and OS (HR, 1.95; 95% CI, 1.32–2.87; P = 0.001), between patients with single- and dual-organ invasion. In addition, higher histological grade (RFS: HR, 2.25; 95% CI, 1.5–3.39; P < 0.001; OS: HR, 2; 95% CI, 1.32–2.92; P = 0.001) and advanced N stage (RFS: HR, 1.73; 95% CI, 1.18–2.53; P = 0.005; OS: HR, 1.47; 95% CI, 1.03–2.1; P = 0.036) remained associated with poor RFS and OS (Table 4).
Discussion
In the new 8th AJCC staging manual, cholangiocarcinoma is classified based on its anatomic location into three subtypes: intrahepatic, perihilar, and distal portion. Importantly, DBD cancers comprise 20–30% of all cholangiocarcinoma and are clinically silent, with symptoms only developing at an advanced stage11. Beginning in the 7th edition, and continuing in the 8th edition, the T and N criteria are different for PBD and DBD and are based on their distinct biological behavior, natural course, and therapeutic plan11,12. In particular, the factors that were specifically associated with patient survival were DOI, nodal metastasis, lymphatic/perineural invasion as well as pancreatic invasion, and resection margin involvement, to name a few1,4,5,6.
Interestingly, among the above prognostic indicators, DOI and number of metastatic regional lymph nodes were applied in the new 8th T and N criteria for DBD cancer, because the previous 7th AJCC staging was described as having vague T criteria resulting in wide inter-observer variation. Therefore, the 8th AJCC staging system suggested a cut-off value of DOI measured in millimeters to reduce inter-observer variation1,13,14. To determine the stage of tumours in various organs (lip and oral cavity, cervix uteri, vulva, and melanoma of the skin), DOI was adopted in the 8th AJCC staging system. These organs have simple anatomical structures and are relatively distant from tumour-adjacent organs. In other words, a long distance from the tumour origin inhibits direct tumour infiltration into other organs. However, the DBD is located near various organs and has a relatively complex anatomical structure. In organs that are close to tumour origins, such as the nasal cavity, paranasal sinus, and larynx, the T criteria are classified based on direct tumour infiltration into adjacent organs. A study of DBD cancer showed that the presence or absence of adjacent organ invasion was associated with a significant difference in survival9. Nevertheless, for DBD cancer, the 7th AJCC staging manual categorizes T criteria based on adjacent organ invasion, but organ invasion is no longer described in the 8th AJCC T criteria, especially T3 stage3. A study by Ebata et al. showed that presence or absence of adjacent organ invasion created a significant difference in survival9. In our results, patients with organ invasion show lower RFS and OS than those without organ invasion. Notably, there were significant differences of RFS or OS between single- and dual-organ invasion. However, there was little survival difference when the 8th AJCC T criteria were adopted for DBD cancer. An explanation for this is that the interval of invasion depth among T1, T2, and T3 groups was widened. In our study, the categories of DOI were as follows: no invasion, 4.5 mm; single-organ invasion, 8.2 mm; dual-organ invasion, 10.7 mm.
The recommended 8th AJCC T criteria for DOI are 5–12 mm and >12 mm in the T2 and T3 groups, respectively. Hong et al. performed a study of 222 patients who underwent surgery at a single center and whose tumours included both perihilar and/or distal tumours (perihilar, 111 cases; distal 101 cases; perihilar and distal, 10 cases)3. In survival models to determine the cut-off value of DOI, only 101 patients had DBD cancer, whereas 110 patients had PBD cancer. The cut-off values for the measured DOI were calculated in both perihilar and DBD cancers without considering their distinct biological behaviors11,12. In a validation study, there was no survival difference between groups (T2 versus T3)15. Recently, a multicenter study of 179 patients with only DBD cancer was designed using a smaller range of DOI and revealed a significant survival difference among groups (<3 mm, 4–10 mm, >11 mm)13. Thus, controversy exists regarding the relationship between clinical outcome and the criteria for DOI.
In summary, patients with dual-organ invasion showed lower survival rate than patients with single-organ invasion in DBD cancer, although there is no survival difference between the T2 and T3 groups based on DOI defined by the 8th AJCC staging system. In the prognosis prediction with advanced T groups, adjacent organ invasion could enhance prognostic accuracy. Consequently, the significant difference in survival between single- and dual-organ invasion could be considered to supplement the T criteria using DOI to guide therapy and standardize the 8th AJCC staging system.
Materials and Methods
Case Selection
Tumour with their center located between the confluence of the cystic duct and common hepatic duct and the Ampulla of Vater (excluding ampullary cancer) are considered DBD cancer in reference with 8th AJCC stage. A total of 404 cases of patients diagnosed with DBD cancer at multi-institutions (Eulji Hospital, Kangbuk Samsung, Hanyang Guri, Hallym University Sacred Heart Hospital, Gangneung Asan) in Korea between January 1, 1996 and December 31, 2013 were collected for this study. This study included data obtained from previously conducted research study13. As for twenty-six patients who died within 90 days after surgery or who had few representative slide for microscopic review were excluded from this study.
The following clinicopathological parameters were recorded: age, gross type, histological grade, size, 8th AJCC stage, lymph node metastasis, adjacent organ invasion (pancreas, duodenum, gallbladder), lymphovascular invasion, perineural invasion, margin involvement, relapse, and survival. Grossly, the tumours were classified as papillary, nodular, and infiltrative, and the tumour size was measured along its greatest dimension. Hematoxylin and eosin-stained slides with representative tumour section were reviewed by at three pathologists (KWM, DHK, EKK). The DOI from the basal lamina of the adjacent normal epithelium to the most deeply advanced tumour cells was measured in reference with previous study3.
The mean and median age of the remaining 378 patients was 63 and 64 years, respectively. The male to female ratio was 253:125. The surgical treatment included the Whipple procedure in 153 (40.5%), pylorus-preserving pancreaticoduodenectomy in 125 (33.1%), and extended bile duct resection in 101 (26.4%) patients. In multi-institutions, the indication of surgical procedure for DBD cancer was as follows: pylorus-preserving pancreaticoduodenectomy was performed on patients with (a) no evidence of tumour extension to the pylorus, (b) chances to preserve pylorus artery and (c) no ulcer in pylorus. Whipple’s operation was conducted when a patient did not belong to the above PPPD indication. Extended bile duct resection was conducted when (a) tumour was positioned at mid-portion and did not invade adjacent organs and (b) safety margin is confirmed by frozen section of tumour.
Statistical Analysis
Correlations between clinicopathological parameters and adjacent organ invasion were analysed using the Chi-square test and the linear-by-linear association. Relapse-free survival is defined as the time elapsed from the date of treatment to the date of progression such as a local recurrence, new lymph node metastasis or distant organ metastasis. Overall survival was defined as the time from the date of treatment to cancer-related death. Relapse-free survival (RFS) and overall survival (OS) curves were generated using the Kaplan-Meier method and were compared using the Log Rank test. Multivariate analysis was performed to confirm independent prognostic factors for patient survival using a Cox proportional hazard model. A 2-tailed P value of <0.05 was considered statistically significant. All data were analysed using SPSS statistics software (version 20.0, Chicago, IL, USA) and R packages (http://www.r-project.org/).
Ethics Approval
This study (involving human participants) was approved by the Ethics Committee of the Eulji Hospital (EMCIRB-2016-10-001) and performed with respect to the ethical standards of the Declaration of Helsinki, as revised in 2008. The IRB review confirmed that the informed consent is not necessary in this study.
Change history
10 August 2018
A correction to this article has been published and is linked from the HTML and PDF versions of this paper. The error has been fixed in the paper.
References
Hong, S. M. et al. Depth of tumor invasion better predicts prognosis than the current American Joint Committee on Cancer T classification for distal bile duct carcinoma. Surgery 146, 250–257, https://doi.org/10.1016/j.surg.2009.02.023 (2009).
Kiriyama, M. et al. Prognostic impact of lymph node metastasis in distal cholangiocarcinoma. Br J Surg 102, 399–406, https://doi.org/10.1002/bjs.9752 (2015).
Hong, S. M., Cho, H., Moskaluk, C. A. & Yu, E. Measurement of the invasion depth of extrahepatic bile duct carcinoma: An alternative method overcoming the current T classification problems of the AJCC staging system. Am J Surg Pathol 31, 199–206, https://doi.org/10.1097/01.pas.0000213384.25042.86 (2007).
Ito, K. et al. Adequate lymph node assessment for extrahepatic bile duct adenocarcinoma. Ann Surg 251, 675–681, https://doi.org/10.1097/SLA.0b013e3181d3d2b2 (2010).
Murakami, Y. et al. Prognostic significance of lymph node metastasis and surgical margin status for distal cholangiocarcinoma. J Surg Oncol 95, 207–212, https://doi.org/10.1002/jso.20668 (2007).
Woo, S. M. et al. Recurrence and prognostic factors of ampullary carcinoma after radical resection: comparison with distal extrahepatic cholangiocarcinoma. Ann Surg Oncol 14, 3195–3201, https://doi.org/10.1245/s10434-007-9537-y (2007).
Murakami, Y. et al. Pancreatoduodenectomy for distal cholangiocarcinoma: prognostic impact of lymph node metastasis. World J Surg 31, 337–342; discussion 343-334, https://doi.org/10.1007/s00268-006-0224-0 (2007).
Yoshida, T. et al. Prognostic factors after pancreatoduodenectomy with extended lymphadenectomy for distal bile duct cancer. Arch Surg 137, 69–73 (2002).
Ebata, T. et al. Pancreatic and duodenal invasion in distal bile duct cancer: paradox in the tumor classification of the American Joint Committee on Cancer. World J Surg 31, 2008–2015, https://doi.org/10.1007/s00268-007-9173-5 (2007).
Ebata, T. et al. Hepatectomy with portal vein resection for hilar cholangiocarcinoma: audit of 52 consecutive cases. Ann Surg 238, 720–727, https://doi.org/10.1097/01.sla.0000094437.68038.a3 (2003).
DeOliveira, M. L. et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 245, 755–762, https://doi.org/10.1097/01.sla.0000251366.62632.d3 (2007).
Nakeeb, A. et al. Cholangiocarcinoma. A spectrum of intrahepatic, perihilar, and distal tumors. Ann Surg 224, 463-473; discussion 473-465 (1996).
Min, K. W. et al. Invasion Depth Measured in Millimeters is a Predictor of Survival in Patients with Distal Bile Duct Cancer: Decision Tree Approach. World J Surg, https://doi.org/10.1007/s00268-016-3687-7 (2016).
Hong, S. M. et al. Analysis of extrahepatic bile duct carcinomas according to the New American Joint Committee on Cancer staging system focused on tumor classification problems in 222 patients. Cancer 104, 802–810, https://doi.org/10.1002/cncr.21236 (2005).
Moon, A., Choi, D. W., Choi, S. H., Heo, J. S. & Jang, K. T. Validation of T Stage According to Depth of Invasion and N Stage Subclassification Based on Number of Metastatic Lymph Nodes for Distal Extrahepatic Bile Duct (EBD) Carcinoma. Medicine (Baltimore) 94, e2064, https://doi.org/10.1097/MD.0000000000002064 (2015).
Acknowledgements
We are grateful to Ina Jeong, an English teacher, for helping to edit this manuscript. This work was supported by SAMJIN PHARM (IIT-13-01) in 2016.
Author information
Authors and Affiliations
Contributions
Min, Kim and Son had full access to all the data in the study and take responsibility for the integrity of the and the accuracy of the data analysis. Acquisition, analysis, or interpretation of data: Min, Kim, Moon, Oh, Kwon, Choi. Drafting of the manuscript: Min, Kim. Critical revision of the manuscript for important intellectual content. Min, Kim, Son. Statistical analysis: Min, Kim. Study supervision: Min, Son.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Min, KW., Kim, DH., Son, B.K. et al. Dual-organ invasion is associated with a lower survival rate than single-organ invasion in distal bile duct cancer: A multicenter study. Sci Rep 8, 10826 (2018). https://doi.org/10.1038/s41598-018-29205-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29205-z
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.