Abstract
This study was conducted to profile the selenoprotein encoding genes or proteins in mouse C2C12 cells and integrate their roles in the skeletal cell damage induced by heat stress (HS). Cells were cultured at 37.0 °C or 41.5 °C for 4, 6 or 8 days. The mRNA expression of 24 selenoprotein encoding genes and abundance of 5 selenoproteins were investigated. HS suppressed myogenic differentiation and impaired the development of muscle myotubes. HS down-regulated (P < 0.01) mRNA abundance of MYOD and MYOGENIN, and decreased (P < 0.01) MYOGENIN protein expression, HS elevated (P < 0.01) HSP70 and (P < 0.01) the ratio of BCL-2 to BAX at both mRNA and protein level. Meanwhile, HS up-regulated (P < 0.01–0.05) expressions of 18, 11 and 8 selenoprotein encoding genes after 4, 6 and 8 days of hyperthermia, and only down-regulated (P < 0.01) DIO2 after 6 and 8 days of hyperthermia, respectively. Furthermore, HS influenced expression of selenoproteins and up-regulated (P < 0.01–0.05) GPX1, GPX4 and SEPN1 after 6 days of HS. The damage to development of mouse skeletal muscle myotubes by HS accompanied with the up-regulation of both selenoprotein encoding genes and proteins, which suggested a potential protective effect of selenoprotein on hyperthermia associated damage in C2C12 cells.
Similar content being viewed by others
Introduction
The climate change with increased surface temperature on the earth occurs globally in the past decades. Heat stress (HS) has been is a challenge of the animal industry. HS can be simply defined as a condition in which the animal cannot dissipate excess heat in the body, either produced by itself or absorbed from the environment, to maintain its body thermal balance1. The disruption of thermal balance by HS negatively impacts animal’s physiology and performance including decreases in feed intake and milk yield, alterations in milk composition and carcass traits, growth retardation and reproduction disorders2,3,4,5,6, which severely influence the animal agriculture. Thus, HS induces financial burden globally7,8, and part of the economic distress derives from decreased carcass value. It has been documented over the past 40 years that the pig under HS has reduced muscle mass and increased adipose tissue9,10,11. Rats exposed to heat stress exhibit a subsequent retardation of muscle development12.
Skeletal muscle is the major component of edible animal products. Skeletal muscle is differentiated from satellite cells and is highly adaptive to stress due to a remarkable regenerative capability, which is attributed to the high proliferation and differentiation rate of satellite cells. Myogenesis is a two-step process including determination of the muscle lineage committed from satellite cells and differentiation of committed myoblasts to myotubes13,14. The C2C12 cells derived from murine skeletal muscle cells is a well-established model to study muscle regeneration and differentiation15. In our study, C2C12 cells were used to investigate the response of skeletal muscle cells to heat stress during differentiation.
During differentiation of C2C12 cells, myoblasts undergo remodeling to form mature myotubes in parallel with the increased expression of muscle specific genes16. This process requires activation of myogenic regulatory factors (MRF), including myogen termination gene (MYOD), MYOGENIN, MRF4, and myogenic factor 5 (MYF5)17. 5′-AMP-activated protein kinase (AMPK) is well known as a sensor for cell energy status18,19,20 and plays an important role in muscle development that the activation of AMPK inhibits myogenesis and hypertrophy of skeletal muscle cells, and decreases muscle mass21. Heat stress has been associated with abnormality of cell function, including inhibition of protein synthesis, changes in protein folding and function, alteration in metabolism and membrane fluidity22,23. The nucleated cell responds to short period of (non-damaging) stress by synthesizing heat shock proteins (HSPs), a family of stress responsive protein. HSP70 is a well characterized marker of cellular stress responding to heat and other stressors in a variety of organism24. Increased cellular content of HSP70 protects cells from stress induced impairment16.
Selenium (Se) is a micronutrient essential for animals. Study shows that Se supplementation alleviates the negative effect of HS25. Selenium exerts most of its biological functions in the form of selenoproteins, which contain at least one selenocysteine (Sec) in their active center26. A total of 25 selenoprotein coding genes have been identified in mammals and 24 in rodents27,28. Selenoproteins have been involved in the regulation of redox balance, protection protein from oxidized damage, immunomodulatory, cell apoptosis, protein folding, and degradation of misfolded proteins in endoplasmatic reticulum (ER)29. Our previous studies showed that selenoprotein encoding genes were influenced by HS in a porcine small intestinal epithelial cell line (IPEC-J2)30, which suggested that they play important roles in cells under HS. However, the metabolic impact of HS on skeletal muscle and expression of selenoprotein encoding genes remain unclear, and it is necessary to explore the impact of HS on expression of selenoproteins using skeletal muscle cells model.
C2C12 isolated from mouse lines by Yaffe and Saxel, which mimics the development of skeletal muscle in vivo, representing an excellent model to study myogenic regulation and response to stimuli14,31. Therefore, we conducted this to determine (1) impact of HS on myogenic differentiation in C2C12 cells; (2) effect of HS on the gene or protein expression of selenoproteins, myoblast differentiation-related protein, apoptosis-related protein and HSP70; (3) impact of HS on antioxidant attributes of C2C12 cells. The results may help to further explore the potential roles of selenoproteins in skeletal cells faced to HS.
Results
Effect of HS on C2C12 cell differentiation
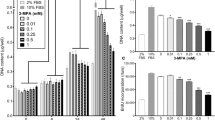
As shown in Fig. S1, serum starvation medium triggered myogenic differentiation as indicated by formation of myotubes after 4 days of induction, while HS impaired development of muscle myotubes that cells became round with dramatically decreased number of myotubes. Compared to control cells, the myotubes incubated at 41.5 °C were poorly formed (Fig. 1A). The fusion index was increased (P < 0.01) from 11.1% to 26.0% after 4, 6 and 8 days of incubation in control cells, whereas it was significantly decreased (P < 0.01) when cells were incubated at 41.5 °C (Fig. 1B).
Morphological changes of differentiated C2C12 cells after exposure to heat stress. (A) Representative images of differentiated C2C12 subject to HS. Blue color indicated DAPI-stained nuclei and red color indicated stained differentiated myotubes. Bars, 200 µm; (B) Changes of fusion index of C2C12 cells after exposure to heat stress. Each column shows means ± SE of 3 independent cultures (n = 3). **P < 0.01.
Effect of HS on expression of differentiation-related genes and AMPK genes
We further investigated effect of HS on mRNA and protein expression of MYOD, MYOGENIN, AMPKα1 and AMPKα2 in the differentiating C2C12 cells (Fig. 2). Compared to the CK groups, HS decreased (P < 0.01) mRNA expression of MYOD by 66%, 60%, 83%, and MYOGENIN by 66%, 63%, 83%, in C2C12 cells at day 4, 6, 8, respectively (Fig. 2A–C). HS also decreased (P < 0.01) the protein abundance of MYOGENIN by 47% at day 6 (Fig. 2D), confirming HS impairs C2C12 cell differentiation. HS increased AMPKα1 (P < 0.01) and AMPKα2 (P < 0.05) mRNA profiles at day 4 (Fig. 2A), but decreased (P < 0.05) mRNA expression of AMPKα2 at day 8 (Fig. 2C) in differentiated C2C12 cells.
Effect of HS on relative mRNA profiles of AMPKα1, AMPKα2, MYOD and MYOGENIN and protein level of MYOGENIN in the differentiated C2C12 cells. mRNA expression at day 4 (A); at day 6 (B); at day 8 (C); Protein level of MYOGENIN at day 6 (D). Values are means ± SE (n = 6 for genes and 3 for protein). **P < 0.01, *P < 0.05.
Effect of HS on expression of HSP70
We investigated effect of HS on mRNA and protein levels of HSP70, which is a sensitive cellular indicator for heat stress. As expected, HS increased (P < 0.01) both mRNA (Fig. 3A) and protein (Fig. 3B) levels of HSP70 in differentiated C2C12 cells, respectively.
Effect of HS on expression of selenoproteins
We explored effect of HS on mRNA abundance of 24 selenoprotein encoding genes in the myogenic differentiated C2C12 cells. HS increased (P < 0.05) mRNA profiles of 18 selenoprotein encoding genes (DIO2, GPX1, GPX3, GPX4, MSRB1, SELENOF, SELENOI, SELENOK, SELENON, SELENOO, SELENOP, SELENOS, SELENOT, SELENOW, SEPHS2, TXNRD1, TXNRD2, TXNRD3) at the early stage (day 4) (Fig. 4A). With prolonged HS challenge, the number of up-regulated selenoprotein encoding genes decreased. HS led to increases in mRNA expression of 11 selenoprotein encoding genes (GPX3, GPX4, SELENOI, SELENOK, SELENOM, SELENON, SELENOO, SELENOS, SEPHS2, TXNRD2, TXNRD3) at day 6 (Fig. 4B) and 8 genes (GPX1, SELENOI, SELENOK, SELENON, SELENOS, SEPHS2, TXNRD1, TXNRD2) at day 8 (Fig. 4D), respectively. Interestedly, DIO2 was up-regulated (P < 0.01) at early stage (day 4) of HS (Fig. 4A), while it was down-regulated (P < 0.01) at late stage (day 6 and 8) of HS (Fig. 4C,E). Furthermore, DIO2 was the only selenoprotein gene that was down-regulated in differentiated C2C12 cells under HS. The profiles of selenoprotein encoding genes are shown in the Table S2.
Effect of HS on relative mRNA levels of selenoprotein encoding genes in the differentiated C2C12 cells. (A) The up-regulated selenoprotein genes under HS for 4 days; (B) The up-regulated selenoprotein genes under HS for 6 days; (C) The down-regulated selenoprotein genes under HS for 6 days; (D) The up-regulated selenoprotein genes under HS for 8 days; (E) The down-regulated selenoprotein genes under HS for 8 days. Data are means ± SE (n = 6). **P < 0.01, *P < 0.05.
We also investigated effect of HS on protein expression of 5 selenoproteins (GPX1, GPX4, SEPS1, SEPN1, and TRXR2) at day 6. Among those selenoproteins investigated, GPX1 and GPX4 have a higher distribution in skeletal tissues and SEPN1 is a selenoprotein relating to muscle development. As shown in Fig. 5, HS increased GPX1 (P < 0.05), GPX4 (P < 0.05) and SEPN1 (P < 0.01) protein abundance, and decreased (P < 0.01) SEPS1 abundance while exhibited no effect on TRXR2 (P > 0.05) in the differentiated C2C12 cells. The limited availability of antibodies in our Lab prevents us from exploring more selenoproteins in the present study.
Effect of HS on cell apoptosis
To determine whether HS induces apoptosis in differentiated C2C12 cells, we investigated effect of HS on expression of BCL-2 and BAX (Fig. 6). The results showed that HS increased both mRNA abundance (P < 0.01) (Fig. 6A) and protein levels (P < 0.05) (Fig. 6B) of BCL-2 and BAX. The ratio of BCL-2/BAX at mRNA and protein level (Fig. 6C) was also significantly increased (P < 0.01) by HS.
Effect of HS on the mRNA and protein levels of BAX and BCL-2 in the differentiated C2C12 cells after incubation for 6 day. (A) The mRNA abundance of the BAX and BCL-2 (n = 6). (B) The protein levels of BAX and BCL-2 (n = 3). (C) The mRNA and protein ratio of BCL-2 to BAX. Values are means ± SE. **P < 0.01, *P < 0.05.
Effect of HS on antioxidant attributes in C2C12 cells
To determine whether HS induces oxidative stress in differentiated C2C12 cells, effect of HS on activity of glutathione peroxidase (GSH-Px), total superoxidase dismutase (T-SOD), and concentration of malondialdehyde (MDA) in differentiated C2C12 cells were investigated, and results are shown in Table 1. Compared to control cells, HS challenge for 6 days significantly increased (P < 0.01) the activity of T-SOD while had no effect on activity of GSH-Px. MDA is an indicative for oxidative stress in cells or organism. The results showed that HS decreased (P < 0.01) the levels of MDA in C2C12 cells.
Discussion
In this study, our target was to investigate effect of HS on the expression of selenoproteins in differentiating C2C12 mouse myoblast. Firstly, we investigated the effect of HS on myogenic differentiation of C2C12 cells. HS impaired the differentiation of C2C12 cells as shown by the suppression of myotube formation in a hyperthermia condition (Fig. 1). Similar results were reported in mouse study that HS impeded the development of myotube in skeletal muscles16. Myotubes were poorly formed when primary human skeletal muscle culture cells, human skeletal muscle myoblasts (HSMMs), and C2C12 mouse myoblasts were cultured at 41 °C32.
We investigated expression of two myoblast differentiation-related genes. MYOD is essential for skeletal muscle differentiation33 through mediating the expression of some muscle-specific genes34. Previous study showed the absence of MYOGENIN resulted in a deficiency of muscle fiber despite muscle cell migration and commitment31. In our study, HS decreased expressions in both mRNA and protein levels of MYOD and MYOGENIN, indicating that HS suppressed the myogenic differentiation of C2C12 cells. We also investigated expression of two AMPK genes. Interestingly, we found HS increased the mRNA expression of AMPKα1 and AMPKα2 at day 4, while decreased AMPKα2 at day 8. The up-regulation of AMPKα1 and AMPKα2 at early stage of HS (at day 4) may reflect an increased energy requirement for adaption of metabolism and cell survival. With prolonged HS, cells gradually lost the adaptive function as cell impairment occurred (Fig. 2C).
Heat shock proteins are considered as a cellular thermometer, which is frequently used to evaluate HS response35. HSPs are expressed globally in a variety of species and are required for cell survival under stress36. The previous studies showed a significant increase in the induction of HSPs, mainly HSP70 and HSP90, in different tissues and cells under HS37,38. Increased cellular HSPs can provide cytoprotection against subsequent stresses16. HSP70 is the most ubiquitous chaperones and is highly conserved in all organisms39. Thus, it has been frequently used to characterize stress response to heat and other stressors in different organisms24,40. It was not surprising that HS increased gene and protein expression of HSP70 (P < 0.01) in the differentiated C2C12 cells (Fig. 3), which was consistent with previous studies30,41.
Selenoprotein encoding genes encode for selenocysteine-containing proteins (selenoproteins), which are involved in a variety of functions including redox homeostasis regulation28. However most of their functions are still unknown. Our previous study showed that both mRNA and protein expression of selenoprotein encoding genes were influenced by HS for 24 h in IPEC-J2 cells, and 4 selenoprotein genes (GPX3, DIO2, SELENOK, SELENOS) were up-regulated (P < 0.05) and six selenoprotein genes (GPX2, GPX6, TXNRD1, SELENOH, SELENOM, MSRB1) were down-regulated (P < 0.05 or as indicated) in IPEC-J2 cells by HS30. Interestingly, in this study, selenoprotein encoding genes (except DIO2) were globally up-regulated by HS in C2C12 cells, which suggesting their potential roles against HS-induced cell damage (Fig. 4). The numbers of these up-regulated genes decreased from 18 to 8 genes from day 4 to day 8 indicating decreased metabolism with exposure duration of HS. It was reported that genes related to cell survival will be turned on, while more unessential genes may be turned off under stress conditions42.
Among those selenoproteins influenced by HS, GPXs contribute to antioxidant system in mammals43. GPX1 deficiency is correlated with increased susceptibility to oxidative stress44. The increased expression of GPX3 may contribute to detoxify reactive oxygen species (ROS) such as phospholipid hydroperoxide and hydrogen peroxide induced by HS45. In this study, HS increased expression of GPX1, GPX3, and GPX4 in the differentiated C2C12 cells, indicating the potential protective effects of these selenoproteins in muscle cells against HS.
SELENOK, SELENOM and SEPS1 are endoplasmic reticulum (ER) transmembrane proteins. SELENOK is an ER stress-regulating protein, which modulates cellular redox balance46,47. SELENOM acts as a thiol-disulfide oxidoreductase involved in protein folding48. SEPS1 induces production of inflammatory cytokines and protects the cell compartment from oxidative stress49. The up-regulation of SELENOS, SEPHS2 and SELENOK in our study suggested an important role of these selenoproteins in protecting cells from the damage of HS. Although mRNA expression of SELENOS was up-regulated, protein level of SEPS1 was down-regulated when cells were challenged with HS for 6 days (Fig. 5). SEPN1 has been involved in muscle physiology as a key regulator of satellite function50,51,52. SEPN1 shows a high expression during the proliferation of fibroblast and myoblast, but it decreases when myoblasts differentiate into myotubes53. Absence of SEPN1 was associated with high susceptibility to H2O2-induced oxidative stress, leading to cell death54. The increased SEPN1 expression by HS in differentiated C2C12 cells suggested SEPN1 may protect C2C12 cells from HS.
Thioredoxin (TRX) is an antioxidant that reduces oxidized moieties55. Thioredoxin reductases (TRXRs) are crucial to regenerate reduced TRX to maintain balance between reduced and oxidized molecules56,57. The up-regulation of TXNRD1, TXNRD2 and TXNRD3 in C2C12 cells suggested that TRX might contribute to maintain the redox balance in muscle cells under HS, these may partly explained why MDA were not increased in the stressed cells (Table 1). The protein levels of TRXR2 were not decreased by HS at the 6th day (Fig. 5), implying a physiological necessity for a constant expression of TRXR2 to deal with HS.
Iodothyronine deiodinase 2 (DIO2) converts thyroxine (T4) to bioactive 3,5,3′-tri-iodothyronine (T3) to initiate the action of thyroid hormone58. DIO family is comprised of 3 isoforms, DIO1, DIO2 and DIO3. DIO2 was the only selenoprotein encoding gene that was down-regulated by HS in C2C12 cells. It has reported that T3 generated from T4 by DIO2 is key to maintain C2C12 cells differentiation, and T3 were essential for the enhanced transcription of MyoD59. In the present study, decreases expression of DIO2, MYOD and MYOGENIN is consistent with the low level of differentiation under HS. Thyroid hormone improves critical protein synthesis60, however cells may have to decrease cell metabolism to survive with extended HS61, which may partly explain the down-regulation of DIO2 (P < 0.01) at late stage (Fig. 4C,E).
Hyperthermia investigations at cellular level showed some types of cell underwent apoptosis in response to heat stress22. BCL-2 genes play important roles in regulating apoptosis, including antiapoptotic protein BCL-2 and proapoptotic protein BAX62. The ratio of BCL-2 to BAX represents the level of apoptosis63. We found that HS increased (P < 0.01) ratios of BCL-2/BAX at both mRNA and protein level in C2C12 cells, which were consistent with previous study in C2C12 cells64. Increased BAX may indicate apoptosis, and increase in the BCL-2/BAX ratio would indicate the anti-apoptosis. The up-regulation of selenoproteins may contribute to anti-apoptosis and prevent cells underwent apoptosis by HS. ROS generated through a variety of extracellular and intracellular actions has drawn attention as novel signal mediator involved in growth, differentiation, progression, and death of cells65. A previous study has shown that HS caused overproduction and accumulation of ROS, leading to the impairment of cells66. Chicken exposed to HS resulted in a significant increase in activities of SOD, CAT and GPx67. In the present study, HS greatly increased activity of T-SOD in C2C12 cells, while decreased levels of MDA (Table 1). GSH-Px showed no response, however it increased in value (P = 0.14) by HS. MDA is used as a biomarker to measure the level of oxidative stress in an organism68, and the decreased levels of MDA indicate cells were not in an oxidative stress condition. Our previous results shows that HS has limited effect on antioxidant measurements in porcine IPEC-J2 cells, and MDA exhibits a decreasing tendency in HS stressed cells30. It seems oxidative stress is not the major factor for C2C12 cells damages induced by HS, possibly the up-regulation of selenoprotein encoding genes contribute to preventing the increasing of MDA.
In summary, HS impairs the differentiation of C2C12 cells and induces selenoprotein responses. Although information available concerning the relations between selenoproteins response and HS in skeletal muscle was still limited, studies yet elucidated was that the increased mRNA and protein expression of HSP70 protected cells from heat stress. Therefore, many selenoprotein encoding genes or proteins were up-regulated in C2C12 cells under HS, which implied the potential protective effect of these selenoproteins against the impairment induced by hyperthermia. The results may also implied the potential of these selenoproteins act as target genes or protein be used to further investigate the effect of husbandry temperature on meat quality or production.
Materials and Methods
Cell culture
The C2C12 mouse myoblast cell line was maintained in medium (DMEM; Gibco, USA) containing 1% penicillin-streptomycin (Gibco, USA) and 10% (v/v) fetal bovine serum (FBS; Gibco, USA). 1 × 105 cells/well of cells were seeded in 12-well plates and cultured at 37 °C under 5% CO2. After reaching to 80% confluence, cells were divided into two groups: cells in control group (CK) were cultured at 37 °C, while cells in HS group (HS) were exposed to a hyperthermia condition at 41.5 °C. Meanwhile, differentiation were triggered by replacing 10% FBS to 2% horse serum (Gibco, USA), and cells were cultured for another 4, 6 or 8 days. The differentiation media were changed every two days.
Immunofluorescence staining
After HS treatment for 4, 6, 8 days during differentiation, cells were washed with warm PBS (37 °C) and fixed in 4% paraformaldehyde at room temperature for 30 min and then applied for immunofluorescent staining for myotubes and 4,6-diamidino-2-phenylindole (DAPI) staining for nuclei as described by Yamaguchi et al.32. The primary antibody was mouse anti-MyHC (1:200; Zen BioScience, China) and the secondary antibody was fluorescence-conjugated goat anti-mouse IgG (1:1000; Millipore, USA). The immunofluorescence stained cells were examined with fluorescent microscope (DMI 4000B; Leica, Germany). The fusion index was defined and determined according to Yamaguchi et al.32.
Real-time quantitative PCR analyses
After HS treatment for 4, 6, 8 days during differentiation, the cells were harvested for total RNA extraction using TRIzol (Invitrogen, USA). Two wells of cells were pooled together and in each treatment six samples were collected (n = 6). The qPCRs procedure and relative mRNA abundance quantification were conducted as previously described using 2−ΔΔCt method30,69. For each measurement, all samples were run on the same plate. Primer Express 3.0 (Applied Biosystems, USA) was used for primers design and primers for 4 myogenic differentiation-related genes (AMPKα1, AMPKα2, MYOD and MYOGENIN), 24 selenoprotein encoding genes, HSP70, 2 apoptosis-related genes (BAX and BCL-2), and 2 reference gene (β-ACTIN and GAPDH) are presented in Table S1.
Western blot analyses
Cell culture and HS treatment were conducted as mentioned above, and cells for protein extraction were grown in 6-well plates. After HS treatment for 6 days, cells were harvested and protein was extracted using RIPA lysis buffer30. Each treatment contain three replicates (n = 3) and four wells of cells were pooled together for each replicate. Western blot was processed as described previously by our group30. The primary antibodies included MYOGENIN (1:800; Zen BioScience, China), HSP70 (1:5000; Abcam, USA), GPX1 (1:1000; Zen BioScience, China), GPX4 (1:2000; Zen BioScience, China), SEPS1 (1:800; Zen BioScience, China), SEPN1 (1:800; Proteintech, China), TRXR2 (1:800; Zen BioScience, China), BAX (1:5000; Proteintech, China), BCL-2 (1:800; Proteintech, China), and β-ACTIN (1:5000; Millipore, USA). The secondary antibodies were horseradish peroxidase-linked goat anti-rabbit IgG (1:10000; CST, USA) or goat anti-mouse IgG (1:20000; Millipore, USA). Electrochemiluminescence (ECL) was used to detect a specific protein signal and western blot bands were analyzed using Image Lab™ software system (Bio-Rad, USA).
Enzyme activity assays
After HS treatment for 6 days, cells were harvested and digested with 0.25% trypsin. Samples (n = 6) were prepared as described previously30. Activity of GSH-Px, T-SOD and concentration of MDA were determined using corresponding kit according to the manufacturer’s instructions. Kits for GSH-Px (No. A005), T-SOD (No. A001–1–1) and MDA (No. A003-4) were purchased from Jiancheng Bioengineering, China, respectively. Protein concentration was determined by the BCA method. The optical density (OD) values were measured with an UV-visible spectrophotometer (SpectraMax 190, MD, USA).
Statistical analysis
Independent t-test (SPSS for Windows 13.0, Chicago, IL) was used to determine the influence of HS on investigated index in C2C12 cells. Data are presented as means ± SE and significance level is set at P < 0.05.
References
Bernabucci, U. et al. The effects of heat stress in Italian Holstein dairy cattle. J. Dairy Sci. 97, 471–486 (2014).
Wheelock, J. B. et al. Effects of heat stress on energetic metabolism in lactating Holstein cows. J. Dairy Sci. 93, 644–655 (2010).
West, J. W. Effects of heat-stress on production in dairy cattle. J. Dairy Sci. 86, 2131–2144 (2003).
Baumgard, L. H. & Rhoads, R. P. Jr. Effects of heat stress on postabsorptive metabolism and energetics. Annu. Rev. Anim. Biosci. 1, 311–337 (2013).
Chand, N. et al. Performance traits and immune response of broiler chicks treated with zinc and ascorbic acid supplementation during cyclic heat stress. Int. J. Biometeorol. 58, 2153–2157 (2014).
Gao, C. Q. et al. Heat stress inhibits proliferation, promotes growth, and induces apoptosis in cultured Lantang swine skeletal muscle satellite cells. J. Zhejiang Univ. Sci. B. 16, 549–559 (2015).
St-Pierre, N. R., Cobanov, B. & Schnitkey, G. Economic losses from heat stress by US livestock industries. J. Dairy Sci. 86, E52–E77 (2003).
Pollman, D. Seasonal effects on sow herds: Industry experience and management strategies. J. Anim. Sci. 88, 9 (2010).
Bridges, T. C., Turner, L. W. & Gates, R. S. Economic evaluation of misting-cooling systems for growing/finishing swine through modeling. Appl. Eng. Agric. 14, 425–430 (1998).
Collin, A. et al. Effect of high temperature and feeding level on energy utilization in piglets. J. Anim. Sci. 79, 1849–1857 (2001).
Qu, H. et al. Heat stress in pigs is accompanied by adipose tissue-specific responses that favor increased triglyceride storage. J. Anim. Sci. 94, 1884–1897 (2016).
Naito, H. et al. Heat stress attenuates skeletal muscle atrophy in hindlimb-unweighted rats. J. Appl. Physiol. 88, 359–363 (2000).
Pownall, M. E., Gustafsson, M. K. & Emerson, C. P. Jr. Myogenic regulatory factors and the specification of muscle progenitors in vertebrate embryos. Ann. Rev. Cell Dev. Biol. 18, 747–783 (2002).
Moran, J. L. et al. Gene expression changes during mouse skeletal myoblast differentiation revealed by transcriptional profiling. Physiol. Genomics 10, 103–111 (2002).
Schöneich, C. et al. Apoptosis in differentiating C2C12 muscle cells selectively targets Bcl-2-deficient myotubes. Apoptosis 19, 42–57 (2014).
Maglara, A. A. et al. Damage to developing mouse skeletal muscle myotubes in culture: protective effect of heat shock proteins. J. Physiol. 548, 837–846 (2003).
Rudnicki, M. A. & Jaenisch, R. The MyoD family of transcription factors and skeletal myogenesis. Bioessays 17, 203–209 (1995).
Hardie, D. G. Energy sensing by the AMP-activated protein kinase and its effects on muscle metabolism. Proc. Nutr. Soc. 70, 92–99 (2011).
Meisse, D. et al. Sustained activation of AMP-activated protein kinase induces c-Jun N-terminal kinase activation and apoptosis in liver cells. FEBS let. 526, 38–42 (2002).
Kefas, B. A. et al. AICA-riboside induces apoptosis of pancreatic beta cells through stimulation of AMP-activated protein kinase. Diabetologia 46, 250–254 (2003).
Lantier, L. et al. Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J. 24, 3555–3561 (2010).
Yonezawa, M. et al. Hyperthermia induces apoptosis in malignant fibrous histiocytoma cells in vitro. Int. J. Cancer 66, 347–351 (1996).
Murtha-Riel, P. et al. Expression of a phosphorylation-resistant eukaryotic initiation factor 2 alpha-subunit mitigates heat shock inhibitor of protein synthesis. J. Biol. Chem. 268, 12946–12951 (1993).
Mayer, M. P. & Bukau, B. Hsp70 chaperones: cellular functions and molecular mechanism. Cell Mol. Life Sci. 62, 670–684 (2005).
Habibian, M., Ghazi, S. & Moeini, M. M. Effects of dietary selenium and vitamin E on growth performance, meat yield, and selenium content and lipid oxidation of breast meat of broilers reared under heat stress. Biol. Trace Elem. Res. 169, 142–152 (2016).
Lobanov, A. V., Hatfield, D. L. & Gladyshev, V. N. Eukaryotic selenoproteins and selenoproteomes. Biochim Biophys Acta 1790, 1424–1428 (2009).
Kryukov, G. V. et al. Characterization of mammalian selenoproteomes. Science 300, 1439–1443 (2003).
Moghadaszadeh, B. & Beggs, A. H. Selenoproteins and their impact on human health through diverse physiological pathways. Physiology 21, 307–315 (2006).
Mujahid, A., Akiba, Y. & Toyomizu, M. Olive oil-supplemented diet alleviates acute heat stress-induced mitochondrial ROS production in chicken skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 297, R690–R698 (2009).
Cao, L. et al. Expression of selenoprotein genes is affected by heat stress in IPEC-J2 cells. Biol. Trace Elem. Res. 172, 354–360 (2016).
Salmon, M., Owens, G. K. & Zehner, Z. E. Over-expression of the transcription factor, ZBP-89, leads to enhancement of the C2C12 myogenic program. Biochim. Biophys. Acta. 1793, 1144–1155 (2009).
Yamaguchi, T. et al. Continuous mild heat stress induces differentiation of mammalian myoblasts, shifting fiber type from fast to slow. Am. J. Physiol. Cell Physiol. 298, C140–C148 (2010).
Rudnicki, M. A. et al. MyoD or Myf-5 is required for the formation of skeletal muscle. Cell 75, 1351–1359 (1993).
Davis, R. L., Weintraub, H. & Lassar, A. B. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell 51, 987–1000 (1987).
Katschinski, D. M. On heat and cells and proteins. News Physiol. Sci. 19, 11–15 (2004).
Leu, J. I. et al. A small molecule inhibitor of inducible heat shock protein 70. Mol. Cell. 36, 15–27 (2009).
Okubo, S. et al. Gene transfer of heat-shock protein 70 reduces infarct size in vivo after ischemia/reperfusion in the rabbit heart. Circulation 103, 877–881 (2001).
Perrone, C. E., Fenwick-Smith, D. & Vandenburgh, H. H. Collagen and stretch modulate autocrine secretion of insulin-like growth factor-1 and insulin-like growth factor binding proteins from differentiated skeletal muscle cells. J. Biol. Chem. 270, 2099–2106 (1995).
Radons, J. The human HSP70 family of chaperones: where do we stand? Cell Stress Chaperones 21, 379–404 (2016).
Daugaard, M., Rohde, M. & Jäättelä, M. The heat shock protein 70 family: Highly homologous proteins with overlapping and distinct functions. FEBS Lett. 581, 3702–3710 (2007).
Dieterich, A. et al. Hsp70 and lipid peroxide levels following heat stress in Xeropicta derbentina (Krynicki 1836) (Gastropoda, Pulmonata) with regard to different colour morphs. Cell Stress Chaperones 20, 159–168 (2015).
Nadal, E., de., Ammerer, G. & Posas, F. Controlling gene expression in response to stress. Nat. Rev. Genet. 12, 833–845 (2011).
Yao, H. et al. Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol. Trace Elem. Res. 161, 318–327 (2014).
Handy, D. E. et al. Glutathione peroxidase-1 regulates mitochondrial function to modulate redox-dependent cellular responses. J. Biol. Chem. 284, 11913–11921 (2009).
Koyama, H. et al. Separation of selenium-containing proteins in human and mouse plasma using tandem high-performance liquid chromatography columns coupled with inductively coupled plasma-mass spectrometry. Anal. Biochem. 267, 84–91 (1999).
Lu, C. et al. Identification and characterization of selenoprotein K: an antioxidant in cardiomyocytes. FEBS Lett. 580, 5189–5197 (2006).
Du, S. et al. SelK is a novel ER stress-regulated protein and protects HepG2 cells from ER stress agent-induced apoptosis. Arch Biochem Biophys 502, 137–143 (2010).
Ferguson, A. D. et al. NMR structures of the selenoproteins Sep15 and SelM reveal redox activity of a new thioredoxin-like family. J. Biol. Chem. 281, 3536–3543 (2006).
Gao, Y. et al. Regulation of the selenoprotein SelS by glucose deprivation and endoplasmic reticulum stress-SelS is a novel glucose-regulated protein. FEBS Lett. 563, 185–190 (2004).
Moghadaszadeh, B. et al. Selenoprotein N deficiency in mice is associated with abnormal lung development. FASEB J. 27, 1585–1599 (2013).
Rederstorff, M. et al. Increased muscle stress-sensitivity induced by selenoprotein N inactivation in mouse: a mammalian model for SEPN1-related myopathy. PloS One 6, e23094 (2011).
Castets, P. et al. Satellite cell loss and impaired muscle regeneration in selenoprotein N deficiency. Hum. Mol. Genet. 20, 694–704 (2011).
Petit, N. et al. Selenoprotein N: an endoplasmic reticulum glycoprotein with an early developmental expression pattern. Hum. Mol. Genet. 12, 1045–1053 (2003).
Arbogast, S. et al. Oxidative stress in SEPN1-related myopathy: from pathophysiology to treatment. Ann. Neurol. 65, 677–686 (2009).
Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid Redox Signal. 2, 811–820 (2000).
Lu, J., Berndt, C. & Holmgren, A. Metabolism of selenium compounds catalyzed by the mammalian selenoprotein thioredoxin reductase. Biochim Biophys Acta 1790, 1513–1519 (2009).
Turanov, A. A. et al. Mammalian thioredoxin reductase 1: roles in redox homoeostasis and characterization of cellular targets. Biochem. J. 430, 285–293 (2010).
Pappas, A. C. et al. Selenoproteins and maternal nutrition. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 151, 361–372 (2008).
Dentice, M. et al. The FoxO3/type 2 deiodinase pathway is required for normal mouse myogenesis and muscle regeneration. The Journal of Clinical Investigation 11, 4021–4030 (2010).
Muller, M. J. et al. Effect of thyroid hormones on oxidative and nonoxidative glucose metabolism in humans. Am. J. Physiol. 255, E146–E152 (1988).
Tomanek, L. & Zuzow, M. J. The proteomic response of the mussel congeners Mytilus galloprovincialis and M. trossulus to acute heat stress: Implications for thermal tolerance limits and metabolic costs of thermal stress. J. Exp Biol 213, 3559–3574 (2010).
Franklin, J. L. Redox regulation of the intrinsic pathway in neuronal apoptosis. Antioxid Redox Signal. 14, 1437–1448 (2011).
Del Poeta, G. et al. Amount of spontaneous apoptosis detected by Bax/Bcl-2 ratio predicts outcome in acute myeloid leukemia (AML). Blood 101, 2125–2131 (2003).
Chen, K. L. et al. The protective effect of rosmarinic acid on hyperthermia-induced C2C12 muscle cells damage. Mol. Biol. Rep. 41, 5525–5531 (2014).
Sena, L. A. & Chandel, N. S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell. 48, 158–167 (2012).
Xu, S. et al. Effects of heat acclimation pretreatment on changes of membrane lipid peroxidation, antioxidantmetabolites, and ultrastructure of chloroplasts in two coolseason turfgrass species under heat stress. Environ Exp. Bot. 56, 274–285 (2006).
Altan, Ö. et al. Effect of heat stress on oxidative stress, lipid peroxidation and some stress parameters in broilers. Br. Poult. Sci. 44, 545–550 (2003).
Rio, D. D., Stewart, A. J. & Pellegrini, N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr. Metab. Cardiovasc Dis. 15, 316–328 (2005).
Zhao, H. et al. Expression of selenoprotein genes is affected by obesity of pigs fed a high-fat diet. J. Nutr. 145, 1394–1401 (2015).
Acknowledgements
This work was supported partly by the National Natural Science Foundation of China (No. 31772643 and 31272468), the Foundation of Educational Department of Sichuan (17ZA0318) and by the Special Research Funding for Discipline Construction in Sichuan Agricultural University (No. 03570126).
Author information
Authors and Affiliations
Contributions
H.Z. conceived the study, designed the experiments and wrote the manuscript; J.T. and A.H. carried out the experiments and analyzed the data; H.Y. contributed to the manuscript writing; G.J., G.L., X.C., J.C., G.T. and H.S. contributed to sample collection. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tang, J., He, A., Yan, H. et al. Damage to the myogenic differentiation of C2C12 cells by heat stress is associated with up-regulation of several selenoproteins. Sci Rep 8, 10601 (2018). https://doi.org/10.1038/s41598-018-29012-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-29012-6
This article is cited by
-
Effects of energy drinks on myogenic differentiation of murine C2C12 myoblasts
Scientific Reports (2023)
-
Synergistic effect of Spirulina platensis and selenium nanoparticles on growth performance, serum metabolites, immune responses, and antioxidant capacity of heat-stressed broiler chickens
Biological Trace Element Research (2022)
-
Cloning and promoter analysis of palladin 90-kDa, 140-kDa, and 200-kDa isoforms involved in skeletal muscle cell maturation
BMC Research Notes (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.