Abstract
CD8+ cytotoxic T-cell (CTL) specific for non-mutated, wild type (wt) sequence p53 peptides derived from wt or mutant p53 molecules expressed in head and neck squamous cell carcinomas (HNSCC) have been detected in the circulation of patients with this disease. The frequency and differentiation/maturation phenotypes of these anti-tumor specific CTL can reflect the host’s immunologic response. Therefore, we investigated the frequency and phenotypes of wt sequence p53 peptide-specific CTL in patients with HNSCC (n = 33) by flow cytometric analysis using HLA-A*0201 tetrameric peptides (tet) complexed with the wt sequence p53264–272 or p53149–157 peptide and co-staining with phenotypic markers. One main finding was that increasing frequencies of tet+ CD8+ T cells in patients’ circulation correlated with increased frequencies of inactive naïve tet+ cells, while those with effector memory and terminally differentiated phenotypes, which are associated with positive anti-tumor immune responses, decreased. We also found that the frequency of circulating tet+ CD8+ T cells negatively correlated with p53 expression in tumor tissues and tumor stage. Our findings support further clinical-based investigations to define the frequencies and phenotypes of wt sequence p53 peptide-specific CD8+ T cells to predict disease severity, enhance selection of patients for inclusion in vaccination trials and highlight prerequisites to enhance immune susceptibility by activation of inactive naïve tet+ T cells and/or enhancing circulating effector T cell activity by checkpoint blockage.
Similar content being viewed by others
Introduction
The development and clinical application of novel biopharmaceutical agents targeting elements of the immune system, such as CTLA-4 and programmed death-1 (PD-1) checkpoint receptors as well as tumor associated cell surface antigens, has revolutionized immunotherapy and the oncologic treatment landscape. Patients with head and neck squamous cell carcinoma (HNSCC) are known to be immunosuppressed. Signaling defects in regulatory T cells (Treg) and cytolytic T lymphoctes (CTL) as well as a higher proportion of apoptotic T cells in these populations, in particular, anti-tumor specific CTL are detected in the peripheral blood of HNSCC patients compared to healthy individuals1,2,3. Thus, judiciously selected T-cell defined epitopes for cancer vaccines have been developed and defined with the aim to induce robust host anti-tumor immunogenicity. TP53, highly frequently mutated gene in HNSCC4, has been an attractive candidate for vaccines potentially capable of inducing immune responses in HNSCC patients directed against tumor-specific antigens. Mutant p53 protein, which accumulates in most HNSCC cells, potentially can yield mutation-specific p53 peptides. Although these epitopes would be tumor-specific, they have limited clinical applicability due primarily to the constraints imposed by antigen processing and presentation. In contrast, non-mutated, wild type (wt) sequence peptides derived from genetically altered p53 molecules in tumors have a greater potential of being processed and presented and represent a more practical approach for developing broadly applicable p53-based cancer vaccines for the prevention and treatment of HNSCC5,6.
Previously, we have demonstrated in vitro that the presentation of wt sequence p53 peptides pulsed on autologous-derived dendritic cells (DC) induced peptide-specific immune responses from peripheral blood lymphocytes obtained from HLA-A2+ normal donors as well as patients with HNSCC7,8,9,10. Dendritic cells (DC)-based wt sequence p53 peptide vaccines have been used for immunotherapy in a variety of human cancers, including HNSCC. In a recent phase I clinical trial5 involving HLA-A2+ patients with HNSCC, patients were treated with a multiple CTL and T helper cell-defined, wt sequence p53 peptide-loaded DC-based adjuvant vaccination. The vaccination was shown to have some beneficial effects on the recipients. In patients with advanced HNSCC, however, there were limited post-vaccination anti-wt sequence p53 peptide-specific immunologic responses. Overall, wt sequence p53 peptide-specific CTL frequencies were increased post-vaccination in 69% of patients, with IFN-γ secretion detected in these cells in 25% of patients, but consistently decreased Treg frequencies relative to pre-vaccination values were also observed in these patients. However, disease free survival (DFS) after vaccination did not correlate with the presence or expression levels of p53 in the patients’ tumor cells nor with frequencies of wt sequence p53 peptide-specific CD8+ T cells in their peripheral circulation. Despite advances in the developing cancer vaccines, these findings are consistent with the poor clinical responses observed in many previous vaccine-based, cancer immunotherapy studies9,11.
To promote further understanding of the nature of wt p53 peptide-specific responses in patients with HNSCC and its relevance to patient survival and p53-based immunotherapy, it is important to determine the frequency and functional activity of wt sequence p53 peptide-specific CTL relative to their differentiation/maturation phenotype in these individuals. T cells have been characterized by their phenotypic and functional profiles into T cell subsets, namely, naïve (TN), central memory (TCM), effector memory (TEM) and terminally differentiated T cells (TTD). One established protocol for identifying these subsets is the differential expression of certain phenotypic markers, such as chemokine receptor 7 (CCR7) and CD45RA12,13. In addition, CTL function can also be assessed by monitoring IFNγ production and CD247/perforin expression. TN CD8+ T cells (CD45RA+CCR7+) are activated when interacting with antigen-presenting cells (APC) in secondary lymph nodes and rapidly proliferate and differentiate into TCM (CD45RA−CCR7+) and TEM (CD45RA−CCR7−). TEM migrate into the peripheral tissues and efficiently differentiate to effector cells TTD (CD45RA+CCR7−) while TCM home to the secondary lymphoid organs and retain the ability to proliferate and differentiate into TEM upon T cell receptor stimulation by antigen12. In this study, we determined the frequency and phenotype of wt sequence p53 peptide-specific T cells in the peripheral circulation of HLA-A*0201+ patients with HNSCC by multicolor flow cytometry using HLA-A*0201 tetramers (tet) complexed with wt sequence p53264–272 or p53149–157 peptides, referred to as tet264–272 and tet149–157, respectively. We further evaluated their correlation with clinicopathological factors as well as p53 and HPV status of the patients’ tumor specimens.
Methods
Ethics Statement and the criteria of patient inclusion
The clinical sample collection was carried out in accordance with the guidelines and protocols approved by the internal ethics board at University of Pittsburgh Cancer Institute (Pittsburgh, PA), and written informed consent was obtained from each participating individual prior to participation in the study. Patients with HNSCC (n = 33) were selected for inclusion into this study. None were receiving treatment at the time of blood draw. All blood samples were drawn pre-therapeutically after histological confirmation of HNSCC before removal of the cancers. All were HLA-A2+, as determined by sero-phenotyping of their peripheral blood mononuclear cells (PBMC) using monoclonal antibodies (mAbs) BB7.2 and MA2.1 (produced by hybridomas obtained from American Type Culture Collection, Manassas, VA)8. The clinicopathologic characteristics of the patients are listed in Table 1. TNM classification of malignant tumors according UICC 7th ed was used.
Collection of peripheral venous blood
Peripheral venous blood (30–50 mL) was drawn into heparinized tubes that were transferred to the laboratory and lymphocyte recovery on Ficoll-Hypaque gradients was immediately conducted. The recovered PBMC were washed, counted, and directly stained for ex vivo flow cytometry. The elapsed time between phlebotomy and PBMC staining for flow cytometry was within 2 hours.
Tetramers, antibodies and staining
The PE-labeled tet264–272 and tet149–157 reagents were obtained through the National Institute of Allergy and Infectious Diseases Tetramer Facility in Atlanta, GA. Titrations of tetramers and specificity assays were as follows: (a) all tetramers were pre-titered on bulk or cloned CD8+ T cell lines with specificity for the wt sequence p53 peptides were available in our laboratories to determine optimal reagent concentrations and to distinguish positive from negative signals; (b) negative controls were used with HLA-A2+ PBMC in all assays; (c) a cut-off for tetramer binding to PBMC of HLA-A2− normal donors (n = 10) was established as previously described by us1. The lower limit of detection (LLD) was defined as the frequency of 1/7800 cells or approximately 0.01%. This LLD was used as a cut-off for evaluating all tetramer results presented in this manuscript.
The following anti-human mAbs were used in this study: CD8-PC5 (Beckman Coulter, Miami, FL), CD45RA-FITC (Immunotech), CCR7-PE and CD247-APC BD Biosciences perforin-FITC; BD Biosciences, San Jose, CA).
The staining for tetramers and cell surface antigens by flow cytometry was performed as previously described8. Briefly, for p53 tet, aliquots of diluted stock (1/100) of tet were added directly to subtly disrupted cell pellets at ambient temperature (5–7 × 106 cells). The cells were incubated for 30 minutes at room temperature in the dark. Next, 5 μl of each mAb was added on the cell subtlety disrupted pellet, followed by 30 minutes incubation at 4 °C in the dark. Cells were washed, centrifuged and resuspended in 500 μl of PBS/0.5% (wt/vol) paraformaldehyde. Flow cytometry was performed within 30 minutes.
Immunohistochemistry
Immunostaining for p53 protein was performed as previously described9. Briefly, formalin-fixed, paraffin-embedded tissue specimens were sectioned (4 μm thick), deparaffinized and rehydrated in a series of graded ethanol. Immunohistochemical staining was performed using a mAb against p53 (DO-7, Dako, Carpinteria, CA, USA), which recognizes an epitope in the N-terminus between amino acid 35 and 45 and reacts with the wt and most mutant forms of p53 protein, followed by the avidin-biotin-peroxidase method to visualize the p53 according to the manufacturer’s instructions (Dako). Positive and negative controls were included in each run for quality control of the immunoreactivity. IgG isotype mAb was used as a negative control. Normal-appearing salivary gland tissue or skeletal muscle from patients with HNSCC served as an internal non-tumor control. A tumor was considered p53 positive when >25% of the tumor cells showed staining intensity of 2+ and higher on a scale of 0–4+. For p16 staining, mouse monoclonal antibody specific for p16 (1:100 dilution, clone DCs-50; neomarkers, Fremont, CA, USA) was used as introduced before14. p16 expression was scored as positive if there was strong and diffuse nuclear and cytoplasmic staining in >60% of the tumor. Three independent experienced observers, who were blinded to the patient clinical information, performed semiquantitative evaluation of the slides.
Statistical analysis
The descriptive statistics were provided using the median/range and box plots. The associations among lymphocyte subsets were tested with the t test or Wilcoxon rank sum test (for two groups) or Kruscal Wallis test (for multiple groups). Reciprocal frequencies of tetramer counts were log transformed (base 10) and tested for differences with the paired t test. Multivariate correlation analysis was performed to determine the relationship between the frequency of p53-specific CTL and clinicopathological parameters. All the reported p-values are based on two-sided tests.
Results
Frequencies of tet264–272 + and tet149–157 + CD8+ T cells in peripheral circulation of HLA-A0201+ patients with HNSCC
The frequencies of tet264–272+ CD8+ T cells in the peripheral circulation of 33 HLA-A0201+ patients with HNSCC was determined by tetramer-based flow analysis, and in a similar manner, the frequency of tet149–157+ CD8+ T cells in samples obtained from 19 of these 33 patients. Based on the lower limit of detection (LLD) of 1/7800, 27/33 patients had detectable frequencies of tet264–272+ CD8+ T cells in their circulation ranging from 1/7800 to 1/483, with an average frequency of 1/2694. Sufficient sample was obtained from 19/33 patients, all of which had detectable levels of tet264–272+ CD8+ T cells for an additional analysis of tet149–157+ CD8+ T cells. These tet+CD8+ T cells were detectable at relatively high frequencies ranging from 1/4283 to 1/859 in all 19 samples. In these 19 patients, the average frequency of tet264–272+ CD8+ T cells was 1/4707 (range 1/7798-1/1239) and 1/2130 for tet149–157+ CD8+ T cells (range 1/5492–1/859). The reactivity with these two tetramers correlated (correlation: 0.636, p = 0.003, n = 19).
For further subgroup analysis, the frequencies of tet264–272+ and tet149–157+ CD8+ T cells detected in the peripheral circulation of patients with HNSCC were divided into 3 groups as follows: high frequency >1/2128; intermediate frequency <1/2128 but >1/4767; low frequency <1/4767. The distribution of high, intermediate and low frequency for each tet+CD8+ T cell specificity is listed in Table 2. In the group of tet264–272+CD8+ T cells, 6 cases were lower than 1/7800 cut off frequency. All frequencies of tet149–157+CD8+ T cells were higher than 1/7800.
Clinicopathological parameters and frequencies of tet264–272 + and tet149–157 + CD8+ T cells in peripheral circulation of patients with HNSCC
The distribution of high, intermediate and low frequency for tet264–272+CD8+ T cells regarding to the clinicopathological parameters is listed in Table 1. Frequencies of tet264–272+CD8+ T cells tended to be higher in patients with T1 while those with low frequencies tended to be advanced T4 (Table 1). However, a significant inverse correlation between the frequency of tet264–272+CD8+ T cells and the tumor p53 accumulation (r = −0.637, p < 0.05) was noted with low T cell frequencies in the circulation and a high p53 accumulation at the tumors site, strongly suggesting T cell depletion in patients with p53+ tumors. Similarly, a significant inverse correlation (r = −0.813, p < 0.05) was found between the frequency of tet149–157+CD8+ T cells and tumor p53 accumulation. There were no significant correlations between any of other clinical parameters listed in Table 2 and the frequencies of wt sequence p53 peptide-specific CD8+ T cells determined in the HNSCC patient’s samples (data not shown).
Disease-free survival relative to clinicopathology parameters and frequencies of tet264–272 + and tet149–157 +CD8+ T cells in peripheral circulation of patients with HNSCC
Among the 33 patients, 14 died of disease (DOD) and 1 died of unknown reason; 17 patients remain alive with no evidence of disease and 1 patient was alive with disease. The median follow-up was 8.82 years (range 2.33–23.42 years). Thus, 3-year DFS was 85% and 5-year DFS was 76% (Fig. 1A). No significant difference in DFS between patients who had p53+ versus p53− tumors was observed (Fig. 1B). As expected, HPV p16 status strongly predicted improved clinical outcome even within this small patient group (Fig. 1C). There was no significant difference of DFS, however, among patients who were stratified between those with no or low frequency of tet+ T cells and those with median or high frequency of tetramer reactive T cells (Fig. 1D).
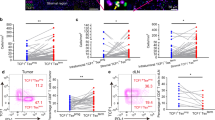
Differential Phenotypes of wt sequence p53 peptide-specific tet+CD8+ and tet−CD8+ T cells in the peripheral circulation of patients with head and neck cancer. Examples of dot plots for patient #1 are shown for (A) CD45RA and CCR7 expression by wt sequence p53264–272-specific or wt sequence p53149-156-specific CD8+ T cells, and differentiation phenotypes of (B) wt sequence p53 tet264-272−CD8+ T cells and wt p53 tet264–272+CD8+ T cells (*p < 0.01), (C) wt sequence p53 tet149–156−CD8+ T cells and tet149–156+CD8+ T cells (*p < 0.01), and (D) CD247/Perforin expression of wt p53 tet264–272−CD8+ T cells and wt p53 tet264–272+CD8+ T cells (#p < 0.05; *p < 0.01).
Differentiation/maturation phenotypes of tet+CD8+ T cells and tet−CD8+ T cells in peripheral circulation of patients with HNSCC
The tet+CD8+ T cells and tet−CD8+ T cells were co-stained for T cell surface marker expression CD45RA and CCR7 and analyzed by flow cytometry to determine their differentiation/maturation status as TN, TCM, TEM, and TTD. Representative dot plots obtained from one patient are shown in Fig. 2A.
As shown in Fig. 2B,C, the subpopulation of TN cells contained in both tet+ CD8+ T cell populations show a significant increase relative to the tet− CD8+ populations in the patients’ circulation. For tet264–272+CD8+ it was p < 0.001 and for tet149–156+CD8+ it was p = 0.005. In contrast, TEM tet+ CD8+ T cells for both specificities are significantly decreased when compared to the tet− population; tet264–272+CD8+ T cells it was p < 0.001 and for tet149–156+CD8+ T cells it was p = 0.001.
TD cell subpopulation was significantly decreased in tet264–272+CD8+ T cell population (p = 0.002) compared to the TD tet− subpopulation. There was no significant difference of TD tet+ CD8+ T cell subpopulations between tet149–156+ and tett− CD8+ T cell populations in the patients’ samples.
Comparison of the percentages of the differentiation/maturation phenotypes of the tet264–272 + and tet149–157 + CD8+ T cell populations and tet− CD8+ T cell populations in patients’ peripheral circulation
The median percentages of the four differentiation/maturation phenotypes of tet264–272+ CD8+ T cells were compared to those of tet− CD8+ T cells relative to whether the patients’ peripheral circulation had high, medium, low, or no detectable levels of tet264–272+ CD8+ T cells in their circulation. The results of this analysis are presented in Table 3. Only in patients with high frequencies of tet264–272+ CD8+ T cells in their peripheral circulation were any significant differences detected: TN cells (CD45RA+/CCR7+) were significantly increased (p = 0.049), while TD cells (CD45RA+/CCR7−) were decreased (p = 0.022) (Table 3). No significant findings between phenotypes of tet149–157+CD8+ T cells and tet− CD8+ T cell populations were detected (data not shown).
Association between clinicopathological parameters and differentiation/maturation phenotypes of tet+ 264–272 CD8+ T cells
Possible associations between clinicopathological parameters and the mean percentage of tet264–272+ CD8+ T cells with different phenotypes detected in the peripheral blood of patients were also investigated (Table 4). A significantly (p = 0.029) lower mean percentage of tet 264–272+ TN CD8+ T cells was found in patients with T3–4 stage. In patients without p53 accumulation at the tumor sites, the percentage of tet 264–272+ CD8+ TD cells declined (p = 0.03) and a trend for increased TN cells (p = 0.06) was seen. No other significant findings relative to the other clinicopathological parameters studies.
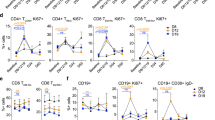
Comparison of the differentiation phenotype (CD247/perforin) of tet274–272 + and tet149–156 +CD8+ T cells versus tet− CD8+ T cells
Sufficient cells were available from 20/33 patients for additional co-staining of CD8+ T cells with tet274–272 and CD247/perforin. The percentage of CD247+perforin+tet264–272+CD8+ T cells was found to significantly decrease while that of CD247+perforin−tet264–272+CD8+ T cells significantly increased when compared to the CD247+perforin+ tet− populations (Fig. 1D). Seven patients had increased CD247−/CD247+ ratio in perforin+tet264–272+CD8+ T cells (data not shown). CD247−perforin+tet264–272+CD8+ T cells were not detected in 11 patients. Two patients had similar percentages of CD247−perforin+tet264–272+CD8+ T cells as their tet− populations. Moreover, the percentage of CD247−perforin+tet264–272+CD8+ T cells was correlated to TD tet+ cells (p = 0.019). Samples from nine of 20 patients were also available for co-staining with p53149–156 tet and CD247/perforin. There were no significant differences in these populations in patients stained with tet149–156 (data not shown).
Discussion
Wt sequence p53 tet+CD8+ T cells identified in patients with HNSCC
Consistent to our earlier findings and those of others9,15 tet264–272+ CD8+ T cells were identified in the circulation of most, but not all patients participating in this study. Furthermore, in patients who had tet264–272+ CD8+ T cells in their circulation, tet149–157+ CD8+ T cells were also detectable, indicative of a polyclonal reaction of T cells to this tumor antigen. The lack of a wt sequence p53 peptide immune response in some patients observed in this study has been noted in previous ones as well and may, in part, be due to the clonal deletion or anergy of effector T cells specific for self-epitopes16 or apoptosis of T-cell receptor (TCR) variable β-chain-restricted antigen-responsive T cells17.
Differentiation/Maturation Phenotypic differences between tet+ and tet− CD8+ T cells in HNSCC patients’ circulation
In this study, we determined that in patients with a high frequency of tet+ CD8+ T cells in their peripheral circulation, tet+ cells with an N phenotype increased while those with the mature EM and TD phenotypes declined compared to those in the tet− populations. Previously, we have shown that tet+ CD8+ T cells in HNSCC patients preferentially localized to the tumor sites and tumor-involved lymph nodes and their frequencies increased in the population of tumor-infiltrating lymphocytes (TIL)8. Therefore, possible trafficking of tet+CD8+ T cells with TD and EM phenotype to peripheral sites might alter the phenotype composition of these effectors in the circulation and enhance the N compartment.
CD247 or CD3-ζchain and perforin expression are widely used markers for T cell activation and the expression of perforin was found to decrease in CD247+ tet+CD8+ cells compared to CD247+ tet− CD8+ T cells. In addition, in some patients, tet+CD8+ T cells displayed a CD247− phenotype and a strong correlation with a TD phenotype and perforin expression. This observation suggests that CD247+ perforin+ tet+CD8+ T cells may have migrated to peripheral sites in these patients concurrent with downregulation of CD247 expression8. A better 5-year survival has been shown in patients with tumors infiltrated by TILs expressing normal levels of CD3-ζchain than those showing loss of CD3-ζchain expression18,19. Although in the current study, CD247−perforin+tet+CD8+ T cells were not correlated to the low frequency tet+CD8+ T cells, it suggests that TD cells that traffic to the peripheral site lack T cell receptor-ζchain and may account for the unsuccessful or limited immune response in some patients.
Frequencies of circulating tet+CD8+ T cells negatively correlated to p53 expression in tumor tissues
In this study, patients who had high frequency of tet+ CD8+ T cells were found to have lower p53 accumulations in their tumor tissues while patients with low frequency of tet+CD8+ T cells had higher p53 accumulations in tumor tissues. This discrepancy is consistent with our previous finding relating frequencies of these tet+ CD8+ T cells in the peripheral circulation of HNSCC patients and p53 mutational sites in their tumors9 and suggests that these CTL in responsive patients could have eliminated tumor cells capable of processing and presenting the targeted epitope resulting in the immunoselection and outgrowth of “epitope-loss” tumors.
Clinical relevance of circulating tet+ CD8+ T cells
The frequency of circulating tet+ CD8+ T cells was found to decrease in HNSCC patients with advanced disease. Furthermore, a decline of the N phenotype subpopulation but similar TD phenotype subpopulation in tet+CD8+ T cells was found in advanced T stage as compared to lower T stage. The reason for this correlation remains presently unknown. As previously shown, the presence and frequency of tet+ CD8+ T cells among TIL did not correlate with tumor stage indicating it was independent of tumor progression in HNSCC8. We speculate that patients with advanced T stage HNSCC may not present the wt sequence p53 peptide epitopes properly or have limited CTL recognition due to the downregulation of expression of antigen-processing machinery components17,20. For example, CTL function may be suppressed by Treg, which are abundant in TIL at tumor sites8,21. Treg frequency, which is responsible for immunosuppression of adaptive and innate immunity and correlates with tumor progression and outcome, increases in patients with HNSCC8,22.
An important parameter that contributes to the complexity of analyzing immune responses of patients to HNSCC is HPV infection, since High Risk-HPV-related HNSCC currently accounts for 25% of HNSCC and up to 70% of oropharyngeal squamous carcinoma23. HPV E6 binds and degrades the p53 while E7 binds and degrades the pRB retinoblastoma tumor suppressor protein24. In HPV+ HNSCC, circulating HPV E7 specific CD8+ T cells are detectable indicating that endogenously-induced E7-specific immunity exists in these patients7. It has also been shown that wt and mutant p53 molecules are sensitive to HPV E6-mediated degradation and in vitro and in vivo results in increased presentation of the wt sequence p53264–272 peptide by HLA-A•0201+ tumors for CTL recognition6. Using p16 as the marker of HPV infection, there was no correlation of HPV expression in patients’ tumors to the frequencies of tet+ CD8+ T cells, in the patients’ circulation. However, consistent with current literature, p16 expression did correlate with improved DFS.
Relevant to the results of this study are the findings obtained from the recent multiepitope wt sequence p53 peptide vaccine clinical trial5, the vaccination of patients with HNSCC resulted in an increased frequency of tet264–272+ CD8+ T cells in their peripheral circulation and decreased levels of Treg. Additionally, circulating tet+ CD8+ T cells were more vulnerable to spontaneous apoptosis suggesting their preferential demise represents a mechanism of immunoescape of tumor cells2. Nonetheless, these patients had a favorable two-year DFS of 88% as compared to 70% of DFS in a similar clinical trial cohort including unvaccinated patients treated with chemoradiation25. Interestingly but unaccounted for at present, was that a limited and weak post-vaccination, wt sequence p53 peptide-specific immunity was observed in 5/16 patients. Overall, the presence or expression levels of p53 in these patients’ tumor tissues and the presence of tet+ CD8+ T cells in their peripheral blood after vaccination did not correlate to DFS. The explanation for this phenomenon is still unclear and requires a more extensive analysis of the nature of the immune response to wt sequence p53 peptide- specific epitopes in patients with HNSCC.
In perspective, our data support findings from the interaction of other solid cancers with the immune system: presence of a range of tumor-specific T cells in various differentiation stages and an immunosuppressive tumor microenvironment on multiple levels8,26. With the advent of checkpoint inhibition treatment, it appears that tumor-specific T cells can be activated more readily and tumor immune suppression ameliorated to some extent. It will be intriguing to see if the empirically observed clinical benefit of checkpoint inhibitors27,28 can be explained by the use of our T cell characterization methods and that they could be used as a read out of patient response or selection for treatment.
In summary, the results of this study further revealed the complex nature of wt sequence p53 peptide-specific immune responses in HNSCC patients and highlight several new parameters, like naïve T cell activation by vaccination and checkpoint inhibition by modulating the PD-1/PD-L1 axis, that should be considered in developing the design and analysis of future vaccination protocols to enhance the efficacy of p53-based immunotherapy of HNSCC.
References
Hoffmann, T. K. et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clinical cancer research: an official journal of the American Association for Cancer Research 8, 2553–2562 (2002).
Albers, A. E. et al. Spontaneous apoptosis of tumor-specific tetramer+ CD8+ T lymphocytes in the peripheral circulation of patients with head and neck cancer. Head & neck 31, 773–781, https://doi.org/10.1002/hed.21031 (2009).
Qian, X. et al. Biology and immunology of cancer stem(-like) cells in head and neck cancer. Critical reviews in oncology/hematology 95, 337–345, https://doi.org/10.1016/j.critrevonc.2015.03.009 (2015).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160, https://doi.org/10.1126/science.1208130 (2011).
Schuler, P. J. et al. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clinical cancer research: an official journal of the American Association for Cancer Research 20, 2433–2444, https://doi.org/10.1158/1078-0432.CCR-13-2617 (2014).
Sirianni, N. et al. Effect of human papillomavirus-16 infection on CD8+ T-cell recognition of a wild-type sequence p53264-272 peptide in patients with squamous cell carcinoma of the head and neck. Clinical cancer research: an official journal of the American Association for Cancer Research 10, 6929–6937, https://doi.org/10.1158/1078-0432.CCR-04-0672 (2004).
Albers, A. et al. Antitumor activity of human papillomavirus type 16 E7-specific T cells against virally infected squamous cell carcinoma of the head and neck. Cancer research 65, 11146–11155, https://doi.org/10.1158/0008-5472.CAN-05-0772 (2005).
Albers, A. E. et al. Immune responses to p53 in patients with cancer: enrichment in tetramer+ p53 peptide-specific T cells and regulatory T cells at tumor sites. Cancer immunology, immunotherapy: CII 54, 1072–1081, https://doi.org/10.1007/s00262-005-0670-9 (2005).
Hoffmann, T. K. et al. Generation of T cells specific for the wild-type sequence p53(264–272) peptide in cancer patients: implications for immunoselection of epitope loss variants. J Immunol 165, 5938–5944 (2000).
Hoffmann, T. K. et al. The ability of variant peptides to reverse the nonresponsiveness of T lymphocytes to the wild-type sequence p53(264–272) epitope. J Immunol 168, 1338–1347 (2002).
Chikamatsu, K. et al. Generation of anti-p53 cytotoxic T lymphocytes from human peripheral blood using autologous dendritic cells. Clinical cancer research: an official journal of the American Association for Cancer Research 5, 1281–1288 (1999).
Sallusto, F., Geginat, J. & Lanzavecchia, A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annual review of immunology 22, 745–763, https://doi.org/10.1146/annurev.immunol.22.012703.104702 (2004).
Sallusto, F., Langenkamp, A., Geginat, J. & Lanzavecchia, A. Functional subsets of memory T cells identified by CCR7 expression. Current topics in microbiology and immunology 251, 167–171 (2000).
Qian, X. et al. Prognostic significance of ALDH1A1-positive cancer stem cells in patients with locally advanced, metastasized head and neck squamous cell carcinoma. Journal of cancer research and clinical oncology 140, 1151–1158, https://doi.org/10.1007/s00432-014-1685-4 (2014).
Hoffmann, T. K. et al. Frequencies of tetramer+ T cells specific for the wild-type sequence p53(264–272) peptide in the circulation of patients with head and neck cancer. Cancer research 62, 3521–3529 (2002).
Hernandez, J., Lee, P. P., Davis, M. M. & Sherman, L. A. The use of HLA A2.1/p53 peptide tetramers to visualize the impact of self tolerance on the TCR repertoire. J Immunol 164, 596–602 (2000).
Albers, A. E. et al. Alterations in the T-cell receptor variable beta gene-restricted profile of CD8+ T lymphocytes in the peripheral circulation of patients with squamous cell carcinoma of the head and neck. Clinical cancer research: an official journal of the American Association for Cancer Research 12, 2394–2403, https://doi.org/10.1158/1078-0432.CCR-05-1818 (2006).
Whiteside, T. L. Signaling defects in T lymphocytes of patients with malignancy. Cancer immunology, immunotherapy: CII 48, 346–352 (1999).
Reichert, T. E., Day, R., Wagner, E. M. & Whiteside, T. L. Absent or low expression of the zeta chain in T cells at the tumor site correlates with poor survival in patients with oral carcinoma. Cancer research 58, 5344–5347 (1998).
Cruz, I., Meijer, C. J., Walboomers, J. M. & Snijders, P. J. & Van der Waal, I. Lack of MHC class I surface expression on neoplastic cells and poor activation of the secretory pathway of cytotoxic cells in oral squamous cell carcinomas. British journal of cancer 81, 881–889, https://doi.org/10.1038/sj.bjc.6690780 (1999).
Schaefer, C. et al. Characteristics of CD4+ CD25+ regulatory T cells in the peripheral circulation of patients with head and neck cancer. British journal of cancer 92, 913–920, https://doi.org/10.1038/sj.bjc.6602407 (2005).
Jie, H. B. et al. CTLA-4(+) Regulatory T Cells Increased in Cetuximab-Treated Head and Neck Cancer Patients Suppress NK Cell Cytotoxicity and Correlate with Poor Prognosis. Cancer research 75, 2200–2210, https://doi.org/10.1158/0008-5472.CAN-14-2788 (2015).
Qian, X. et al. ALDH1-positive cancer stem-like cells are enriched in nodal metastases of oropharyngeal squamous cell carcinoma independent of HPV status. Oncology reports 29, 1777–1784, https://doi.org/10.3892/or.2013.2340 (2013).
Albers, A. E., Hoffmann, T. K., Klussmann, J. P. & Kaufmann, A. M. [Prophylactic and therapeutic vaccines against human papilloma virus]. Hno 58, 778–790, https://doi.org/10.1007/s00106-010-2118-6 (2010).
Argiris, A. et al. Induction docetaxel, cisplatin, and cetuximab followed by concurrent radiotherapy, cisplatin, and cetuximab and maintenance cetuximab in patients with locally advanced head and neck cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 28, 5294–5300, https://doi.org/10.1200/JCO.2010.30.6423 (2010).
Li, J. et al. Tumor-infiltrating Tim-3(+) T cells proliferate avidly except when PD-1 is co-expressed: Evidence for intracellular cross talk. Oncoimmunology 5, e1200778, https://doi.org/10.1080/2162402X.2016.1200778 (2016).
Ferris, R. L. et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375, 1856–1867, https://doi.org/10.1056/NEJMoa1602252 (2016).
Kansy, B. A. et al. PD-1 Status in CD8(+) T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer research 77, 6353–6364, https://doi.org/10.1158/0008-5472.CAN-16-3167 (2017).
Author information
Authors and Affiliations
Contributions
A.E.A and A.B.D. designed the study. A.E.A performed the experiments. A.E.A. and R.F.L. collected the data. A.E.A., X.Q. and A.M.K. analyzed data. A.E.A., X.Q. and A.M.K. wrote the main manuscript text. D.M., R.F.L, T.K.H. and A.B.D. wrote this manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Albers, A.E., Qian, X., Kaufmann, A.M. et al. Phenotype of p53 wild-type epitope-specific T cells in the circulation of patients with head and neck cancer. Sci Rep 8, 10716 (2018). https://doi.org/10.1038/s41598-018-29067-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29067-5
This article is cited by
-
Translating p53-based therapies for cancer into the clinic
Nature Reviews Cancer (2024)
-
Head and Neck Squamous Cell Carcinoma: Risk Factors, Molecular Alterations, Immunology and Peptide Vaccines
International Journal of Peptide Research and Therapeutics (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.