Abstract
To date, no methodology has been described for predicting the age of Aedes albopictus Skuse mosquitoes, commonly known as Asian tiger mosquitoes. In this study, we report the potential of near-infrared spectroscopy (NIRS) technique for characterizing the age of female laboratory reared Ae. albopictus. Using leave-one-out cross-validation analysis on a training set, laboratory reared mosquitoes preserved in RNAlater for up to a month were assessed at 1, 3, 7, 9, 13, 16, 20 and 25 days post emergence. Mosquitoes (N = 322) were differentiated into two age classes (< or ≥ 7 days) with 93% accuracy, into three age classes (<7, 7–13 and >13 days old) with 76% accuracy, and on a continuous age scale to within ±3 days of their actual average age. Similarly, models predicted mosquitoes (N = 146) excluded from the training model with 94% and 71% accuracy to the two and the three age groups, respectively. We show for the first time that NIRS, with an improved spectrometer and fibre configuration, can be used to predict the age of laboratory reared female Ae. albopictus. Characterization of the age of Ae. albopictus populations is crucial for determining the efficacy of vector control interventions that target their survival.
Similar content being viewed by others
Introduction
Over the last three decades, the mosquito Aedes albopictus Skuse commonly referred to as the Asian tiger mosquito, has rapidly spread to new regions; notably Europe, the Americas, Africa and the Caribbean1. Ae. albopictus is a competent vector of dengue, Zika and chikungunya viruses2,3,4. With an estimated 390 million cases each year in over 100 countries, dengue has increasingly become a public health problem over the last five decades putting half of the world’s population at risk5,6,7. Ae. albopictus is also a highly competent vector of chikungunya virus which has been responsible for major outbreaks on Reunion Island8, Gabon9, the Caribbean, South and Central America10 and more recent outbreaks in Italy11,12,13. The ability of Ae. albopictus to thrive in newly invaded regions is largely due to a broad thermal tolerance. Unlike the primary dengue vector Ae. aegypti, Ae. albopictus is not limited to tropical regions, and its invasion has already redefined the distribution of arboviral diseases, for example, bringing chikungunya to temperate regions of Europe12,13. There is a widespread concern about what the increased distribution of the species means for the burden of several mosquito-borne arboviruses3.
Mosquito survival is a critical determinant of its vectorial capacity. Only those mosquitoes that have survived longer than the extrinsic incubation period of the pathogen they are carrying (defined as the interval between the acquisition of an infectious agent by a vector and the vector’s ability to transmit the agent to a new, susceptible host14) can transmit diseases. According to the vectorial capacity model15,16, a slight change in mosquito survival can lead to exponentially larger changes in pathogen transmission. Because mosquito age plays a significant role in disease transmission, it is crucial to establish the survival characteristics of Ae. albopictus particularly in newly invaded areas.
To date, no technique has been described to predict the age and or survivorship of Ae. albopictus. While classical age grading techniques such as those described by Detinova17 and Polovodova18 to determine the gonotrophic history of females mosquitoes could theoretically be applied to Ae. albopictus, these techniques are laborious, technically demanding and can be highly inaccurate19. Moreover it remains unclear whether these classical age grading techniques are applicable to Ae. albopictus.
Near-infrared spectroscopy (NIRS) has been used to predict the age of the major African malaria vectors Anopheles gambiae and An. arabiensis20,21,22,23,24,25, and to predict the age of wild type Ae. aegypti and Ae. aegypti infected with Wolbachia26,27. NIRS has also been used to detect Wolbachia28 and Zika virus29 infections in Ae. aegypti. This technique detects molecular vibrations resulting from brief exposure of a sample to light in the near infrared spectrum (approximately 700–2500 nm). The spectrum is obtained from a <10 s scan from the head and thorax, providing a characteristic spectral signature. The spectral signature is defined by the composition and concentration of chemical compounds within the samples being analysed. The NIRS age grading technique is rapid, non-destructive, and cost effective.
NIRS can classify mosquitoes into groups of young (<7 days old) and old (≥7 days old) mosquitoes23,25, representing those less likely to be infectious and those potentially infectious, respectively, with accuracies exceeding 90%. Here we describe for the first time, the use of NIRS for determining the age of laboratory reared female Ae. albopictus.
Results
We tested the capability of NIRS to predict the age of Ae. albopictus preserved in RNAlater for a month. We analysed spectra collected from the heads and thoraces of eight age groups of female laboratory reared mosquitoes using a colony established with collections from the Torres Strait, northern Australia. To develop age prediction models, we used leave-one-out cross-validation analysis of a training set, where one sample is removed from the population and the rest of the samples are used to predict the age of the removed sample and the process is repeated for all the samples. The model was validated on a subset of mosquito samples that were excluded from the training model. NIRS allowed a rapid age identification of these mosquitoes into multiple age categories that can be used to define their potential for transmitting pathogenic arboviruses.
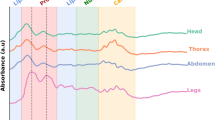
Typical spectra collected from the head and thorax of Ae. albopictus are shown in Fig. 1 and regression coefficient plots used for predicting the age of female Ae. albopictus into various age groups are shown in Fig. 2. The spectrometer and fibre-optic probe used in this study have improvements in the light source, optics, electronics, and fibre material resulting to an improved spectra with less noise compared to those reported in previous studies21,23,24,25,26,28,30.
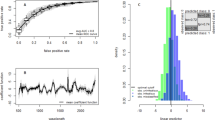
The total number of mosquitoes used in the training set was 322 and the model was tested on a total of 146 mosquitoes. Using cross-validation analysis, female Ae. albopictus were predicted into < or ≥7 days age groups with 93% accuracy (Table 1 and Fig. 3), into <7, 7–13 and >13 days age groups with 76% accuracy (Table 1) or generally into ±3 days of the actual average age (Table 2 and Fig. 4). Similarly, mosquitoes that were excluded from the model were predicted into two and three age groups with 94% and 71% accuracy, respectively, (Table 1) and to within ±3 days of their actual average age (Table 2 and Fig. 4). The predicted age of very young mosquitoes (one and three day old) was significantly different from all other age groups (Table 2). Results from analysis of variance (ANOVA) indicated mosquitoes could generally be clustered into three statistically distinct age groups (<7, 7–13 and >13 d old), as indicated in Table 2.
The highest classification accuracy of 94% was achieved when mosquitoes were simply grouped into two age groups (<or ≥7 days age group). Seven days was chosen because it provided the highest classification accuracy of mosquitoes into two age groups and because dissemination efficiency of some viruses such as chikungunya in Ae. albopictus has been reported to peak at 7 days post infection31. Mosquitoes were less accurately predicted if three or more age groups were used in the analysis. Additionally, the younger age groups (1–3 d and 7–9 d old mosquitoes) were more easily differentiated from the older age groups (>13 days old mosquitoes) than they were from each other. This could be due to minimal biochemical changes occurring between closely related age groups as previously described32,33.
The age prediction accuracy of female Ae. albopictus was slightly better than the prediction for laboratory reared female Ae. aegypti26 or female An. gambiae mosquitoes reared under laboratory23,25 or semi-field30 environments. This could be attributed to innate differences in biochemical composition or improved spectra collected using the current spectrometer and fibre configuration compared to those used in previous studies. Peaks around 1100, 1300, 1550, 1600, 1700, 1900, 1950, 2050, 2150 and 2300 nm appeared to be the major spectral regions distinguishing the ages of Ae. albopictus (Fig. 2). These regions comprise C-H and N-H functional groups and have previously been shown to play a role in age grading Aedes and Anopheline mosquitoes32,34,35,36,37.
Discussion
Characterising the age structure of Ae. albopictus is a crucial determinant of the efficacy of mosquito control interventions, particularly those designed to reduce mosquito survival. Defining mosquito population age structure is also critical as it helps to define the risk of arbovirus transmission38. This is due to the fact that mosquitoes must live long enough to allow for the pathogens they acquire through a blood meal to replicate in mesenteronal epithelial cells, disseminate to other tissues and finally to the salivary glands where they can be transmitted to a susceptible host during blood feeding14. Ae. albopictus is a vector of several important human pathogens including dengue, Zika, yellow fever and chikungunya viruses. Vector control tools are designed to interrupt the normal development of these viruses inside the mosquito by reducing the survival of the mosquito vector. For example, pathogenic bacteria (Wolbachia) shortens mosquito’s lifespan, reducing the time available for the virus to develop within the mosquito. Alternatively, Wolbachia induces resistance against dengue and chikungunya infection therefore blocking their transmission39,40.
To date, age grading options for Ae. albopictus remain poorly investigated. The classical age grading techniques based on gonotrophic history of mosquitoes17,18 have not been assessed for Ae. albopictus. Similarly, the potential of more accurate age grading techniques developed for Ae. aegypti such as analysis of changes in abundance of transcriptional profiles41,42 and cuticular hydrocarbons43 have not been attempted for age grading Ae. albopictus. Changes in abundance of protein age biomarkers as a potential age grading technique for Ae. albopictus has only been recently described44 and it’s still early in development.
Here we describe an easy to use and rapid method for age grading Ae. albopictus. NIRS is field-portable, cost effective and could be useful in comparative studies to establish longevity of Ae. albopictus in various regions as well as its survival across different seasons following invasion. The technique allows hundreds of samples to be analysed in a day without consuming any reagents. After training models have been established, only minimal computer skills are required.
Although the cost for buying NIRS instruments are high ($60,000), the cost can easily be justified through the programmatic use of the technique for multiple applications including species identification, age grading, and infection detection. In comparison with the polymerase chain reaction technique which costs ~10 dollars/mosquito, the costs of buying the NIRS instrument could easily be recovered after analysis of approximately 10,000 samples.
This study provides a platform for the future development of this technique for investigating the age of Ae. albopictus. Further work is required to establish the technique’s accuracy under varying environmental conditions to account for factors such as varying diet, temperature and humidity. Following assessments on laboratory mosquitoes, the next phase of validation for age grading techniques can involve comparison of predictions of wild caught specimens against predictions made with a standard age grading method. However, as there are no alternative age grading techniques for Ae. albopictus, we recommend validating NIRS using wild Ae. albopictus larvae/pupae reared to known ages in field cages.
We conclude that the use of NIRS for age prediction of Ae. albopictus is consistent with accuracies previously reported for other mosquito species including Ae. aegypti and An. gambiae, thereby extending the range of mosquito vectors that can be age graded using this technology.
Materials and Methods
Ethics statement
Ethics approval for routine blood feeding of mosquito colonies was obtained from QIMR Berghofer Medical Research Institute (QIMR HREC P1162). All experiments were carried out in accordance with relevant human ethics guidelines and regulations. Informed consent was obtained from volunteers involved in blood feeding and blood feeding volunteers were free to withdraw their participation at any time.
Mosquito rearing
Mosquitoes were obtained from a colony of Ae. albopictus established in the insectary at QIMR Berghofer Medical Research Institute from materials collected on Hammond Island, Torres Strait, Australia in 2014. These mosquitoes are routinely maintained at 27 °C, 70% humidity with 12:12 hr day:night lighting and 30 min dawn/dusk periods. Approximately 50 larvae were reared in aged tap water in plastic containers (17 × 12 × 7 cm). Larvae were provided ground Tetramin tropical flakes fish food at the following rate; first and second instar larvae were fed on 0.16 mg/larva/day whereas 3rd and 4th instar larvae were fed on 0.32 mg/larva/day. Approximately 200 pupae were transferred into small cages measuring 31 × 20 × 20 cm for adult emergence. Adult mosquitoes were given a 48 hr time window to emerge into the cage before the remaining pupae were removed. Thereafter, adult female mosquitoes were collected 24 hr after emergence and at 3, 7, 9, 13, 16, 20 and 25 days post emergence. Mosquitoes were supplied with 10% sucrose ad libitum, and blood fed on a human volunteer for 15 min every 7 d. Female Ae. albopictus were knocked down with carbon dioxide and stored whole in RNAlater® solution for about 4 weeks before scanning21.
Mosquito scanning using NIR spectrometer
Residual RNAlater was removed from the mosquito specimens by gently blotting the mosquito with a paper towel prior to scanning. At least 48 mosquitoes at each age were scanned as described previously23 using a LabSpec 4Si NIR spectrometer (ASD Inc, Boulder, CO) and a 3.2 mm-diameter bifurcated fibre-optic probe which contained a single 600 micron collection fibre surrounded by six 600 micron illumination fibres.
Data Analysis
All spectra were analysed using partial least squares (PLS). Analysis was limited to within the near-infrared region between 700–2350 nm to exclude a region of high spectral ‘noise’ observed from 2350 to 2500 nm. At least 40 mosquitoes were included in the analysis at each age. Mosquitoes were divided into a training set (N = 322) and a validation set (N = 146). The training set classification accuracy along with the predicted residual error sum of squares (PRESS) and regression coefficient plots were used to select the number of factors used in the training set for predicting the ages of mosquitoes in the validation set.
Three classification models, each with 10 factors chosen from the cross-validation analysis, were developed to predict mosquitoes into 1) two groups (< or ≥7 days old), 2) three groups (< 7, 7–13 and >13 days old) and 3) on a continuous age scale. Ten factors were chosen as they provided maximum accuracy with minimal noise in the regression coefficient graph when compared to models with >10 factors. Each of the models was then applied to predict the age of samples in the validation set. We tested whether the mean predicted ages differed significantly between age groups using ANOVA and Tukey post hoc analysis in Statistical Package for Social Sciences 22 (IBM, Armonk, NY).
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author.
References
Kraemer, M. U. et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. Elife 4, e08347, https://doi.org/10.7554/eLife.08347 (2015).
Simmons, C. P., Farrar, J. J., van Vinh Chau, N. & Wills, B. Dengue. New England Journal of Medicine 366, 1423–1432, https://doi.org/10.1056/NEJMra1110265 (2012).
Gratz, N. G. Critical review of the vector status of Aedes albopictus. Medical and Veterinary Entomology 18, 215–227, https://doi.org/10.1111/j.0269-283X.2004.00513.x (2004).
Metselaar, D. et al. An Outbreak of Type-2 Dengue Fever in the Seychelles, Probably Transmitted by Aedes-Albopictus (Skuse). B World Health Organ 58, 937–943 (1980).
Gubler, D. J. The global emergence/resurgence of arboviral diseases as public health problems. Arch Med Res 33, 330–342, https://doi.org/10.1016/S0188-4409(02)00378-8 (2002).
Rigau-Perez, J. G. et al. Dengue and dengue haemorrhagic fever. Lancet 352, 971–977, https://doi.org/10.1016/S0140-6736(97)12483-7 (1998).
Bhatt, S. et al. The global distribution and burden of dengue. Nature 496, 504–507, https://doi.org/10.1038/nature12060 (2013).
Borgherini, G. et al. Outbreak of Chikungunya on Reunion Island: Early clinical and laboratory features in 157 adult patients. Clin Infect Dis 44, 1401–1407, https://doi.org/10.1086/517537 (2007).
Pages, F. et al. Aedes albopictus mosquito: the main vector of the 2007 Chikungunya outbreak in Gabon. PLoS One 4, e4691, https://doi.org/10.1371/journal.pone.0004691 (2009).
Morrison, T. E. Reemergence of Chikungunya Virus. Journal of Virology 88, 11644–11647, https://doi.org/10.1128/Jvi.01432-14 (2014).
Bonilauri, P. et al. Chikungunya virus in Aedes albopictus, Italy. Emerg Infect Dis 14, 852–854, https://doi.org/10.3201/eid1405.071144 (2008).
Marano, G. et al. Ten years since the last Chikungunya virus outbreak in Italy: history repeats itself. Blood Transfus 15, 489–490, https://doi.org/10.2450/2017.0215-17 (2017).
Venturi, G. et al. Detection of a chikungunya outbreak in Central Italy, August to September 2017. Euro Surveill 22, https://doi.org/10.2807/1560-7917.ES.2017.22.39.17-00646 (2017).
Hardy, J. Susceptibility and resistance of vector mosquitoes. The arboviruses: epidemiology and ecology 1, 87–126 (1988).
Macdonald, G. The Epidemiology and Control of Malaria. (Oxford University Press, 1957).
Garrett-Jones, C. Prognosis for interuption of malaria transmission through assessment of the mosquito’s vectorial capacity. Nature 204, 1173–1175 (1964).
Detinova, T. Age-grouping methods in Diptera of medical importance, with special reference to some vectors of malaria. Monogr Ser World Health Organ 47, 13–191 (1962).
Polovodova, V. P. The determination of the physiological age of female Anopheles by number of gonotrophic cycles completed. Med Parazitol Parazitar Bolezni 18, 352–355 (1949).
Hugo, L. E., Quick-miles, S., Kay, B. H. & Ryan, P. A. Evaluations of Mosquito Age Grading Techniques Based on Morphological Changes. Journal of medical entomology 45, 353–369, doi:10.1603/0022-2585(2008)45[353:EOMAGT]2.0.CO;2 (2008).
Sikulu, M. Non-destructive near infrared spectroscopy for simultaneous prediction of age and species of two major African malaria vectors. An. gambiae and An. arabiensis. NIR news 25, 4–6 (2014).
Dowell, F. E., Noutcha, A. E. M. & Michel, K. The effect of preservation methods on predicting mosquito age by near Infrared spectroscopy. Am J Trop Med Hyg 85, 1093–1096, https://doi.org/10.4269/ajtmh.2011.11-0438 (2011).
Mayagaya, V. S. et al. Evaluating preservation methods for identifying Anopheles gambiae ss and Anopheles arabiensis complex mosquitoes species using near infra-red spectroscopy. Parasite Vector 8, 1–6 (2015).
Mayagaya, V. S. et al. Non-destructive determination of age and species of Anopheles gambiae s.l. Using near-infrared spectroscopy. Am J Trop Med Hyg 81, 622–630, https://doi.org/10.4269/ajtmh.2009.09-0192 (2009).
Ntamatungiro, A. et al. The influence of physiological status on age prediction of Anopheles arabiensis using near infra-red spectroscopy. Parasite Vector 6, 298 (2013).
Sikulu, M. et al. Near-infrared spectroscopy as a complementary age grading and species identification tool for African malaria vectors. Parasite Vector 3, 49 (2010).
Sikulu-Lord, M. T. et al. Near-infrared spectroscopy, a rapid method for predicting the age of male and female wild-type and Wolbachia infected Aedes aegypti. PLoS Negl Trop Dis 10, e0005040 (2016).
Liebman, K. et al. The Influence of Diet on the Use of Near-Infrared Spectroscopy to Determine the Age of Female Aedes aegypti Mosquitoes. Am J Trop Med Hyg 92, 1070–1075 (2015).
Sikulu-Lord, M. T. et al. Rapid and Non-destructive Detection and Identification of Two Strains of Wolbachia Aedes aegypti by Near-Infrared Spectroscopy. PLoS Negl Trop Dis 10, e0004759, https://doi.org/10.1371/journal.pntd.0004759 (2016).
Fernandes, J. N. et al. Rapid, non-invasive detection of Zika virus in Aedes aegypti mosquitoes by near-infrared spectroscopy. Science Advances 4, https://doi.org/10.1126/sciadv.aat0496 (2018).
Sikulu, M. T. et al. Using a Near-infrared spectrometer to estimate the age of Anopheles mosquitoes exposed to pyrethroids. PLoS One 9, e90657, https://doi.org/10.1371/journal.pone.0090657 (2014).
Vega-Rúa, A., Zouache, K., Girod, R., Failloux, A.-B. & Lourenço-de-Oliveira, R. High level of vector competence of Aedes aegypti and Aedes albopictus from ten American countries as a crucial factor in the spread of Chikungunya virus. Journal of virology 88, 6294–6306 (2014).
Hugo, L. E. et al. Proteomic biomarkers for ageing the mosquito Aedes aegypti to determine risk of pathogen transmission. PloS one 8, e58656 (2013).
Sikulu, M. T. et al. Proteomic changes occurring in the malaria mosquitoes Anopheles gambiae and Anopheles stephensi during aging. Journal of proteomics 126, 234–244 (2015).
Sikulu, M. T. et al. Proteomic changes occurring in the malaria mosquitoes Anopheles gambiae and Anopheles stephensi during aging. J Proteomics, https://doi.org/10.1016/j.jprot.2015.06.008 (2015).
Caputo, B. et al. Identification and composition of cuticular hydrocarbons of the major Afrotropical malaria vector Anopheles gambiae s.s. (Diptera: Culicidae): analysis of sexual dimorphism and age-related changes. J Mass Spectrom 40, 1595–1604 (2005).
Desena, M. L. et al. Potential for aging female Aedes aegypti (Diptera: Culicidae) by gas chromatographic analysis of cuticular hydrocarbons, including a field evaluation. J. Med. Entomol. 36, 811–823 (1999).
Hugo, L. E., Kay, B. H., Eaglesham, G. K., Holling, N. & Ryan, P. A. Investigation of cuticular hydrocarbons for determining the age and survivorship of Australian mosquitoes. Am J Trop Med Hyg 74, 462–474 (2006).
Xiao, F. Z. et al. The effect of temperature on the extrinsic incubation period and infection rate of dengue virus serotype 2 infection in Aedes albopictus. Arch Virol 159, 3053–3057, https://doi.org/10.1007/s00705-014-2051-1 (2014).
McMeniman, C. J. et al. Stable introduction of a life-shortening Wolbachia infection into the mosquito Aedes aegypti. Science 323, 141–144, https://doi.org/10.1126/science.1165326 (2009).
Ritchie, S. A., Townsend, M., Paton, C. J., Callahan, A. G. & Hoffmann, A. A. Application of wMelPop Wolbachia strain to crash local populations of Aedes aegypti. PLoS Negl Trop Dis 9, e0003930, https://doi.org/10.1371/journal.pntd.0003930 (2015).
Cook, P. E. et al. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proceedings of the National Academy of Sciences 103, 18060–18065 (2006).
Cook, P. E. et al. The use of transcriptional profiles to predict adult mosquito age under field conditions. Proc Nat Acad Sci 103, 18060–18065, https://doi.org/10.1073/pnas.0604875103 (2006).
Desena, M. L., Edman, J. D., Clark, J. M., Symington, S. B. & Scott, T. W. Aedes aegypti (Diptera: Culicidae) age determination by cuticular hydrocarbon analysis of female legs. Journal of medical entomology 36, 824–830 (1999).
Iovinella, I., Caputo, B., Michelucci, E., Dani, F. & Della Torre, A. Candidate biomarkers for mosquito age-grading identified by label-free quantitative analysis of protein expression in Aedes albopictus females. Journal of proteomics 128, 272–279 (2015).
Acknowledgements
We would like to acknowledge USDA for providing the NIR instrument used in this study. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture. USDA is an equal opportunity provider and employer.
Author information
Authors and Affiliations
Contributions
Designed the experiment-All authors. Run the experiment-M.T.S.-L., F.E.D. Run analysis-M.T.S.-L. Wrote the manuscript-M.T.S.-L. Prepared Figures-M.T.S.-L. Provided reagents-G.J.D., F.E.D. Reviewed the manuscript-All authors.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sikulu-Lord, M.T., Devine, G.J., Hugo, L.E. et al. First report on the application of near-infrared spectroscopy to predict the age of Aedes albopictus Skuse. Sci Rep 8, 9590 (2018). https://doi.org/10.1038/s41598-018-27998-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27998-7
This article is cited by
-
Rapid classification of epidemiologically relevant age categories of the malaria vector, Anopheles funestus
Parasites & Vectors (2024)
-
Age, sex, and mating status discrimination in the sand fly Lutzomyia longipalpis using near infra-red spectroscopy (NIRS)
Parasites & Vectors (2024)
-
Using transfer learning and dimensionality reduction techniques to improve generalisability of machine-learning predictions of mosquito ages from mid-infrared spectra
BMC Bioinformatics (2023)
-
Ability of near-infrared spectroscopy and chemometrics to predict the age of mosquitoes reared under different conditions
Parasites & Vectors (2020)
-
Using mid-infrared spectroscopy and supervised machine-learning to identify vertebrate blood meals in the malaria vector, Anopheles arabiensis
Malaria Journal (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.