Abstract
Chemical pesticides are widely used around the world, but at the same time, they may cause direct or indirect risks to many non-target organisms. Recent increased use of insecticides in coastal areas, for example to control invasive tawny crazy ants, raises concern that insecticides may affect ecologically and/or commercially important species found in estuaries. Here, we investigated the lethal and sub-lethal effects of fipronil on juvenile brown shrimp Farfantepenaeus aztecus over 29 days at five different nominal concentrations (0.1, 1.0, 3.0, 6.4, and 10.0 µg/L) in a laboratory experiment. Exposure to all of the fipronil treatments resulted in all individuals dying before the end of the experiment; whereas, no individual died in the control (0.0 µg/L). The 96-hour LC50 was determined to be 1.3 µg/L. Shrimp also experienced weight loss under all of the fipronil treatments. Inter-moult interval was increased from 12.2 ± 1.64 day in the control group to 15.5 ± 0.53 day in the 1.0 μg/L treatment. Lipid content of shrimp increased significantly in a concentration-dependent manner. Finally, behavioral and body color changes were also observed under the fipronil treatments. We conclude F. aztecus is very sensitive to fipronil and monitoring is needed in coastal areas.
Similar content being viewed by others
Introduction
Chemical pesticides are commonly used for both agricultural and household purposes world- wide to control pests. However, they are known to have negative side effects on non-target organisms, including terrestrial organisms such as birds1,2,3 and insects4,5,6 as well as aquatic organisms such as fish7,8,9 and arthropods10,11. A rapid increase in pesticides use in recent years has resulted in enormous pressure on the ecosystems12,13. Pesticides are expected to have a much higher effect on the aquatic environments compared with terrestrial environments, because water bodies are the eventual recipients of these chemicals14. The adverse effects of chemical pesticides may be lethal (acute) or sub-lethal (chronic), and the effects can vary depending on species15. However, the majority of ecotoxicological studies have focused on the investigation of their lethal effects, neglecting sub-lethal effects. These studies also focus on a few selected model organisms, neglecting effects on other non-target organisms, which may play an important role for ecosystem functions and/or are important for commercial purposes16,17,18.
Currently, fipronil is considered one of the most effective phenylpyrazole insecticides, which are used widely, and it is considered to affect arthropods selectively19,20. It is used increasingly for the protection of crops such as rice, corn, cotton, potatoes, turnips, and rutabagas from herbivorous insects and for controlling ticks and fleas on animals13,21,22. In particular, the use of fipronil has increased in the U.S.A. in recent years in many different states such as California, Louisiana, South Carolina, and Texas. For example, the United States Environmental Protection Agency (U.S. EPA) issued a quarantine exemption to the Texas Department of Agriculture in 2016, allowing the expanded use of fipronil in southeastern counties of Texas to control tawny crazy ants Nylanderia fulva (Fig. 1)23.
Fipronil can flow into creeks, rivers, and estuaries because it is mobile in soils and soluble in water24. Many recent studies have demonstrated the occurrence of fipronil and its degradation products, which have the same or greater toxic properties and are more stable than fipronil itself21,25,26,27, in the aquatic environment at levels ranging between 0.001–10.004 µg/L, often exceeding the acute level (0.1 µg/L) of fipronil in the aquatic life benchmark of the U.S. EPA22,28,29,30,31. A nationwide survey from 2002 to 2011 conducted by Stone et al.32 found that fipronil concentrations exceeded its chronic aquatic life benchmark concentration (0.01 µg/L) in about 70% of 125 monitored streams sometime during the survey.
Several studies tested the toxicity of fipronil on non-target aquatic crustaceans such as blue crab Callinectes sapidus33, Chinese mitten crab Eriocheir sinensis and giant river prawn Macrobranchium rosenbergii34, red swamp crayfish Procambarus clarkia35,36, white river crayfish Procambarus zonangulus35, grass shrimp Palaemonetes pugio37,38, water flea Daphnia pulex39, and estuarine mysid shrimp Americamysis bahia24. However, the number of species studied is still limited, and most focused on lethal effects.
The aim of this study was to investigate both lethal and sub-lethal effects of fipronil on the brown shrimp Farfantepenaeus aztecus. F. aztecus is one of the most important commercial fishery species in the U.S., found along the Atlantic coast of the southeastern United States and in the Gulf of Mexico (GOM)40,41 with a commercial landing value of $166,542 million in 201642. They are especially abundant along the coasts of Texas and Louisiana, U.S.A. In addition to their economic importance, brown shrimp play an important ecological role for supporting other species41,43,44. They are estuarine-dependent during a juvenile stage40,41; this potentially exposes them to pesticides that are used on land, because their residues end up in the runoff. The effects of fipronil on penaeid shrimp such as brown shrimp are particularly a concern because of its increased use in coastal communities. In this study, we estimated the effects of fipronil on survivorship, weight gain, inter-moult interval, behavioral changes, and body chemical composition under different nominal concentrations in controlled conditions. The concentrations were selected based on those previously reported for the aquatic environment. We also determined the nominal median lethal concentration (LC50) and the nominal median lethal time (LT50) of fipronil. These results will fill our knowledge gap in potential effects of fipronil on estuarine crustacean.
Results
Water quality
Mean values of water quality parameters were the following: temperature, 20.84 ± 0.24 °C; salinity, 16.20 ± 0.10‰; pH, 8.69 ± 0.15; and dissolved oxygen (DO), 5.67 ± 0.24 mg/L (Table 1). There were no significant differences among treatments for all water quality parameters measured during the experiment, which lasted 29 days, and all of them were within appropriate ranges of the environmental requirements of shrimp45.
Survival, median lethal time (LT50), and acute toxicity test (LC50)
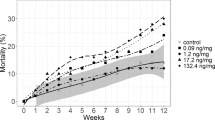
Our results showed that survival of juvenile shrimp decreased significantly with fipronil concentration, from 0.1 µg/L to 10.0 µg/L (Kaplan-Meier survival curve analysis followed by the non-parametric Log-Rank test, P < 0.0001) as shown in Table 2 and Fig. 2. Starting from week 1, significant differences were detected between a control treatment (with survival rate of 100%) and all other fipronil treatments except 0.1 µg/L treatment, which showed a survival rate of 72.2% over the week. After week 1, all treatments were significantly different from the control (Kaplan-Meier survival curve analysis followed by the non-parametric Log-Rank test, P < 0.0001).
Under the higher concentrations of fipronil (6.4 µg/L and 10 µg/L), shrimp showed faster reduction in survivorship where all individuals died by day 8 and day 4, respectively. Under lower fipronil concentrations (0.1 µg/Land 1.0 µg/L), survivorship declined with time at a slower rate and all individuals died by day 28 and 23, respectively. Under all concentrations of fipronil, the survival rate of juvenile shrimp over the duration of the experiment was 0.0% (Kaplan-Meier survival curve analysis followed by the non-parametric Log-Rank test, P < 0.0001). In comparison, none of the shrimp died in any replicate under the control treatment (0.0 µg/L).
The median lethal time LT50 (the time required for 50% of the animals to die at a particular exposure concentration, and also called median time to death) of juvenile shrimp under fipronil treatments ranged between 1.66 ± 0.57 day in the 10.0 µg/L treatment to 6.66 ± 3.51 day in the 0.1 µg/L treatment. One-way Analysis of Variance ANOVA (P < 0.05) showed that all treatments were significantly different from the control which showed no mortality among the shrimp during the experiment (Table 2). Fipronil 96-h LC50 (lethal concentration to reach 50% mortality within 96 hours) of juvenile shrimp was 1.3 µg/L with the 95% confidence interval ranging from (1.0 to 1.5). Table 3 compares the results from this study with those from previous studies obtained by others.
Weight gain and growth rate
At the beginning of the experiment, there was no significant difference in the initial weight among the treatments; initial weight of shrimp ranged between 0.78 ± 0.08 g in the 1.0 µg/L treatment and 0.82 ± 0.08 g in the 6.4 µg/L treatment (Table 4).
The final weight in Table 4 was calculated for each treatment by taking the final weight measured before the death of all shrimp. However, the week of death of the last shrimp was different among the treatments. For example, the final weights of the 0.1 µg/L and 1.0 µg/L treatments were measured at the end of week 3; whereas, final weights of 3.0 µg/L and 6.4 µg/L treatments were measured at the end of week 1 of fipronil exposure because all shrimp in these treatments died before reaching week 2. However, final weight and percent weight gain clearly showed the effect of fipronil. In all treatments, a significant reduction in growth was observed after the first week of fipronil exposure (ANOVA, P < 0.05) (Table 4 and Fig. 3a).
(a) Average weight (g wet weight per individual) of juvenile shrimp as a function of the duration of the exposure to fipronil. The horizontal axis represents the experiment period per week (week 0–week 4) while the vertical axis represents the average wet weight (g) per individual shrimp in each treatment. Error bars indicate the standard errors (n = 18). One-way Analysis of Variance (P < 0.05) showed that, after one week of fipronil exposure all treatments were significantly different from the control. (b) Inter-moult intervals of juvenile shrimp exposed to different concentrations of fipronil for 29 days. The horizontal axis represents the six fipronil concentrations (µg/L) including the control used during the experiment while the vertical axis represents time (per day) of the inter-moult intervals of juvenile shrimp. Error bars indicate the standard errors (n = 18). m is the average number of moults of individual shrimp in each treatment. Non- parametric Kruskal-Wallis test followed by the pairwise Wilcoxon rank sum test (P < 0.0001) showed that means in columns not sharing the same letter were significantly different.
The percent weight gain showed significant differences among all treatments (ranging from −21.42 g in the 1.0 µg/L treatment to 2.77 ± 19.64 g in the 3.0 µg/L treatment) and the control (60.19 ± 15.44 g) (ANOVA, P < 0.05). Weight loss occurred (−8.97, −21.42, and −16.87 ± 29.57 g) in three concentrations (0.1, 1.0, and 6.4 µg/L, respectively), indicating the final weight was less than the initial weight under all of these treatments. Final weight and percent weight gain in the 10.0 µg/L treatment were not measured because all individuals died during the first days of exposure before measuring the weight in week 1 (Table 4).
Inter-moult interval
Control shrimps showed the highest number of moults per individual (m = 2.6); whereas, the number of moults decreased progressively with fipronil concentration. Inter-moult interval of the control treatment (12.2 ± 1.64 day) was significantly shorter than 0.1 μg/L treatment (14.0 ± 0.85 day) and the 1.0 μg/L treatment (15.5 ± 0.53 day) according to the Kruskal-Wallis rank sum test (P < 0.0001) (Fig. 3b).
We could not calculate the inter-moult intervals for shrimp in treatments of higher fipronil concentrations (3.0, 6.4, and 10.0 μg/L) because they died before they have two consecutive moults of any individual during the experiment.
Behavioral and physical changes
Shrimp under high fipronil concentrations (3.0, 6.4, and 10.0 µg/L) showed behavioral changes after only one day. These changes were observed in the following order: (1) shrimp in these treatments started moving in circles with no control on their movements; (2) shrimp stopped moving in circles and sprawled on their sides or backs on the bottom of the aquarium with only their swimming legs moving in continuous involuntary movements; and (3) shrimp stopped moving their swimming legs and died. All of these abnormal swimming and feeding behaviors were recorded to compare them with shrimp in the control treatment. It is important to note that during all of these stages of abnormal behaviors, shrimp were not able to feed effectively. This was clearly observable under low fipronil concentrations (0.1 and 1.0 µg/L) in which shrimp survived for a longer period and exhibited the behavioral changes progressively and slowly.
The visual examinations of the physical changes in shrimp bodies at the end of the experiment indicated a clear difference in their body color. Figure 4 shows shrimp body color gradient from bright color of shrimp in the control (0.0 µg/L) to gray and dark color of shrimp in the 0.1 µg/L and 1.0 µg/L treatments. This result indicates that fipronil affected shrimp body color in a concentration-dependent manner.
Analysis of whole-body composition
Figure 5 shows the analysis of protein, lipid, and ash composition (dry basis) of juvenile shrimp in all treatments. Our results revealed that there were some significant differences (ANOVA, P < 0.05) among treatments in all components analyzed. There was an overall significant decrease (ANOVA, P < 0.05) in the percentage of body protein for all treatments compared to the control (0.0 µg/L) which showed the highest level of protein 71.69 ± 0.23%, although there was not a clear trend (Fig. 5a).
Analysis of body composition of juvenile shrimp under different fipronil concentrations. The horizontal axes in (6.a, 6.b, and 6.c) represent the six fipronil concentrations (µg/L) used during the experiment while the vertical axes represent the protein % (in 6.a), lipid % (in 6.b), and ash % (in 6.c) in bodies of juvenile shrimp measured at the end of the experiment. Dashed lines are fitted regression lines. Error bars indicate the standard errors (n = number of samples analyzed from each treatment). One-way Analysis of Variance (P < 0.05) showed that means in columns not sharing the same letter are significantly different.
The ANOVA (P < 0.05) of lipid percentage indicated that there was no difference among treatments with the five lower concentrations, including the control. Similarly, there was no difference among treatments (ANOVA, P < 0.05) with the four higher concentrations (Fig. 5b). However, a linear regression analysis (P = 0.0017) indicated that lipid percentage increased significantly with increasing concentration of fipronil (see Supplementary Fig. S1).
For ash percentage, our analysis showed that the differences among groups in this case appear to be random and not associated with the insecticide exposure; although, control treatment had the lowest ash percentage (15.78 ± 0.53%) and differed significantly (ANOVA, P < 0.05) from most of the treatments (Fig. 5c).
Discussion
Fipronil is known to cause lethal and sub-lethal effects on non-target invertebrates in both aquatic and terrestrial ecosystems46. However, studies are often conducted with a limited number of model organisms. Consequently, there is a gap of knowledge in the effects on a large number of non-target invertebrates, especially from coastal and marine ecosystems47. Fipronil desulfinyl (a photodegradation product of fipronil) was detected in the eggs of the Atlantic blue crab Callinectes sapidus off the coast of South Carolina (the Eastern coast of the United States), and it may be one of the causes of C. sapidus decline to the lowest historical levels over the past decade33. In Texas, fipronil has been reported in several recent studies conducted by the U.S. Geological Survey (USGS) and U.S. EPA in different cities including Houston-Galveston48, Austin49, San Antonio50, and College Station51, in concentrations ranging between 0.021 μg/L and 0.075 μg/L. All of these studies have reported the detection of fipronil and two or more of its degradation products (i.e., fipronil sulfide, fipronil sulfone, desulfinylfipronil, and desulfinylfipronil amide) in surface water and urban streams in levels exceeding the chronic level of the U.S. EPA aquatic life benchmark for invertebrates (0.01 μg/L). To the best of our knowledge, this study is the first to report the effects of fipronil on commercially and ecologically important penaeid shrimp F. aztecus.
All nominal fipronil concentrations tested in this study were within the range of concentrations found in the environment by other researchers in streams, rivers, and estuaries in the U.S. and other countries22,30,52,53 (see Supplementary Table S1). Our results showed fipronil caused significant lethal and sub-lethal effects on juvenile F. aztecus. Results also showed that survival of shrimp was concentration-dependent (Table 2 and Fig. 2). All individuals died during the 29 days of exposure under all the fipronil concentrations tested; whereas, no individual died in the control. The nominal 96-h LC50 of fipronil for juvenile F. aztecus was estimated at 1.3 μg/L. This result suggests F. aztecus have an intermediate sensitivity to fipronil among marine invertebrates, but they are far more sensitive than freshwater invertebrates studied so far (Table 3).
In our study, final weight and percent weight gain of shrimp showed significant differences (P < 0.05) between the control and all other concentrations (Table 4 and Fig. 3a). Growth reduction of aquatic arthropods under the exposures to toxicants also has been reported in other studies with sand shrimp Metapenaeus ensis54 and freshwater crayfish Cherax quadricarinatus55. A similar reduction in body growth of F. aztecus has been documented by Rozas et al.56, who found the reduction in the growth of juvenile F. aztecus and white shrimp Litopenaeus setiferus held for 7 days in field mesocosms contaminated with the nonlethal concentrations of petroleum hydrocarbons from an oil spill. On the contrary, Goff et al.33 found that juvenile blue crabs Callinectes sapidus exposed to different nominal concentrations of fipronil and fipronil desulfinyl resulted in significant increases in growth in all treatments compared to controls in a short-term (96-h) experiment.
There are several reasons that may explain the decrease in the growth of juvenile F. aztecus in our study. For instance, animals affected by environmental stressors, such as the chemical toxicants, utilize the energy in the detoxification processes, thus affecting the metabolism of protein and carbohydrate and eventually growth performance55. Shrimp derive energy more expeditiously from protein than from lipids and carbohydrate57; therefore, exposing F. aztecus to fipronil may have resulted in a reduced protein level in exposed shrimp compared with those in the control (Fig. 5a), which might have, in turn, reduced growth (Fig. 3a). On the other hand, fipronil is a phenylpyrazole insecticide, which acts by blocking the chloride channels, disrupting the central nervous system activity13, which may have inhibited feeding activity of juvenile F. aztecus under exposure.
Moulting is one of the important physiological processes for arthropods allowing them to grow normally58,59. Because moulting in crustaceans is mainly controlled by the interaction of moult-stimulating hormones (ecdysteroids), moult-inhibiting hormones (produced in the eyestalks), and nervous system secretions, endocrine disrupting chemicals, including fipronil38 in our study, are expected to have adverse effects on moulting60. In this study, fipronil affected F. aztecus moulting process in a concentration-dependent manner. Inter-moult intervals of shrimp under the control (12.2 ± 1.64 day) were significantly shorter (P < 0.0001) than those in other fipronil treatments (Fig. 3b). Increased inter-moult intervals suggest the development of shrimp is delayed by exposure to sub-lethal levels of fipronil in water.
Similar delay in moulting has been reported with other arthropods exposed to pesticides. Betancourt-Lozano et al.61 showed significant increase in inter-moult intervals of juvenile Pacific white shrimp Litopenaeus vannamei under the exposure to Tilt (a commercial formulation of the fungicide propiconazole). Snyder & Mulder62 showed delayed moulting of American lobster Homarus americanus larvae exposed to cyclodiene pesticide heptachlor. Baldwin et al.63 reported that juveniles and adults of the freshwater crustacean Daphnia magna exhibited reduced moulting frequency after they were chronically exposed to diethylstilbestrol (DES). Moreover, there are also reports of reduced moulting intervals, for example, with freshwater shrimp Caridina nilotica under exposure to the herbicide Roundup®59 and grass shrimp P. pugio under exposure to sodium pentachlorophenate and Aroclor 124264. These studies suggest potentially complex mechanisms of pesticides affecting the moulting of arthropods.
Behavioral changes are often the first indication of the harmful impacts of pesticides on living organisms, and even at low doses of pesticides, long-term behavioral changes can be observed. This effect is magnified especially if the pesticide exposure occurred during the developmentally critical periods of the organism’s life65. In the present study, behavioral changes were observed under all fipronil concentrations compared with those under the control, starting from day 1 in the high concentration treatments and later in lower concentration treatments. Change in swimming (mobility) and feeding activities were the main observed changes. Similar results have been reported by other researchers. For example, Stratman et al.27 showed that the chironomid midge Cricotopusle betis Sublette exposed to fipronil exhibited abnormal behaviors, movement restriction, and feeding reduction. Overmyer et al.66 observed abnormal behavior and muscle control in the aquatic insect Simulium vittatum under all fipronil concentrations tested in the study.
Color changes were clearly observed in both the exoskeleton and abdominal muscle (Fig. 4). Because the body color of shrimp under 1.0 µg/L fipronil (Fig. 4e,f) were darker than those under 0.1 µg/L fipronil (Fig. 4c,d), which were, in turn, darker than that in the control (Fig. 4a,b), we concluded that the effect of fipronil on the color of juvenile F. aztecus was concentration-dependent. In crustaceans, and especially shrimp, many environmental factors are known to affect body color by affecting pigment dispersion (movement) within the chromatophores67. However, we note that the factors that are known to have an effect on body color of shrimp, such as temperature, light intensity, and background color, were carefully controlled in our study (Table 1). Body color in shrimp is often considered a sign of shrimp health, and consequently, influencing its commercial value68; for the same reason, color change in crustaceans, which is a hormonally-regulated process, can be used as a biomarker of environmental health64.
Some changes in body chemical compositions were observed under the exposure to fipronil in our study. A linear regression analysis showed a significant increase (P = 0.0017) in lipid percentage with fipronil concentration (see Supplementary Fig. S1). Similar results were found with juvenile mud crab Rhithropanopeus harrisii exposed to the insecticide fenoxycarb69, Pacific white shrimp L.vannamei exposed to oxytetracycline (OTC)70, and freshwater crayfish C. quadricarinatus exposed to glyphosate acid and polyoxyethylenamine (POEA)55, and freshwater amphipod Gammarus pulex exposed to the insecticide imidacloprid71. Protein percentage may also have been affected by fipronil (Fig. 5a). Although the protein percentage under the control (71.69 ± 0.23%) did not differ with those in the 6.4 µg/L treatment (70.84 ± 0.41%), it may be because of the fact that those in higher concentrations died early in the first days of the experiment, and they did not have enough time to exhibit a measurable reduction in protein percentage. Both protein and lipid metabolism are potentially affected by detoxification process55. If so, we would expect the effects to be concentration-dependent. However, they are also affected by the duration of exposure and feeding rate, which are also affected by toxicants. Further studies are needed for determining the existence of effects of fipronil on body chemical composition as well as potential mechanisms.
Conclusion
Results of the present study revealed that the insecticide fipronil under concentrations found in the environment caused both lethal (acute) and sub-lethal (chronic) effects on F. aztecus juveniles. In particular, we found the effects on shrimp survival, growth (weight and moulting), swimming (mobility), feeding behavior, exterior appearance (body color), and body composition. Because of the detection of fipronil in estuarine waters, expected increased use of fipronil in areas adjacent to estuarine and coastal areas in the U.S.A and other countries, degradation of fipronil in the environment to multiple metabolites that pose equal or greater toxicity than fipronil itself, the high possibility of fipronil bioaccumulation in non-target organisms, and high commercial and ecological value of penaeid shrimp and their sensitivity to fipronil, we recommend the following: (1) monitoring fipronil concentration around the coastal regions in and out of the U.S.A., (2) trying to limit the use of fipronil during the peak periods of shrimp migration to estuaries, (3) investigating the effects of fipronil on different penaeid species in other countries that are using fipronil, and (4) conducting further studies of the effects of fipronil and its major metabolites on other non-target organisms using concentrations below chronic levels established by the U.S. EPA for marine invertebrates.
Materials and Methods
Test organisms and acclimation to laboratory conditions
Juvenile brown shrimp F. aztecus (weight 0.80 ± 0.06 g, total length 5.0 ± 0.67 cm) were collected from Gangs Bayou, Sportsman Road (N 29.25549; W 94.91575) in Galveston Bay, Texas, using a 3-m bag seine (0.6 cm mesh size) on May 6, 2016. Shrimp were transported in 45-liter coolers equipped with air pumps to the laboratory in Texas A&M University, College Station, Texas. After equilibrating water temperature of the transportation coolers with laboratory temperature over approximately 5 hours (see Supplementary Fig. S2a), active shrimp were selected and moved to 53-liter plastic tanks filled with aerated artificial brackish water, which was prepared with dechlorinated tap water and Instant Ocean® Sea Salt (see Supplementary Fig. S2b).
Shrimp were acclimated to laboratory conditions in the tanks for 10 days at temperature, 19.93 ± 0.15 °C; salinity, 15.75 ± 0.16‰; pH, 8.14 ± 0.18; and photoperiod, 12 hour: 12 hour light: dark cycle (see Supplementary Fig. S2a). During the acclimation period, shrimp were fed on API® Bottom Feeder Shrimp Pellets, which fit the nutritional requirements of shrimp72, twice a day. The acclimation tanks were cleaned daily to remove feces and uneaten food and approximately 30–40% of water was changed with newly prepared brackish water. At the end of the acclimation period, shrimp were moved to test aquariums to begin the experiment.
Experimental design and water quality parameters
The experiment lasted 29 days from May 17, 2016 to June 14, 2016. The system consisted of 18 glass aquariums (six treatments x three replicates) of 9.5 liter (30.7 × 15.4 × 20.5 cm) (see Supplementary Fig. S2c), one aquarium was treated as one replicate. An aquarium was filled with 7 liters of test solution, equipped with air pumps (Topfin® AIR-8000), and covered with a glass lid to prevent shrimp from escaping. Each aquarium was divided equally into six cells (see Supplementary Fig. S2d), and one individual was assigned to each cell to prevent cannibalism among shrimp and to follow moulting of each shrimp individually73. The divider was made of a polypropylene plate and fiberglass screen (see Supplementary Fig. S2d); both are commonly used for aquaculture purposes. The screen maintained the flow of water, which distributed dissolved oxygen among the cells. Additionally, aquariums were covered from all sides with aluminum foil sheets to minimize the degradation of fipronil due to light exposure during daytime (see Supplementary Fig. S2c). The aquariums were placed randomly in three rows. During the experiment, shrimp were fed twice daily. Food amount was adjusted according to the body weight, which was measured weekly, based on the published feeding tables for shrimp72. Dissolved oxygen concentration (mg/L), salinity (‰), temperature (°C), and pH were measured every other day using YSI® Professional Plus Multi-parameter Meter.
Insecticide, concentrations and test solutions
Fipronil (5-amino-1-[2, 6-dichloro4-4(trifluoromethyl) phenyl]-4[(trifluoromethyl) sulfinyl]-1H-pyrazole-3-carbonitrile), CAS number 120068-37-3 and purity limit ≥97% (HPLC), was purchased from Fisher Scientific Co. L.L.C., PA, US. Six nominal concentrations, including the control, were used for this experiment: 0.0, 0.1, 1.0, 3.0, 6.4, and 10.0 µg/L. These concentrations were selected based on those previously observed by other researchers in the environment22,28,30,52 (see Supplementary Table S1). Each treatment (concentration) was conducted in triplicate.
The nominal experimental solutions were prepared by making a 1 liter of highly homogenized 100 mg/L fipronil suspension; this suspension was made by mixing 0.1 g of fipronil powder in 1 liter of artificial brackish water using a magnetic stirrer. Then, all of the nominal experimental concentrations (0.1, 1.0, 3.0, 6.4, and 10.0 µg/L) were prepared by diluting specific quantities of 100 mg/L fipronil suspension with artificial brackish water. For example, to prepare 0.1 µg/L fipronil solution, we took 10 ml of 100 mg/L fipronil suspension and mixed it with 990 ml of prepared water to create 1 mg/L fipronil solution, and then, we took 2.1 ml of 1 mg/L fipronil solution and mixed it with 21 liters of prepared water. Dilutions of all nominal experimental concentrations are shown in Supplementary Table S2. For each nominal concentration, 21 liters of fipronil solution was created for three aquariums (replicates). To maintain the fipronil concentrations under all treatments during the experiment, 100% of test solutions were replaced every two days.
Experimental measurements
All assays were conducted using the static-renewal method and according to the guidelines of the U.S. EPA74. The number of shrimp in each replicate and the number of replicates were determined referring to previous studies34,73.
Survival, median lethal time (LT50), and acute toxicity test (LC50)
Survivorship of shrimp was measured by monitoring shrimp movements in the aquariums during feeding periods. Dead shrimp were removed, counted, and weighed. The weight of dead shrimp was used to adjust food amounts for remaining live shrimp. Shrimp were considered dead if they lay down on their side or back with no noticeable movement and they did not make any response (such as jumping, moving their legs, or flipping their tails) after taking them out of water. The dead individuals were placed in a freezer for later body chemical composition analysis. We used survivorship data to estimate the median lethal time (LT50) and also the acute toxicity of fipronil (LC50) on shrimp under 96-h of exposure.
Weight gain and growth rate
Shrimp were weighed every week to observe the effect of fipronil concentrations as well as to adjust the amount of food. Shrimp were weighed individually after gently removing water with paper towel and placed in a beaker with known amount of brackish water. The weekly weight gain of shrimp was calculated using equation (1):
Inter-moult interval
We calculated the inter-moult interval of shrimp under each concentration by counting the number of days between each two consecutive moults of the same individuals. This was possible because we isolated juvenile shrimp in cells (within the same aquarium) and covered the aquarium with a glass lid to prevent the movement of individuals among cells. Then, the date of moulting of each individual was recorded.
Behavioral and physical changes
At each feeding time (morning and afternoon) and also at night, any abnormalities in shrimp activities as well as any changes in physical appearance compared with shrimp in the control were noted and recorded on video.
Analysis of whole-body composition
At the end of the experiments, live shrimp were collected, euthanized by freezing them, and kept in freezer (at −18 °C). Eighteen individuals under each treatment was combined to create two samples. For each sample, dry matter of whole body of shrimp was measured first by accurately weighing 2.0 g of shrimp in a pre-weighed porcelain crucible, placing the samples in an oven at 135 °C for 3 hours75, and weighing them again. Then, porcelain lab mortar and pestle were used to prepare a highly homogenized shrimp powder to be used in subsequent analyses. The crude protein content of shrimp body was determined through Dumas protocol using a LECO protein analyzer to measure total nitrogen as described in76. Lipids were estimated using chloroform/methanol 2:1 extraction method77. Ash was determined by placing dry matter samples in a muffle furnace at 550 °C for 3 hours75.
Statistical analysis
One-way Analysis of Variance (ANOVA) and linear regression were used to test for the significant differences among all treatments compared to the control. In some measurements such as the survivorship and inter-moult interval of shrimp, the data were not normally distributed, and non- parametric tests were used. Kaplan–Meier estimator was conducted to estimate shrimp survivorship followed by the non-parametric Log-Rank test to compare the survival distribution among treatments. Probit analysis described by Finney78 was used to calculate the LC50, using log concentration as dependent variable and probit as independent variable, then we used the parametric bootstrap method to calculate the 95% confidence intervals of the LC50 toxicity test78 (see Supplementary Fig. S3). Non- parametric Kruskal-Wallis test followed by the pairwise Wilcoxon rank sum test were used to test for differences among the means of treatments of the inter-moult intervals. All of these statistical analyses were conducted at significance level α = 0.05 using JMP® Pro 201679 (ANOVA, Kaplan–Meier, and Kruskal-Wallis tests), Matlab R2017a80 (LC50 calculations), and Microsoft Excel 2016 (linear regression test, and to draw all figures).
Data Availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mineau, P. & Palmer, C. The impact of the nation’s most widely used insecticides on birds. American Bird Conservancy, 97 p. (2013).
Goulson, D. Pesticides linked to bird declines. Nature 511, 295–296 (2014).
Hallmann, C. A., Foppen, R. P., van Turnhout, C. A., de Kroon, H. & Jongejans, E. Declines in insectivorous birds are associated with high neonicotinoid concentrations. Nature 511, 341–343 (2014).
Krupke, C. H., Hunt, G. J., Eitzer, B. D., Andino, G. & Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PloS one 7, e29268, https://doi.org/10.1371/journal.pone.0029268 (2012).
Whitehorn, P. R., O’Connor, S., Wackers, F. L. & Goulson, D. Neonicotinoid pesticide reduces bumble bee colony growth and queen production. Science 336, 351–352 (2012).
Krupke, C. H. & Long, E. Y. Intersections between neonicotinoid seed treatments and honey bees. Curr. Opin. Insect. Sci. 10, 8–13 (2015).
Ghisi Nde, C. et al. Evaluation of genotoxicity in Rhamdia quelen (Pisces, Siluriformes) after sub-chronic contamination with Fipronil. Environ. Monit. Assess. 180, 589–599 (2011).
Beggel, S., Werner, I., Connon, R. E. & Geist, J. P. Impacts of the phenylpyrazole insecticide fipronil on larval fish: time-series gene transcription responses in fathead minnow (Pimephales promelas) following short-term exposure. Sci. Total Environ. 426, 160–165 (2012).
Clasen, B. et al. Effects of the commercial formulation containing fipronil on the non-target organism Cyprinus carpio: implications for rice-fish cultivation. Ecotoxicol. Environ. Saf. 77, 45–51 (2012).
Roessink, I., Merga, L. B. & Zweers, H. J. & Van den Brink, P. J. The neonicotinoid imidacloprid shows high chronic toxicity to mayfly nymphs. Environ. Toxicol. Chem. 32, 1096–1100 (2013).
Osterberg, J. S., Darnell, K. M., Blickley, T. M., Romano, J. A. & Rittschof, D. Acute toxicity and sub-lethal effects of common pesticides in post-larval and juvenile blue crabs, Callinectes sapidus. J. Exp. Mar. Biol. Ecol. 424–425, 5–14 (2012).
Tano, Z. J. In Pesticides in the Modern World - Risks and Benefits (ed Margarita Stoytcheva) 129–143 (inTech, 2011).
Simon-Delso, N. et al. Systemic insecticides (neonicotinoids and fipronil): trends, uses, mode of action and metabolites. Environ. Sci. Pollut. Res. 22, 5–34 (2015).
Pritchard, J. B. Aquatic toxicology: past, present, and prospects. Environ. Health Perspect. 100, 249–257 (1993).
Laboy-Nieves, E. N., Schaffner, F. C., Abdelhadi, A. H. & Goosen, M. F. A. Environmental Management, Sustainable Development and Human Health. London, UK. (CRC Press/Balkema) 596 p., (2009).
Abbott, L. C. Selecting optimal animal models to investigate environmental toxicology. Poult. Fish. Wildl. Sci. 1, https://doi.org/10.4172/2375-446X.1000e102 (2013).
Ottinger, M. A. et al. Assessing effects of environmental chemicals on neuroendocrine systems: potential mechanisms and functional outcomes. Gen. Comp. Endocrinol. 190, 194–202, https://doi.org/10.1016/j.ygcen.2013.06.004 (2013).
Shaw, J. R. et al. Daphnia as an emerging model for toxicological genomics. Adv. Exp. Biol. 2, 165–328, https://doi.org/10.1016/s1872-2423(08)00005-7 (2008).
Hainzl, D., Cole, L. M. & Casida, J. E. Mechanisms for Selective Toxicity of Fipronil Insecticide and Its Sulfone Metabolite and Desulfinyl Photoproduct. Chem. Res. Toxicol 11, 1529–1535 (1998).
Chaton, P. F., Ravanel, P., Tissut, M. & Meyran, J. C. Toxicity and bioaccumulation of fipronil in the nontarget Arthropodan fauna associated with subalpine mosquito breeding sites. Ecotoxicol. Environ. Saf. 52, 8–12 (2002).
Wirth, E. F. et al. The effects of the contemporary-use insecticide (fipronil) in an estuarine mesocosm. Environ. Pollut. 131, 365–371 (2004).
Mize, S. V., Porter, S. D. & Demcheck, D. K. Influence of fipronil compounds and rice-cultivation land-use intensity on macroinvertebrate communities in streams of southwestern Louisiana, USA. Environ. Pollut. 152, 491–503 (2008).
USEPA. Exemption of fipronil use in Texas. United States Environmental Protection Agency. Office of Chemical Safety and Pollution Prevention. 2 p (Washington DC. USA, 2016).
USEPA. New pesticide fact sheet: fipronil. United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances. 10 p (Washington DC. USA, 1996b).
Tingle, C. C. D., Rother, J. A., Dewhurst, C. F., Lauer, S. & King, R. P. Fipronil: environmental fate, ecotoxicology, and human health concerns. Rev. Environ. Contam. Toxicol. 176, 1–66 (2003).
Gunasekara, A. S., Truong, T., Goh, K. S., Spurlock, F. & Tjeerdema, R. S. Environmental fate and toxicology of fipronil. J. Pestic. Sci. 32, 189–199 (2007).
Stratman, K. N., Wilson, P. C., Overholt, W. A., Cuda, J. P. & Netherland, M. D. Toxicity of fipronil to the midge, Cricotopus lebetis Sublette. J. Toxicol. Environ. Health, Part A 76, 716–722 (2013).
Gan, J., Bondarenko, S., Oki, L., Haver, D. & Li, J. X. Occurrence of fipronil and its biologically active derivatives in urban residential runoff. Environ. Sci. Technol. 46, 1489–1495 (2012).
Ensminger, M., Budd, R., Kelley, K. C. & Goh, K. S. Pesticide occurrence and aquatic benchmark exceedances in urban surface waters and sediments in three urban areas of California, USA, 2008–2011. Environ. Monit. Assess. 185, 3697–3710 (2013).
Ruby, A. Review of pyrethroid, fipronil and toxicity monitoring data from california urban watersheds. California Stormwater Quality Association (CASQA), 90 p. (2013).
Budd, R., Ensminger, M., Wang, D. & Goh, K. S. Monitoring fipronil and degradates in California surface waters, 2008–2013. J. Environ. Qual. 44, 1233–1240 (2015).
Stone, W. W., Gilliom, R. J. & Ryberg, K. R. Pesticides in U.S. streams and rivers: occurrence and trends during 1992–2011. Environ. Sci. Technol. 48, 11025–11030 (2014).
Goff, A. D. et al. The effects of fipronil and the photodegradation product fipronil desulfinyl on growth and gene expression in juvenile blue crabs, Callinectes sapidus, at different salinities. Aquat. Toxicol. 186, 96–104, https://doi.org/10.1016/j.aquatox.2017.02.027 (2017).
Shan, Z. et al. Impact of fipronil on crustacean aquatic organisms in a paddy field-fishpond ecosystem. Bull. Environ. Contam. Toxicol. 70, 746–752 (2003).
Schlenk, D. et al. Toxicity of fipronil and its degradation products to Procambarus sp.: field and laboratory studies. Arch. Environ. Contam. Toxicol. 41, 325–332 (2001).
Biever, R. C. et al. Icon *rice seed treatment toxicity to crayfish (Procambarus clarkii) in experimental rice paddies. Environ. Toxicol. Chem. 22, 167–174 (2003).
Key, P. B., Chung, K. W., Opatkiewicz, A. D., Wirth, E. F. & Fulton, M. H. Toxicity of the insecticides fipronil and endosulfan to selected life stages of the grass shrimp (Palaemonetes pugio). Bull. Environ. Contam. Toxicol. 70, 533–540 (2003).
Volz, D. C. et al. Effects of fipronil and chlorpyrifos on endocrine-related endpoints in female grass shrimp (Palaemonetes pugio). Bull. Environ. Contam. Toxicol. 71, 497–503 (2003).
Stark, J. D. & Vargas, R. I. Toxicity and hazard assessment of fipronil to Daphnia pulex. Ecotoxicol. Environ. Saf. 62, 11–16 (2005).
Ditty, J. G. Young of Litopenaeus setiferus, Farfantepenaeus aztecus and F. duorarum (Decapoda: Penaeidae): a re-assessment of characters for species discrimination and their variability. J. Crustac. Biol. 31, 458–467 (2011).
Montero, J. T., Chesney, T. A., Bauer, J. R., Froeschke, J. T. & Graham, J. Brown shrimp (Farfantepenaeus aztecus) density distribution in the Northern Gulf of Mexico: an approach using boosted regression trees. Fish. Oceanogr. 25, 337–348 (2016).
NMFS. Commercial fisheries statistics. National Marine Fisheries Service. Available at: https://www.st.nmfs.noaa.gov/commercial-fisheries/commercial-landings/annual-landings-with-group-subtotals/index (Accessed: 9th February 2018) (2017).
Sheridan, P. F. & Ray, S. M. Report of the workshop on the ecological interactions between shrimp and bottomfishes, April 1980. National Marine Fisheries Service (NMFS). Southeast Fisheries Center. 138 p. (Galveston, TX, 1981).
Fujiwara, M., Zhou, C., Acres, C. & Martinez-Andrade, F. Interaction between penaeid shrimp and fish populations in the gulf of mexico: Importance of shrimp as forage species. PloS one 11, e0166479 (2016).
Lassuy, D. R. Species profiles: Life histories and environmental requirements (Gulf of Mexico), brown shrimp. U.S. Fish and Wildlife Service. Coastal Engineering Research Center, 15 P. (1983).
Pisa, L. W. et al. Effects of neonicotinoids and fipronil on non-target invertebrates. Environ. Sci. Pollut. Res. Int. 22, 68–102 (2015).
CCME. Canadian water quality guidelines: imidacloprid. Scientific supporting document. Winnipeg, Manitoba. Canadian Council of Ministers of the Environment, 60 p. (2007).
Sneck-Fahrer, D. A. & East, J. W. Water-quality, sediment-quality, stream-habitat, and biological data for Mustang Bayou near Houston, Texas, 2004–05. U.S. Geological Survey Data Series 263, 90 (2007).
Mahler, B. J. et al. Fipronil and its Degradates in Indoor and Outdoor Dust. Enviro. Sci. Technol. 43, 5665–5670 (2009).
Opsahl, S. P. (U.S. Geological Survey Data Series 740. 20 p., 2012).
Mosier, D. G. (AgVise Laboratories, Northward, ND; and Stone Environmental, Inc. Montpelier, VT. MRID 46733902. 32 p., 2005).
Hayasaka, D. et al. Cumulative ecological impacts of two successive annual treatments of imidacloprid and fipronil on aquatic communities of paddy mesocosms. Ecotoxicol. Environ. Saf. 80, 355–362 (2012).
USGS. Fipronil and degradation products in the rice-producing areas of the mermentau river basin, Louisiana, February–September 2000. Fact Sheet FS-010-03. U.S. Geological Survey (USGS), 6 p (2003).
Wong, C. K., Cheung, J. K. Y. & Chu, K. H. Effects of copper on survival, development and growth of Metapenaeus ensis Larvae and postlarvae (Decapoda: Penaeidae). Mar. Pollut. Bull. 31, 416–419 (1995).
Frontera, J. L., Vatnick, I., Chaulet, A. & Rodriguez, E. M. Effects of glyphosate and polyoxyethylenamine on growth and energetic reserves in the freshwater crayfish Cherax quadricarinatus (Decapoda, Parastacidae). Arch. Environ. Contam. Toxicol. 61, 590–598 (2011).
Rozas, L. P., Minello, T. J. & Miles, M. S. Effect of deepwater horizon oil on growth rates of juvenile penaeid shrimps. Estuar. Coast. 37, 1403–1414 (2014).
Gauquelin, F. et al. Effect of dietary protein level on growth and energy utilization by Litopenaeus stylirostris under laboratory conditions. Aquaculture 271, 439–448 (2007).
Lachaise, F., Le Roux, A., Hubert, M. & Lafont, R. The molting gland of crustaceans: localization, activity, and endocrine control (Review). J. Crustac. Biol. 13, 198–234 (1993).
Mensah, P. K., Muller, W. J. & Palmer, C. G. Using growth measures in the freshwater shrimp Caridina nilotica as biomarkers of Roundup® pollution of South African freshwater systems. Phys. Chem. Earth, Parts A/B/C 50-52, 262–268 (2012).
OECD. Detaild review paper on aquatic Arthropods in life cycle and two-generation toxicity tests. Organisation for Economic Co-operation and Development, 135 p (2005).
Betancourt-Lozano, M., Baird, D. J., Sangha, R. S. & Gonzalez-Farias, F. Induction of morphological deformities and moulting alterations in Litopenaeus vannamei (Boone) juveniles exposed to the triazole-derivative fungicide tilt. Arch. Environ. Contam. Toxicol. 51, 69–78 (2006).
Snyder, M. J. & Mulder, M. J. Environmental endocrine disruption in decapod crustacean larvae: hormone titers, cytochrome P450, and stress protein responses to heptachlor exposure. Aquat. Toxicol. 55, 177–190 (2001).
Baldwin, W. S., Milam, D. L. & Leblanc, G. A. Physiological and biochemical perturbations in Daphnia magna following exposure to the model environmental estrogen diethylstilbestrol. Environ. Toxicol. Chem. 14, 945–952 (1995).
Fingerman, M., Jackson, N. C. & Nagabhushanam, R. Hormonally-regulated functions in crustaceans as biomarkers of environmental pollution (Review). Comp. Biochem. Physiol. Part C 120, 343–350 (1998).
Raley-Susman, K. M. In Pesticides - Toxic Aspects (ed Sonia Soloneski) 29 p (inTech, 2014).
Overmyer, J. P., Mason, B. N. & Armbrust, K. L. Acute toxicity of imidacloprid and fipronil to a nontarget aquatic insect, Simulium vittatum Zetterstedt cytospecies IS-7. Bull. Environ. Contam. Toxicol. 74, 872–879 (2005).
O’Halloran, M. J. In Tested Studies for Laboratory Teaching Vol. 11 (ed Goldman, C. A.) 15–26 (1990).
Martinez, A. et al. The effect of copper on the color of shrimps: redder is not always healthier. PloS one 9, e107673 (2014).
Nates, S. F. & McKenney, C. L. Jr Growth, lipid class and fatty acid composition in juvenile mud crabs (Rhithropanopeus harrisii) following larval exposure to Fenoxycarb®, insect juvenile hormone analog. Comp. Biochem. Physiol. Part C 127, 317–325 (2000).
Bray, W. A., Williams, R. R., Lightner, D. V. & Lawrence, A. L. Growth, survival and histological responses of the marine shrimp, Litopenaeus vannamei, to three dosage levels of oxytetracycline. Aquaculture 258, 97–108 (2006).
Nyman, A. M., Hintermeister, A., Schirmer, K. & Ashauer, R. The insecticide imidacloprid causes mortality of the freshwater amphipod Gammarus pulex by interfering with feeding behavior. PloS one 8, e62472 (2013).
Lovell, T. Nutrition and Feeding of Fish. 2nd edn, 270 p (Springer Science + Business Media, LLC, 1998).
USEPA. Fipronil environmental fate and ecological effects assessment and characterization for section 18 registration of in-furrow applications to rutabaga and turnips. United States Environmental Protection Agency. Environmental Fate and Effects Division. 72 p (Washington DC. USA, 2007).
USEPA. Methods for measuring the acute toxicity of effluents and receiving waters to freshwater and marine organisms. United States Environmental Protection Agency. Office of Water. 275 p (Washington DC. USA, 2002).
AOAC. Official Methods of Analysis. Association of Official Analytical Chemists, Arlington, VA, USA. 1298 p. (1990).
AOAC. Official method of Analysis, method 935.14 and 992.24. Association of Officiating Analytical Chemists, Washington DC, USA. (2005).
Folch, J., Lees, M. & Sloane Stanley, G. H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem 226, 497–509 (1957).
Finney, D. J. Probit analysis. Cambridge University Press. Journal of the Institute of Actuaries (1886–1994) 78, 388–390 (1952).
JMP® Pro v. 13.1.0 (SAS Institute Inc., Cary, North Carolina, U.S.A., 2016).
MATLAB v. 9.2 (The MathWorks, Inc., Natic, Masacchusetts, U.S.A., 2017).
Overmyer, J. P. et al. Toxicity of fipronil and its enantiomers to marine and freshwater non-targets. J. Environ. Sci. Health, Part B 42, 471–480 (2007).
Chandler, G. T. et al. Fipronil effects on estuarine copepod (Amphiascus tenuiremis) development, fertility, and reproduction: a rapid life-cycle assay in 96-well microplate format. Environ. Toxicol. Chem. 23, 117–124 (2004).
Konwick, B. J., Fisk, A. T., Garrison, A. W., Avants, J. K. & Black, M. C. Acute Enantioselective Toxicity of Fipronil and its Desulfinyl Photoproduct to Ceriodaphnia dubia. Environ. Toxicol. Chem. 24, 2350–2355 (2005).
USEPA. Fipronil: environmental assessment, current for the turf registration. United States Environmental Protection Agency. Office of Prevention, Pesticides and Toxic Substances. 20 p (Washington DC. USA, 1996a).
Acknowledgements
We thank National Marine Fisheries Service Galveston Laboratory for providing shrimp for the experiment. We especially would like to extend our thanks to Shawn Hillen for assisting us; without his help, this research could have not been completed. We would also like to thank Fiala Bumpers, who created the map of Texas, and David Wells, who provided comments on the proposal for conducting this research.
Author information
Authors and Affiliations
Contributions
A.A.A., M.F., and M.A.M. designed the experiments. A.A.A. and M.F. contributed to sample collection, data analyses, and interpretation. A.A.A. performed the experiment and chemical analysis, prepared figures, and wrote the manuscript. D.M.G. advised and provided material for body chemical composition analysis. All authors reviewed, edited and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Al-Badran, A.A., Fujiwara, M., Gatlin, D.M. et al. Lethal and sub-lethal effects of the insecticide fipronil on juvenile brown shrimp Farfantepenaeus aztecus. Sci Rep 8, 10769 (2018). https://doi.org/10.1038/s41598-018-29104-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29104-3
This article is cited by
-
β-1,3-glucan improved the health and immunity of juvenile African catfish (Clarias gariepinus) and neutralized the histological changes caused by lead and fipronil pollutants
BMC Veterinary Research (2023)
-
Biodegradation of the Pesticides Bifenthrin and Fipronil by Bacillus Isolated from Orange Leaves
Applied Biochemistry and Biotechnology (2023)
-
The potential ameliorative impacts of cerium oxide nanoparticles against fipronil-induced hepatic steatosis
Scientific Reports (2021)
-
Identification, Elucidation, and Toxicity Assessment of Nontarget Disinfection By-products from Fipronil Chlorination
Water, Air, & Soil Pollution (2021)
-
Exposure to environmental concentrations of fipronil induces biochemical changes on a neotropical freshwater fish
Environmental Science and Pollution Research (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.