Abstract
Streptococcus mutans and Streptococcus sobrinus are the main causative agents of human dental caries. Current strategies for treating caries are costly and do not completely eradicate them completely. Passive immunization using nonhuman antibodies against Streptococcal surface antigens has shown success in human trials, however they often invoke immune reactions. We used phage display to generate human antigen-binding fragments (Fabs) against S. mutans and S. sobrinus. These Fabs were readily expressed in E. coli and bound to the surface S. mutans and S. sobrinus. Fabs inhibited sucrose-induced S. mutans and S. sobrinus biofilm formation in vitro and a combination of S. mutans and S. sobrinus Fabs prevented dental caries formation in a rat caries model. These results demonstrated that S. mutans and S. sobrinus Fabs could be used in passive immunization strategies to prevent dental caries. In the future, this strategy may be applied towards a caries therapy, whereby Fabs are topically applied to the tooth surface.
Similar content being viewed by others
Introduction
Dental caries is one of the most common global chronic diseases caused by the formation of biofilms on the tooth surface1. Gram-positive acidogenic and aciduric bacterial species, most often mutans streptococci in humans, have been associated with caries initiation and progression2. In addition to mutans streptococci (Streptococcus mutans and Streptococcus sobrinus), other microbial species have been isolated from caries including: salivarius group streptococci (S. salivarius, S. vestibularis) and S. parasanguinis as well as lactobacilli (L. gasseri, L. johnsonii, L. casei, L. paracasei) and Veillonella species (V. atypica, V. dispar, V. parvula)2,3,4,5,6. Modern molecular and microbial culture techniques have revealed that a range of bacteria can contribute to the caries process at different stages7.
Dental caries are a complex biofilm-mediated disease that occurs when acidogenic/aciduric bacteria obtain a selective advantage over other members of the oral flora, disrupting the balance of the plaque biofilm and initiating the caries8. Mutans streptococci and lactobacilli can dominate advanced stages of caries formation where increased biofilm acidification results in the biofilm becoming less microbially diverse9.
The process of caries formation is modulated by complex interactions between acid-producing bacteria and host factors9,10,11. Streptococcal surface fibrillar adhesins (e.g. antigen I/II) control attachment to the surface of the tooth by adhering to salivary agglutinin. Cell-wall-associated glucosyltransferases (GTFs) produce adhesive glucans from sucrose, which cell wall-associated glucan-binding proteins (GBP) of S. mutans bind, resulting in glucan-mediated aggregation12. Water insoluble glucans produced by S. mutans GTFs are the most important for building the biofilm structure13. Other isoforms of GTFs and glucan produced by various oral streptococci contribute to biofilm formation in a synergistic manner14. S. mutans metabolism and acid production also contribute to the pathogenesis of dental caries9,10,11,12. S. mutans can metabolize a wide variety of carbohydrates, leading to the production of lactic acid. The acid diffuses into tooth enamel and dissolves the mineral underneath the tooth surface. If the mineral dissolution is not reversed, then early lesions result in caries15. New caries preventive therapies may be developed by inhibiting biofilm formation through suppression of mutans streptococci.
Despite efforts in promoting oral hygiene and fluoridation, approximately 35% of the global population suffers from tooth decay and cavities in permanent teeth16. Current strategies for treating caries are limited to removal of the diseased part of the tooth and placing a suitable restoration, which is costly and does not eradicate caries on other teeth17,18. As a result, dentistry is beginning to move from the surgical model for treating tooth decay (placing restorations) to identification of early carious lesions and preventing or treating them with non-surgical methods19,20.
Strategies for caries prevention, such as brushing, professional cleaning, antimicrobial peptides, sugar substitutes, and chemoprophylactic agents such as classical antibiotics, chlorhexidine, and triclosan are effective against dental biofilm, but their retention in the oral cavity is poor21,22,23,24. Passive immunization by applying mucosal vaccinations such as S. mutans and S. sobrinus antigens GTFs, antigen I/II, GBP, and virulence-associated immunomodulatory extracellular proteins (VIP) at inductive sites leads to increases in IgA secretion and can be effective in preventing caries formation18,21. Many other vaccine immunogens such as synthetic peptides, DNA-based active vaccines, and mucosal adjuvants have been successful in animal models25,26,27,28. The murine monoclonal antibody Guy’s 1329, which specifically recognizes the SAI/II protein of S. mutans and S. sobrinus, has been used to prevent caries formation in non-human primates and in human clinical trials30. A humanized scFv based on Guy’s 13 antibody is capable of aggregating S. mutans in vitro31. To date, no vaccines have been brought to market, mainly due to the difficulty in inducing and maintaining protective antibody in oral fluids18.
There has been a growing interest in using antibody fragments in passive immunization strategies to treat caries32. Advantages of using antibody fragments are their low production costs and enhanced tissue penetration. We used phage display to generate Fabs against S. mutans and S. sobrinus. By selecting the Fab-phage library against live bacteria, we were able to generate Fabs against antigens presented in their native context in an unbiased manner. Using this strategy, we obtained Fabs that bound to S. mutans and S. sobrinus, blocked biofilm formation in vitro, and prevented dental caries formation in rat caries model.
Results and Discussion
Selection of antigen-binding fragments (Fabs) against S. mutans and S. sobrinus
To generate synthetic human Fabs against S. mutans and S. sobrinus, we conducted five rounds of phage display selection using a synthetic Fab phage library33 against S. mutans and S. sobrinus (Fig. 1a). We observed an increase in phage retention on the bacteria with sequential rounds of selection up to the fifth round (Fig. 1b). During the fourth and fifth rounds of selection, we included subtractive binding steps. For the S. sobrinus and S. mutans selections, we removed members of the Fab-phage library that bound E. coli and S. mutans and S. sobrinus, respectively. During selection rounds with subtractive panning, we observed a modest drop in the number of phage retained on S. mutans or S. sobrinus (Fig. 1b). Subsequent rounds of selection resulted in increased phage retention for subtracted phage pools (Fig. 1b). At the end of fifth round, we sequenced 20 phage clones from each selection.
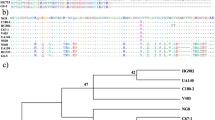
Phage display selection of Fabs against S. mutans and S. sobrinus. (a) Phage display selection scheme for isolating Fabs against S. mutans and S. sobrinus. Five rounds of phage display were performed against S. mutans or S. sobrinus. During the fourth and fifth rounds of selection, subtractive panning steps were included. For the S. sobrinus and S. mutans selections, members of the Fab-phage library were removed that bound E. coli and S. mutans and S. sobrinus, respectively. (b) The frequency of Fab-phage eluted during different rounds of selection against S. mutans and S. sobrinus. (c) Frequency of the top CDRL3 and CDRH3 sequences of Fabs against S. mutans and S. sobrinus.
For the S. mutans selection, sequences present at the highest frequencies were Fabs SM-1, SM-10, and SM-12 (Fig. 1c) and for the S. sobrinus selection Fabs SS-2 and SS-14 were most frequent sequences (Fig. 1c). The highest frequency Fabs in both selections, SM-1 and SS-2, had identical sequences. We also performed a competitive panning against S. mutans and S. sobrinus using a similar procedure as described above. For competitive panning, we induced S. mutans and S. sobrinus to form a biofilm by growing them in presence of sucrose for 4 hours. We then blocked the cell surface of S. mutans biofilm with Fabs SM-1, SM-10, and SM-12 and the S. sobrinus biofilm with Fabs SS-2 and SS-14. After 5 rounds of selection, we sequenced 20 clones from the S. mutans and S. sobrinus competitive selections. For the S. mutans selection Fab SM-C1 and SM-C-4 were most frequent Fabs and for the S. sobrinus selection, Fabs SS-C-1 and SS-C-10 were the most frequent clones. The S. mutans Fab SM-C-1 and the S. sobrinus Fab SS-C-10 were the same sequence and were the second most frequent Fab sequence for both S. mutans and S. sobrinus competitive selections. Based on their high frequency, we cloned and purified Fab SM-10, SM-12, SM-C-1 (same as SS-C-10), SM-C-4, SS-2 (same as SM-C-1), and SS-C-1 for further characterization. We expressed Fabs in E. coli and purified them using Protein L chromatography (Fig. S1). Expression yields of these Fabs were in the range of 5–20 mg/L of bacterial culture.
Fabs binding to S. mutans and S. sobrinus
To test the binding selectivity of S. mutans and S. sobrinus Fabs, we used ELISA, immunfluorescence, and flow cytometry. We performed the ELISA by adding a fixed concentration of Fabs to S. mutans, S. sobrinus, E. coli, and bovine serum albumin (BSA) immobilized on ELISA plates. Fabs interacted with both S. mutans and S. sobrinus and in most cases Fabs bound slightly better to S. mutants relative to S. sobrinus (Fig. 2a). SM-C-1 had the highest ELISA signals for both S. mutans and S. sobrinus (Fig. 2a). SM-12 Fab bound weaker to both S. mutans and S. sobrinus, and SM-C-4 Fab bound non-specifically to S. mutans and S. sobrinus, E. coli, and BSA (Fig. 2a). Except for SM-C-1 and SM-12, Fabs generally bound better to the bacteria that they were selected against, but also showed cross reactivity to S. sobrinus, indicating that antigens targeted by the Fabs were shared between S. mutans and S. sobrinus.
Binding Analyses of anti-S. mutans and ant-S. sobrinus Fabs. (a) Fab ELISA of Fab SM-10, SM-12, SM-C-1, SM-C-4, SS-2, and SS-C-1 against S. mutans and S. sobrinus. E. coli stain DH10B and bovine serum albumin (BSA) were used as negative controls. Binding of Fabs to bacteria- and BSA-coated microtiter plates was detected using HRP-conjugated anti-His antibody. Error bars represent the standard deviation from three independent experiments. (b) Immunofluorescence of selected Fabs against S. mutans and S. sobrinus. Non-specific Fab against the human cell surface receptor EphB6 was used as a negative control. Binding of Fabs to bacteria-coated microtiter plates were detected via FITC-conjugated anti-FLAG antibody.
To visualize Fab binding to S. mutans and S. sobrinus, we performed immunofluorescence with Fabs SM-10, SM-12, SMC-1, SS-2, and SS-C-1. We coated 106 CFU/mL of bacterial cell suspension on ELISA plates and added 5 μg purified Fabs. Consistent with ELISA results, Fabs bound to both S. mutans and S. sobrinus (Fig. 2b).
To confirm that Fabs bound to single cell suspensions of S. mutans and S. sobrinus, we disrupted filaments of S. mutans and S. sobrinus cells by sonication. We added 1 μM Fabs to 106 CFU/tube of suspended bacterial and analyzed binding using flow cytometry with an anti-FLAG R-Phycoerythrin-conjugated secondary antibody. Consistent with ELISA and immunofluorescence results, we observed that SM-10, SM-12, SS-2, and SS-C1 Fabs bound to both S. mutans and S. sobrinus with Fabs binding better to the bacteria that they were selected against (Fig. 3a,b). These results were generally consistent with binding results observed in the ELISA. Although the flow cytometry results indicated that the binding of SM-12 to S. mutans was tighter than SS-2 and SS-C-1, it was the least strongest interaction in the ELISA assay. A nonspecific Fab against human cell surface receptor EphB6 was used as a negative control for Fab binding and it did not bind to S. mutans and S. sobrinus. To check the specificity of these Fabs against S. mutans and S. sobrinus, a gram-negative E. coli strain was used as a negative control for bacteria binding and none of the Fabs interacted with E. coli (Fig. 3c).
In general Fabs bound better to the bacteria that they were selected against, although the S. mutans Fabs still bound to S. sobrinus and vice versa, indicating antigens that Fabs targeted were shared between S. mutans and S. sobrinus. S. mutans Fabs SM-1 and SM-C-1 were identical to S. sobrinus Fabs SS-2 and SS-C-10, respectively, confirming that these Fabs recognized common antigens expressed on S. mutans and S. sobrinus. The cross reactivity of Fabs was not unexpected as it has previously been shown that a murine monoclonal antibody against SAI/II protein of S. mutans recognizes the SAI/II protein of both S. mutans and S. sobrinus26. Further, a monoclonal antibody against antigen B of S. downei is specific for both S. sobrinus and S. downei34.
S. mutans and S. sobrinus Fabs reduced in vitro biofilm formation
To assess the effect of S. mutans and S. sobrinus Fabs on biofilm formation, we used an in vitro biofilm formation assay that measures biofilm formation on a polystyrene plate surface. We added Fabs (3 μM) to cell suspensions of S. mutans or S. sobrinus in BHI media with 1% sucrose in a 96 well plate and incubated at 37 °C for 20 hours. S. mutans or S. sobrinus cultured in BHI with 1% sucrose were used as positive controls for biofilm formation. S. mutans or S. sobrinus cultured in BHI without any sucrose were used as negative controls. Fab SM-10 showed the best blocking of S. mutans biofilm formation, followed by Fab SM-12 (Fig. 4a). The Fab SS-2 showed the highest inhibition of S. sobrinus biofilm formation, followed by Fab SS-C-1 (Fig. 4a). None of the Fabs interfered with the normal growth of S. mutans and S. sobrinus. SM-10, which blocked S. mutans biofilm the most, was also the tightest binder to S. mutans. Fabs SS-2 and SS-C-1 showed the lowest S. mutans biofilm inhibition, which was consistent with their weak binding properties to S. mutans. SS-2, which showed the highest S. sobrinus biofilm reduction, was the strongest binder to S. sobrinus. In general, we found that Fabs with strong binding properties showed high biofilm inhibition. Previously reports have also shown that polyclonal and monoclonal antibodies to Streptococcal surface antigens inhibit adherence of S. mutans cells to tooth surfaces35,36,37 and prevent caries formation in non-human primates and in human clinical trials30.
Inhibition of in vitro biofilm formation. In vitro inhibition of biofilm formation by anti-S. mutans or anti-S. sobrinus Fabs. S. mutans and S. sobrinus were cultured in brain-heart infusion (BHI) with 1% sucrose to induce biofilm formation in presence of Fabs. Positive control: S. mutans or S. sobrinus on BHI with 1% sugar. Negative control: S. mutans or S. sobrinus on BHI. Error bars indicate standard deviation from three independent experiments.
S. mutans and S. sobrinus Fabs prevent dental caries formation in rat caries model
For the rat caries model, we used a combination of S. mutans and S. sobrinus Fabs that showed the greatest biofilm formation blocking effects in vitro (SM-10 and SS-2). Three groups of 20-day old (day 1 after weaning) rats were used for this study. All experimental groups (groups 1, 2 and 3) were infected with S. mutans and S. sobrinus at day 1 of the experiment and then two times per week. All groups were also given the cariogenic diet for a total of 4 weeks. For the treatment, we used 50 µg of Fabs and delivered Fabs twice a week in the oral cavity for the duration of the experiment (total of 4 weeks). Group 1 did not receive Fabs (Fig. 5a, a,b). Group 2 only received 2 weeks of Fab treatment (Fig. 5a, c,d). Group 3 received 4 weeks of Fab treatment (Fig. 5a, e,f). At the end of the 4th week, we sacrificed rats and caries were detected by micro-CT. Compared to control group, we observed a significant higher inhibition of caries formation in rats treated with Fabs. Caries were found in four rats out of five in the control group (Fig. 5b), where we observed a total of five caries per sixty molars (Fig. 5c). In contrast, caries were detected only in 1 rat out of five for groups 2 and 3 (Fig. 5b), where we observed one caries per sixty molars (Fig. 5c). There was no difference between 2 weeks and 4 weeks of Fab treatment (local application), suggesting that Fabs show efficacy in this model as early as 2 weeks following the initial application. This experiment was repeated two times with similar results showing an overall 5-fold reduction in total number of dental caries for rats treated with Fabs. This in vivo rat model study indicated that combined application of Fabs SM-10 and SS-2 significantly reduced caries formation despite rats receiving a cariogenic diet.
Inhibition of dental caries in rat model. (a) Micro-CT analysis of interproximal caries in S. mutans- and S. sobrinus-infected rats. For in vivo experiments, 15 infection-free rats were randomly divided equally into three groups (group 1, 2 and 3). All groups were infected with S. mutans and S. sobrinus. Group 1 did not receive Fabs. Group 2 only received 2 weeks of Fab treatment. Group 3 received 4 weeks of Fab treatment. At the end of the fourth week, rats were sacrificed, mandibles and maxilla were collected and fixed with 70% ethanol for 24 hours and analyzed by micro-CT. (b) Number of rats in each group showing caries. Caries were detected and counted by micro-CT. (c). Percentage of molars showing caries. Each rat has 12 molars. We checked for caries using micro-CT in a total of 60 molar teeth in each group (5 rats in each group). This experiment was repeated 2 times. Error bars indicate standard deviation. * represents P-values < 0.0001.
In conclusion, we isolated Fabs that cross-reacted with gram-positive S. mutans and S. sobrinius although at different levels and not with the gram-negative E. coli, suggesting that they recognize a shared epitope between the gram-positive bacteria. Future work will aim at defining Fab epitopes to determine whether they are specific for mutans streptococci. Epitopes shared by a range of cariogenic bacteria may be show better efficacy in clinical trials, especially for caries when mutans streptococci are present at low levels.”
We showed that synthetic human Fabs could be generated against the cell surface of oral pathogenic bacteria and that oral application of Fabs reduced dental caries in a rat caries model. This strategy allows Fab production in large quantities in microbial culture systems, which would be more suitable to scale up for the routine control of dental caries. Secretory IgEs are generally more preferable for oral applications as they are most abundant in saliva, resistant to proteolysis, and have higher retention time in oral cavity compare to IgGs or Fabs38. Since synthetic Fabs are made using recombinant DNA technology, our system allows Fabs to be engineered easily to make human secretory IgE or IgG. This would prevent adverse immune responses seen with nonhuman antibodies. Passive immunization using synthetic Fabs is much safer and easier to administer locally compared with approaches using active vaccination. In the future, this strategy might lead towards an inexpensive and convenient general treatment for dental caries.
Methods
Bacterial strains and culture conditions
S. mutans (ATCC700610) and S. sobrinus (ATCC27351) were cultured on brain heart infusion (BHI) agar (Difco) for 24–48 h at 37 °C and 5% CO2. Single colonies were isolated and used to inoculate 3 mL of BHI and cultured overnight at 37 °C with 5% CO2. For phage display, 10 mL of BHI was inoculated with 0.5 mL of an overnight culture and grown at 37 °C with 5% CO2 to an OD600 of 1.0. For biofilm- induced competitive panning, 10 mL of BHI with 1% sucrose was inoculated with 0.5 mL of an overnight culture and grown at 37 °C with 5% CO2 for 3–4 hours. For biofilm assay, overnight cultures of S. mutans and S. sobrinus were prepared to a final concentration of 106 CFU/mL and grown in BHI with 1% sucrose for 20 hrs at 37 °C in a 5% CO2 aerobic atmosphere.
Selection protocol
The selection protocol is outlined in Fig. 1a. Target antigens for panning were live Streptococcus mutans and Streptococcus sobrinus cells. The subtraction panning was performed using E. coli (DH10B) and S. mutans (in case of S. sobrinus) or S. sobrinus (in case of S. mutans). For the selection, 10 mL of BHI was inoculated with overnight cultures of S. mutans or S. sobrinus and cultured until they reached an OD600 of 1. Bacteria were pelleted by centrifugation and used for selections. For sucrose-induced biofilm competitive selections, 10 mL of BHI supplemented with 1% sucrose was inoculated with overnight cultures of S. mutans or S. sobrinus and cultured at 37 °C with 5% CO2 for 4 hours. Bacteria were centrifuged and cell pellets were collected for selections. Approximately 109 bacterial cells were washed twice with phosphate-buffered saline (PBS), re-suspended in 1 ml of PBS containing 1% BSA, and incubated in a 1.5 mL micro-centrifuge tube on a rotator for 1 hr at room temperature. This and subsequent incubations were carried out using micro-centrifuge tubes that had been blocked with 1% BSA in PBS for 1 hr at room temperature. After 1 hr, 1013 phage particles containing antibody fragments (Fabs) were added to the tube containing 109 cells in 500 uL PBS-1% BSA and rotated for an additional 1 hr at room temperature to allow phages to bind to the bacteria. After incubation, selection tubes were washed eight times with PBS containing 0.05% Tween-20 (PBT) to remove unbound phages. During final wash, re-suspended bacteria cells were moved to a fresh tube that was pre-blocked with 1% BSA in PBS. To elute bound phages, the cell pellet was re-suspended in 500 uL of 100 mM HCl and rotated at room temperature for 5 min. The tube was centrifuged for 2 min at 10,000 RPM and the supernatant containing eluted phages was transferred to a new micro-centrifuge tube and neutralized with 1 M Tris–HCl pH 8.0. Eluted phages were used to infect NEBrαF’ E. coli cells (NEB). Phage were either amplified for use in the next round of selection or plated on LB media containing carbenicillin and kanamycin to isolate E. coli harboring individual phagemids. Subtractive panning was performed at rounds 4 and 5. During subtractive panning, phage pools amplified from round three were first incubated with E. coli and then the unbound phage pool was further incubated with S. mutans (in case of S. sobrinus) or S. sobrinus (in case of S. mutans). During sucrose-induced biofilm competitive selections, suspended bacteria were first incubated with Fabs that were isolated from non-biofilm selections and then phage particles were added to the bacteria.
DNA sequencing
At the end of the fifth round of selection, phage infected NEBrαF‘ E. coli cells were plated on LB media containing antibiotic carbenicillin and kanamycin to isolate single colonies. Colony PCR was performed to confirm the correct size of the Fab. For Sanger sequencing, phagemids from twenty E. coli colonies after fifth round of panning were isolated. Phagemids were sequenced on 3500 Genetic Analyzer (Thermo Fisher Scientific) using TGS163 and TGS164 primers (Table S1).
Fab Cloning, Expression, and Purification
Selected Fabs were PCR-amplified using TGS157 and TGS160 primers (Table S1) from phagemids. The PCR-amplified Fab amplicon was cloned into SacI- and XhoI-digested pCW-LIC Fab expression vector (Addgene) using Gibson assembly39. Each Fab contained the light chain signaling sequence, light chain (VL-CL), FLAG tag, heavy chain signaling sequence, heavy chain (VH-CH1), GGS linker, and C-terminal His6 tag in the order listed. Fab expression plasmids were transformed into BL21 (DE3) competent E. coli cells (NEB) and plated on 2YT media containing carbenicillin. Single E. coli colonies were cultured in overnight expression Instant TB media (Novagen) for 20–24 hrs40. Cells were pelleted and suspended in Protein L Binding buffer (Sodium Phosphate 20 mM, 0.15 M NaCl, pH 8.0). Cells were disrupted at 35 Kpsi using the cell disruptor (Constant System LTD. USA). Cell debris was removed by centrifuging at 12,000 RPM for 20 min. Supernatant was collected and filtered through a 0.45-micron membrane filter (Minisart, Sartorius stedim). Fabs were purified on a GE Healthcare AKTA FPLC system using a HiTrap Protein L column (GE Heathcare). Fabs were eluted from the Protein L column using IgG elution buffer (Fisher Scientific) and neutralized with neutralization buffer (1 M Tris-HCl pH 9.0). Purified Fabs were dialyzed overnight in PBS and concentrated using 10 K MWCO filter (Millipore). Fabs were filter sterilized and stored at −20 °C. Fabs were quantified using the Bicinchonic acid assay (Pierce™ BCA Protein Assay Kit, Thermo Scientific) following manufacturer’s instructions.
Enzyme linked immunosorbent assay (ELISA)
To test the binding of phage display generated Fabs, 96-well Maxisorp ELISA plates (Nunc) were coated with bacterial cell suspension containing 107 cells per well in PBS for 1 hr at 37 °C followed by overnight at 4 °C. Plates were washed three times with PBS and blocked with 2% milk powder in PBS for 2 hrs at room temperature. Purified Fabs (5 μg per well) were incubated with cells for 2 hrs at room temperature. After incubation with Fabs, the plate was washed three times with PBST (PBS containing 0.01% Tween 20) followed by an additional three washes with PBS. Cell bound Fabs were detected using anti-His horseradish peroxidase-conjugate (Amersham Biosciences, Piscataway, NJ, USA). ELISAs were developed with 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma). Reactions were stopped by the addition of H2SO4 after 20 min and readings taken at OD450nm using plate reader (Spectra Max, Molecular Devices).
Immunofluorescence
Bacterial cell suspensions at a concentration of 106 CFU/mL were coated on 96-well plate and blocked with 2% milk powder in PBS for 1 hr at room temperature. Purified Fabs (5 μg per well) were incubated with bacteria cells for 1 hr at room temperature. Following three washes with 1× PBS, FITC conjugated Anti-FLAG antibody was added at 1:5000 dilution to each well and incubated for 1 hr at room temperature. Bacteria were washed three times in PBST followed by three additional washes with PBS. Fluorescence images were taken using fluorescence microscope (EVOS, Advance Microscopy Group).
Flow cytometry
Bacteria growing on BHI were centrifuged and washed once with PBS. To break filaments of S. mutans and S. sobrinus, bacteria cells were re-suspended in 1 mL of ice-cold PBS and sonicated using a microtip probe sonicator for 20 secs at 10% power with a setting of a 0.5 second impulse, followed by a 0.5 sec break. For flow cytometry analyses, bacterial cell suspensions at a concentration of 106 CFU/tube were incubated with different Fabs (1 μM) at room temperature for 60 min. Bacteria cells were washed three times with PBS, pH 7.4. Anti-FLAG® R-Phycoerythrin Conjugate (ProZyme) was added at 1:3000 dilution to each tube and incubated for 30 min at room temperature. Bacteria cells were washed three times in PBST followed by three additional washes with PBS and re-suspended in 500 μL of PBS. Bacteria cell fluorescence was monitored using a flow cytometer (MACS Quant, Miltenyi Biotec) with 561 nm excitation and 586–541 nm emission wavelengths. Flow cytometry data were analyzed using Flowjo (Tree Star).
In vitro biofilm assay
The effect of different Fabs on S. mutans and S. sobrinus biofilm formation were examined using the microdilution method41. Briefly, overnight culture of S. mutans and S. sobrinus were prepared to a final concentration of 106 CFU/mL and grown in BHI for 24 hrs at 37 °C in a 5% CO2 aerobic atmosphere with the addition of 1% sucrose for biofilm development. Different Fabs were added to the culture at 0 hour at a final concentration of 3 μM. After 24 hours, before biofilm quantification, the culture growth was measured at OD600nm using a plate reader. The cell suspension was carefully removed and each well was washed three times with 1× PBS. The remaining attached cells were fixed with 96% ethanol for 15 min and then plates were dried. Wells were stained with 120 μL of 0.1% crystal violet (Fisher) for 20 minutes. The crystal violet stain was aspirated and wells were washed three times with 150 μL of PBS. Wells were air-dried and retained crystal violet was solubilized with 150 μL of 30% (v/v) glacial acetic acid (Fisher). The plate was incubated at room temperature for 20 min with shaking. The biofilm formation was quantified by measuring the OD590nm using a plate reader.
In vivo rat caries model
The animal experiment protocol was reviewed and approved by the Ethical Committee on Animal Research at the University of Michigan. Fifteen days old Sprague-Dawley rats were purchased from Charles River (Wilmington, MA). Rats then were screened for infection with S. mutans and S. sobrinus. Fifteen infection-free rats were given S. mutans and S. sobrinus (kindly proved by Dr. Christopher Fenno, School of Dentistry, University of Michigan) twice a week for 4 weeks starting at day 21 after birth. All rats were fed high cariogenic diet 2000 (Envigo, Madison, WI) and 5% sucrose water ad libitum42. Rats were randomly divided into three groups. Each group had 5 rats. All groups received the bacteria mixture applied directly on their teeth with a brush twice a week for total 4 weeks. Rats in the first group were treated with a mixture of Fabs (SM-10 and SS-2) applied directly on teeth surface using a brush starting at day 1 of the experiment. The second group was treated with the same Fabs mixture twice a week starting from the 3rd week for a total of two weeks; the last group was the vehicle control group; rats in this group were treated with PBS twice a week for 4 weeks. Upon completion of the 4 weeks the rats were sacrificed under anesthesia with a ketamine/xylazine mixture and mandibles and maxilla collected, fixed with 70% ETOH for 24 hours and analyzed by micro-CT.
Micro-CT
The dissected samples were scanned with micro-computed tomography (SkyScan 1272 Micro-CT at 60 kV, with 6.0 um pixel size). The scanned images were reconstructed with Nrecon (Bruker, Manning Park, MA) and DataViewer (Bruker).
Availability of data and materials
The datasets used and/or analyzed during the current study available from the corresponding author on reasonable request.
Change history
09 September 2020
An amendment to this paper has been published and can be accessed via a link at the top of the paper.
References
Petersen, P. E., Bourgeois, D., Ogawa, H., Estupinan-Day, S. & Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull World Health Organ. 83, 661–669 (2005).
Philip, N., Suneja, B. & Walsh, L. Beyond Streptococcus mutans: Clinical implications of the evolving dental caries aetiological paradigms and its associated microbiome. British dental journal. 224, 219 (2018).
Marsh, P. D. Are dental diseases examples of ecological catastrophes? Microbiology. 149, 279–294 (2003).
Marsh, P. D., Moter, A. & Devine, D. A. Dental plaque biofilms: communities, conflict and control. Periodontology 2000. 55, 16–35 (2011).
Gross, E. L. et al. Bacterial 16S sequence analysis of severe caries in young permanent teeth. Journal of clinical microbiology. 48, 4121–4128 (2010).
Gross, E. L. et al. Beyond Streptococcus mutans: dental caries onset linked to multiple species by 16S rRNA community analysis. PloS one. 7, e47722 (2012).
Tanner, A. C., Kressirer, C. A. & Faller, L. L. Understanding Caries From the Oral Microbiome Perspective. Journal of the California Dental Association. 44, 437–446 (2016).
Marsh, P. D. & Martin, M. V. The resident oral microflora; in Marsh PD, Martin VM (eds): Oral Microbiology. Woburn, Reed Educational and Professional Publishing. pp 7–33 (1999).
Takahashi, N. & Nyvad, B. Caries ecology revisited: microbial dynamics and the caries process. Caries research. 42, 409–418 (2008).
Selwitz, R. H., Ismail, A. I. & Pitts, N. B. Dental caries. Lancet. 369, 51–59 (2007).
Filoche, S., Wong, L. & Sissons, C. H. Oral biofilms: emerging concepts in microbial ecology. J. Dent. Res. 89, 8–18 (2010).
Mitchell, T. J. The pathogenesis of streptococcal infections: from tooth decay to meningitis. Nature Reviews Microbiology. 1, 219–230 (2003).
Kalesinskas, P., Kačergius, T., Ambrozaitis, A., Pečiulienė, V. & Ericson, D. Reducing dental plaque formation and caries development. A review of current methods and implications for novel pharmaceuticals. Stomatologija. 16, 44–52 (2014).
Tamesada, M., Kawabata, S., Fujiwara, T. & Hamada, S. Synergistic effects of streptococcal glucosyltransferases on adhesive biofilm formation. Journal of dental research. 83, 874–879 (2004).
Featherstone, J. D. The continuum of dental caries—evidence for a dynamic disease process. J. Dent. Res. 83, C39–42 (2004).
Marcenes, W. et al. Global burden of oral conditions in 1990–2010: a systematic analysis. Journal of dental research. 92, 592–7 (2013).
Gambhir, R. S. et al. Vaccine against dental caries-an urgent need. J Vaccines Vaccin. 3, 36 (2012).
Yan, H. M. Salivary IgA enhancement strategy for development of a nasal-spray anti-caries mucosal vaccine. Science China. Life Sciences 56, 406 (2013).
Innes, N. P. et al. Managing carious lesions: consensus recommendations on terminology. Advances in dental research. 28, 49–57 (2016).
Abrams, S. New Approaches to Diagnosis, Management and Treatment of Dental Caries. Oral Health. 94, 13–18 (2004).
Chen, F. & Dong, W. Novel technologies for the prevention and treatment of dental caries: a patent survey.”. Expert opinion on therapeutic patents 20, 681–694 (2010).
Balakrishnan, M., Simmonds, R. S. & Tagg, J. R. Dental caries is a preventable infectious disease. Aust. Dent J. 45, 235–45 (200).
Shapiro, S., Giertsen, E. & Guggenheim, B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 36, 93–100 (2002).
Moshrefi, A. Chlorhexidine. J. West Soc. Periodontol Periodontal Abstr. 50, 5–9 (2002).
Fan, M. W. et al. A DNA vaccine encoding a cell-surface protein antigen of Streptococcus mutans protects gnotobiotic rats from caries. J Dent Res. 81, 784–787 (2002).
Jia., R. et al. Immunogenicity of CTLA4 fusion anti-caries DNA vaccine in rabbits and monkeys. Vaccine. 24, 5192–5200 (2006).
Smith, D. J. Prospects in caries vaccine development. J Dent Res. 91, 225–226 (2012).
Xu, Q. A. et al. Protective efficacy of a targeted anti-caries DNA plasmid against cariogenic bacteria infections. Vaccine. 25, 1191–1195 (2007).
Smith, R. & Lehner, T. Characterization of monoclonal antibodies to common protein epitopes on the cell surface of Streptococcus mutans and Streptococcus sobrinus. Oral Microbiol. Immunol. 4, 153–158 (1989).
Lehner, T., Caldwell, J. & Smith, R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect. Immun. 50, 796–799 (1985).
Kuepper, M. B. et al. Generation of human antibody fragments against Streptococcus mutans using a phage display chain shuffling approach. BMC biotechnology. 5, 4 (2005).
Oluwayelu, D. O. & Adebiyi, A. I. Plantibodies in human and animal health: a review. African health sciences. 16, 640–5 (2016).
Persson, H. et al. CDR-H3 diversity is not required for antigen recognition by synthetic antibodies. Journal of molecular biology. 425, 803–811 (2013).
Van Raamsdonk., M., De Soet, J. J., Jones, C. L. & De Graaff, J. Effect of antibodies on the chain length and growth of Streptococcus sobrinus. Caries research. 31, 35–40 (1997).
Brady, L. J., Piacentini, D. A., Crowley, P. J., Oyston, P. C. & Bleiweis, A. S. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect. Immun. 60, 1008–17 (1992).
Brady, L. J., Crowley, P. J., Piacentini, D. A. & Bleiweis, A. S. The interactions of the cell surface P1 adhesion molecule of Streptococcus mutans with human salivary agglutinin. J. Microbiol. Methods. 18, 181–96 (1993).
van Raamsdonk, M., van der Mei, H. C., de Soet, J. J., Busscher, H. J. & de Graaff, J. Effect of polyclonal and monoclonal antibodies on surface properties of Streptococcus sobrinus. Infect. Immun. 63, 1698–702 (1995).
Koga, T., Oho, T., Shimazaki, Y. & Nakano, Y. Immunization against dental caries. Vaccine. 20, 2027–2044 (2002).
Gibson, D. G. Synthesis of DNA fragments in yeast by one-step assembly of overlapping oligonucleotides. Nucleic acids research. 37, 6984–6990 (2009).
Alam, M. K. et al. Synthetic Modular Antibody Construction by Using the SpyTag/SpyCatcher Protein‐Ligase System. ChemBioChem 18, 2217–2221 (2017).
da Silva, B. R. et al. Antibacterial activity of a novel antimicrobial peptide [W7] KR12-KAEK derived from KR-12 against Streptococcus mutans planktonic cells and biofilms. Biofouling. 33, 835–846 (2017).
Bowen, W. H., Madison, K. M. & Pearson, S. K. Influence of desalivation in rats on incidence of caries in intact cagemates. Journal of dental research 67, 1316–1318 (1988).
Acknowledgements
We thank the members of the Geyer and the Papagerakis laboratories for critical review of this manuscript and sharing their experiences. This work is supported by the operating grants from the Grand Challenges Canada 0497-01.
Author information
Authors and Affiliations
Contributions
C.R.G. and P.P. conceived the idea. M.K.A. performed phage display, generated the antibodies, purified, and validated the antibodies. K.A. performed ELISA, immunofluorescence, flow cytometry and in vitro biofilm experiments. L.Z., R.W. and S.P. designed and/or performed in vivo rat study. M.K.A., C.R.G., and P.P. interpreted experimental observations, designed figures, and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Alam, M.K., Zheng, L., Liu, R. et al. Synthetic antigen-binding fragments (Fabs) against S. mutans and S. sobrinus inhibit caries formation. Sci Rep 8, 10173 (2018). https://doi.org/10.1038/s41598-018-28240-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-28240-0
This article is cited by
-
Inhibitory and preventive effects of Arnebia euchroma (Royle) Johnst. root extract on Streptococcus mutans and dental caries in rats
BDJ Open (2024)
-
Antibodies generated against dextransucrase exhibit potential anticariostatic properties in Streptococcus mutans
Applied Microbiology and Biotechnology (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.