Abstract
Sexual selection allows male individuals to adopt different evolutionary strategies in mating system. In this study, we determined whether dominance affected reproductive fitness of male crickets Velarifictorus aspersus during both pre-copulatory and post-copulatory selection when we excluded male–male competition. The results showed that females mated more often with male winners only during the first 2 h after a fight when male winners were more likely to produce courtship songs than losers. However, females did not retain the attached spermatophores of male winners longer than those of male losers, and the fecundity and fertilization success also did not differ significantly between females mated different times with male winners and losers. Instead, the fertilization success was positively correlated with male body weight. These results suggest that a recent wining experience increases reproductive fitness of males during pre-copulatory selection, but females may prefer larger males rather than winners during post-copulatory selection. The incoordination between pre- and post-copulatory selection may allow males to adopt different evolutionary strategies in mating system.
Similar content being viewed by others
Introduction
Sexual selection is one of the most powerful force in determining the reproductive success of all individuals. Darwin has proposed that sexual selection operates in two mechanisms: male-male competition and female mate choice1,2. Because these two mechanisms can work simultaneously on the same species, males may adopt different evolutionary strategies in mating system. For example, in Giant hissing cockroaches Gromphadorhina oblongonota, some males invest heavily in weapons which allows them to be more successful in male-male competition, but some others invest heavily in testes development which may give them advantages in sperm competition3.
Fighting behavior is common among male animals. The fighting behavior comprises a sequential escalating series of behaviors, which ends when one male surrenders. The outcome of a fight determines the ownership of resources and it can also dramatically change the subsequent behavior of males. For example, male crickets that win a fight can become more aggressive and have a greater chance of winning another fight, whereas losers become non-aggressive and tend to lose other fights4,5. This phenomenon is known as the “winner–loser” effect and it has also been reported in many other species, where it usually last from minutes to hours6,7.
Male winners usually have a greater chance of copulating by suppressing the male losers8, suggesting that dominance positively affects competition for access to mating partners. However, it is unclear whether male dominance affects the mating choice of females. Some studies have shown that the male winners of fights are the preferred mates because they are of higher quality9. However, some studies found no correlation between male fighting ability and attractiveness10,11. Sexual selection continues post-copulation if the females mate multiple times, which is known as sperm competition. For example, female crickets retain the attached spermatophores of preferred males longer than those of less desirable males, thereby resulting in greater sperm transfer and increased fertilization success12,13,14,15. The post-copulatory selection can act to reinforce pre-copulatory selection16 or oppose it17,18.
The fighting behavior of male crickets is highly impressive and it has been studied extensively19,20,21. The burrowing cricket Velarifictorus aspersus Walker (Gryllidae) distributes widely in China. Males of this species have extremely large mandibles and they often fight with other males using their mandibles when competing for burrows and females22. A previous study showed that the courtship behavior of male losers was suppressed by the presence of male winners, and that females mated more often with male winners than losers, thereby suggesting that males can increase their reproductive fitness by winning a male–male competition22. In the present study, to determine whether male dominance affects reproductive fitness of male crickets during both pre-copulatory and post-copulatory selection when male–male competition is excluded, we investigated the mating behaviors of males and females when male winners and losers were kept separately with a sexually mature female at different times after fighting. We also compared the spermatophore retention time, fecundity, and fertilization success of females mated with winners or losers to determine whether male dominance affected the post-copulatory reproductive fitness.
Results
Effect of dominance on the courtship behavior of males
During the first 2 h after fighting, more than 90% of the male winners produced courtship songs, whereas only 55% and 67.5% of male winners produced courtship songs during 3–5 h and 10–12 h after fighting, respectively. Among the losers, 60%, 47.5%, and 50% of males produced courtship songs during the periods 0–2 h, 3–5 h, and 10–12 h after fighting, respectively. Courtship rate was significantly affected by fight outcome and the time after fight (GLM with binomial errors, fight outcome: χ2 = 12.517, P < 0.001; time after fight: χ2 = 14.841, P < 0.001). Male losers were less likely to produce courtship songs than male winners during the first 2 h after fighting, but not during 3–5 h and 10–12 h after fighting (Fig. 1A). Male winners and losers required more than 60 min to initiate courtship songs, and there were no significant differences between winners and losers (Two-way ANOVA, fight outcome: df = 1, F = 3.700, P = 0.056). However, males took longer to produce courtship song after a recent fight than they did if the fight was 10-12 hours prior (Two-way ANOVA, time after fight: df = 2, F = 6.089, P = 0.003) (Fig. 1B).
Effect of dominance on female mating behavior
During the first 2 h after fighting, females mated with more male winners than male losers, whereas they mated with equal numbers of male winners and losers during the periods 3–5 h and 10–12 h after fighting (GLM with binomial errors, fight outcome: χ2 = 6.937, P = 0.008; time after fight: χ2 = 2.263, P = 0.322) (Fig. 2A). Fight outcome did not influence spermatophore retention time, but the spermatophore retention time of males fought 10–12 h previously was significantly shorter than that of males fought recently (Two-way ANOVA, fight outcome: df = 1, F = 0.254, P = 0.615; time after fight: df = 2, F = 9.550, P < 0.001) (Fig. 2B).
Effects of male dominance and body weight on the fecundity and fertilization success of females
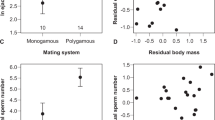
Five females died without laying any eggs, so these females were excluded from the analyses of fecundity and fertilization success. Females mated with male winners or male losers at different times after fighting laid similar amounts of eggs during the next 20 days (Two-way ANOVA, fight outcome: df = 1, F = 0.005, P = 0.942; time after fight: df = 2, F = 1.984, P = 0.055) (Fig. 3A). Less than half of the eggs hatched and the fertilization success did not differ significantly between the females mated with male winners and losers, but the fertilization success of females mated with males fought recently was higher than that of females mated with males fought 10–12 h previously (Two-way ANOVA, fight outcome: df = 1, F = 0.415, P = 0.521; time after fight: df = 2, F = 3.169, P = 0.046) (Fig. 3B). There was no significant correlation between the male body weight and the fecundity of females (linear regression analysis: F1,113 = 0.04, P = 0.85) (Fig. 4A), but the fertilization success of females was positively correlated with male body weight (linear regression analysis: F1,113 = 4.52, P = 0.04) (Fig. 4B), thereby suggesting that male body size could affect reproductive fitness during post-copulatory selection.
Effect of dominance on the capacity for multiple mating
Fecundity did not differ significantly between females mated two or three times with male winners and male losers (t-test: two copulations: t33 = 0.16, P = 0.87; three copulations: t19 = −0.01, P = 0.99) (Fig. 5A). About two-thirds of the eggs hatched successfully and there were no significant differences in the fertilization success of females that mated two or three times with male winners and male losers (t-test: two copulations: t33 = 0.24, P = 0.81; three copulations: t19 = −0.71, P = 0.48)(Fig. 5B). These results indicate that dominance did not affect the capacity for multiple mating with males.
Discussion
Courtship songs are a primary component of the mating behavior by male crickets and females usually do not mate with males without courtship songs. In this study, we found that losing a fight did not affect the production of courtship songs by male crickets V. aspersus, but winning a fight could enhance the courtship songs of males. Similarly, in crickets Gryllus bimaculatus, the courtship behavior of male losers recovered when male winners were absent and male winners were more sensitive to the initiation of courtship songs when stimulated by female wings where they required a shorter time to initiate courtship songs when stimulated by females23. Interestingly, this winning effect was only observed during the first 2 h after fighting, but not during the periods 3–5 h and 10–12 h after fighting, thereby suggesting that the winning experience only briefly enhanced the courtship behavior of males. Activation of a specific motor pattern briefly affects an unrelated subsequent behaviour has been reported in other species. For example, flying could briefly activated octopamine nervous system which promotes male aggression20,24. Behavioural modulation of motivational aspects of brain function may yield new insight as to how and why evolutionary adaptation has connected behaviours that were previously unrelated.
Pre-sexual selection is one of the primary forces that drive the evolution of extravagant phenotypic traits in males. Some studies have shown that females prefer to mate with male winners than losers. For example, male winners of the house cricket Acheta domesticus are significantly more likely to court females than male losers25. There is great diversity in the signals employed by females to discriminate male winners from losers. In some insects, females discriminate the winners from losers based on pheromones26. Other studies have shown that females incite male competition to facilitate mate choice27,28, thereby suggesting that females may discriminate winners from losers by watching the males fighting. By contrast, female Teleogryllus commodus do not prefer to mate with males that win fights11. Our results showed that female crickets V. aspersus mated more often with male winners during the first 2 h after fighting when the male winners were more likely to produce courtship songs than losers, suggesting that fight outcome affects reproductive fitness of males during pre-copulatory selection. However, females mated with equal number of male winners and losers during 3–5 and 10–12 hours after fighting. It appears that this difference in the courtship singing behavior may cause females V. aspersus to mate with more male winners than losers.
Our results also showed that reproductive fitness in post-copulatory selection was affected by male body weight but not by male dominance. It seems that females may prefer to store sperms of larger males, or larger males transfer more sperm or seminal fluid products that increase fertilization success. In the cricket G. bimaculatus, females that mated with potentially more dominant males laid more eggs, which suggests that the fighting ability of males may have a positive effect on post-copulatory female selection29. Thomas and Simmons26 also found that subordinate male crickets Teleogryllus oceanicus produced lower quality ejaculates than dominant males and they sired less offspring when competing for fertilization. In these previous studies, males were forced to fight several rounds, so the body quality of the males that won all of their fights may have been much higher than that of the males that lost all of their fights. In the current study, the males only fought once, so the difference in quality between male winners and losers might not have been sufficiently large. Alternatively, the fighting ability of males can be affected by many factors, including their body size, health status, reproductive development, weapon size, and personality30,31,32,33. Some traits may also be positively correlated with the sperm number or quality, thereby directly or indirectly increasing the reproductive fitness of females. For example, sperm number was significantly higher in larger males in mosquitofish34 and crickets35. However, some other traits may be negatively correlated with investment in sperm number or quality. For example, in two species of hissing cockroaches, Gromphadorhina oblongonota and Aeluropoda insignis, individuals invest more heavily in weapon length at the expense of the testes mass3. Therefore, mating with a dominant male might not increase the reproductive fitness in some circumstances and females should prefer males that develop better traits correlated with sperm number or quality after copulation.
Interestingly, the time after a fight also influenced mating behavior of both males and females. Males after a recent fight required more time to initiate courtship songs, but females retained the attached spermatophores of males fought recently longer than males fought 10–12 h previously. It seems that males may need more time to produce larger spermatophores when they recently experienced a fight. Similarly, the presence of potential competitors causes males to increase sperm number in other cricket species, G. bimaculatus and Gryllodes sigillatus, and cockroach Nauphoeta cinerea36,37. However, we did not compare the spermatophore size between males fought recently and males fought 10–12 h previously, so this inference needs to be further tested.
Methods
Experimental individuals
Crickets V. aspersus were collected from Hainan Province in China, and reared in our laboratory under the condition of LD 16:8 h, 30 °C. Nymphs were kept in groups (50 nymphs/container), and provided with artificial insect feed (DaRui Co., Changsha, China) and water. Male adults were kept separately until they were used in the following experiments. The fighting trials were conducted on day 8–9 of adulthood, because males were sexually matured at 7 days after molting to adulthood38.
Effects of dominance on the courtship behavior of males and female mating choice
Body weight of males were measured with a digital scale (0.0001 g) and about 200 pairs of males matched by body weight (difference of less than 2%) were used in fighting experiment. To discriminate each male within the pair, the males were marked with red or black color on their pronotum using a marker pen. Each pair of males were allowed to fight in a transparent round plastic container following the method described in Zeng et al.22. Dominance was established when one male sung and chased around the other. Fighting trials were observed within a period of 30 min and those where the clear establishment of dominance did not occur were excluded. After fighting, the winners and losers were returned to their own containers and 120 pairs of winners and losers were used to conduct mating trials. Mating trials were performed over three periods comprising 0–2 h, 3–5 h, and 10–12 h after fighting, and 40 pairs of winners and losers were used for each time period. In each mating trial, a sexually mature virgin female (15 days after adulthood) was introduced into the container with the male and the mating behavior was observed for 2 h. We recorded the courtship singing rate and time required to initiate singing by males, as well as the mating rates of females and the spermatophore retention time.
Effects of male dominance and body weight on fecundity and fertilization success of females
To examine whether the fecundity and fertilization success of females differed when mated with male winners or losers, the females that mated successfully in mating trials were housed separately and provided with ovipositional substrates. Eggs laid over the following 20 days were collected and hatched under 25 °C. After no further eggs hatched over 10 successive days, the remaining eggs were observed and counted under a microscope (Leica, Germany). Male body weight is positively correlated with spermatophore size, so we also analyzed the correlations between male body weight and female fecundity and fertilization success.
Effect of dominance on the capacity for multiple mating
Previous study has shown that this species is polygamous and that multiple copulation increases the fertilization success of females39. Therefore, we examined the effects of dominance on the fecundity and fertilization success of females when mated two or three times. We introduced 120 pairs of males matched by weight into a transparent round plastic arena to fight and establish clear dominance. Next, 60 pairs of winners and losers were kept separately with a female for 4 h to copulate twice, and 60 pairs of winners and losers were kept separately with a female for 6 h to copulate three times. After the mating trial, the females that mated two or three times were housed separately and provided with ovipositional substrates. Eggs laid over the following 20 days were collected and hatched at 25 °C. After no further eggs hatched over 10 successive days, the remaining eggs were observed and counted under a microscope.
Data analysis
Effects of fight outcome and time after a fight on courtship singing rate and mating rate were analyzed by Generalized Linear Models (GLM) with binomial errors. Effects of fight outcome and time after a fight on the time required to initiate courtship songs, spermatophore retention time, fecundity, and fertilization success results were analyzed by Two-way ANOVA. Comparisons of fecundity and fertilization between females mated multiple times with male winners and male losers were analyzed by Student’s t-test. Correlations between male body weight and the fecundity or fertilization success (data were log transformed) of females were analyzed by linear regression.
References
Darwin, C. The descent of man and selection in relation to sex. John Murray, London (1871).
Andersson, M. Sexual selection. Princeton Univ. Press, Princeton (1994).
Durrant, K. L., Skicko, I. M., Sturrock, C. & Mowles, S. L. Comparative morphological trade-offs between pre- and post-copulatory sexual selection in Giant hissing cockroaches (Tribe: Gromphadorhini). Sci. Rep. 6, 36755 (2016).
Rillich, J. & Stevenson, P. A. Winning fights induces hyperaggression via the action of the biogenic amine octopamine in crickets. PLoS One 6, e28891 (2011).
Rillich, J. & Stevenson, P. A. A fighter’s comeback: Dopamine is necessary for recovery of aggression after social defeat in crickets. Horm. Behav. 66, 696–704 (2014).
Hsu, Y. Y., Earley, R. L. & Wolf, L. L. Modulation of aggressive behaviour by fighting experience: mechanisms and contest outcomes. Biol. Rev. 81, 33–74 (2006).
Rutte, C., Taborsky, M. & Brinkhof, M. W. G. What sets the odds of winning and losing? Trends Ecol. Evol. 21, 16–21 (2006).
Ogawa, Y. & Sakai, M. Fighting changes courtship activity in the male cricket. Comp. Biochem. Physiol. B 151, 453 (2008).
Berglund, A., Bisazza, A. & Pilastro, A. Armaments and ornaments: an evolutionary explanation of traits of dual utility. Biol. J. Linn. Soc. 58, 385–399 (1996).
Moore, A. J., Gowaty, P. A., Wallin, W. G. & Moore, P. J. Sexual conflict and the evolution of female mate choice and male social dominance. Proc. R. Soc. B 268, 517–523 (2001).
Shackleton, M. A., Jennions, M. D. & Hunt, J. Fighting success and attractiveness as predictors of male mating success in the black field cricket, Teleogryllus commodus: the effectiveness of no-choice tests. Behav. Ecol. Sociobiol. 58, 1–8 (2005).
Simmons, L. W. Female choice in the field cricket, Gryllus bimaculatus (De Geer). Anim. Behav. 34, 1463–1470 (1986).
Simmons, L. W. Sperm competition as a mechanism of female choice in the field cricket. Gryllus bimaculatus. Behav. Ecol. Sociobiol. 21, 197–202 (1987).
Sakaluk, S. K. & Eggert, A. K. Female control of sperm transfer and intraspecific variation in sperm precedence: antecedents to the evolution of a courtship food gift. Evolution 50, 694–703 (1996).
Gershman, S. N. Postcopulatory female choice increases the fertilisation success of novel males in the field cricket. Gryllus vocalis. Evolution 63, 67–72 (2009).
Bateman, P. W., Gilson, L. N. & Ferguson, J. W. H. Male size and sequential mate preference in the cricket Gryllus bimaculatus. Anim. Behav. 61, 631–637 (2001).
Danielsson, I. Antagonistic pre- and post-copulatory sexual selection on male body size in a water strider (Gerris lacustris). Proc. R. Soc. Lond. B 268, 77–81 (2001).
Parker, G. A., Lessells, C. M. & Simmons, L. W. Sperm competition games: A general model for precopulatory male–male competition. Evolution 67, 95–109 (2013).
Simmons, L. W. Inter-male competition and mating success in the field cricket, Gryllus bimaculatus (De Geer). Anim. Behav. 34, 567–579 (1986).
Hofmann, H. A. & Stevenson, P. A. Flight restores fight in crickets. Nature 403, 613 (2000).
Stevenson, P. A. & Rillich, J. Adding up the odds-Nitric oxide signaling underlies the decision to flee and post-conflict depression of aggression. Sci. Adv. 1, e1500060 (2015).
Zeng, Y., Zhu, D. H. & Kang, W. N. Variation in fighting strategies in male wing-dimorphic crickets (Gryllidae). Behav. Ecol. Sociobiol. 70, 429–435 (2016).
Ogawa, Y. & Sakai, M. Calling and courtship behaviors initiated by male-male contact via agonistic encounters in the cricket Gryllus bimaculatus. Zool. Sci. 26, 517–524 (2009).
Stevenson, P. A., Dyakonova, V., Rillich, J. & Schildberger, K. Octopamine and experience-dependent modulation of aggression in crickets. J. Neurosci. 25, 1431–1441 (2005).
Savage, K. E., Hunt, J., Jennions, M. D. & Brooks, R. C. Male attractiveness covaries with fighting ability but not prior fight outcome in house crickets. Behav. Ecol. 16, 196–200 (2005).
Thomas, M. L. & Simmons, L. W. Male dominance influences pheromone expression, ejaculate quality, and fertilization success in the Australian field cricket. Teleogryllus oceanicus. Behav. Ecol. 20, 1118–1124 (2009).
Cox, C. R. & LeBoeuf, B. J. Female incitation of male competition: a mechanism in sexual selection. Am. Nat. 111, 317–335 (1977).
Montgomerie, R. & Thornhill, R. Fertility advertisement in birds: a means of inciting male–male competition. Ethology 81, 209–220 (1989).
Bertman, A., Rodriguez-Munoz, R. & Tregenza, T. Male dominance determines female egg laying rate in crickets. Biol. Lett. 2, 409–411 (2006).
Dixon, K. A. & Cade, W. H. Some factors influencing male–male aggression in the field cricket Gryllus integer (time of day, age, weight and sexual maturity). Anim. Behav. 34, 340–346 (1986).
Kortet, R. & Hedrick, A. A behavioural syndrome in the field cricket Gryllus integer: intrasexual aggression is correlated with activity in a novel environment. Biol. J. Linn. Soc. 91, 475–482 (2007).
Judge, K. A. & Bonanno, V. L. Male weaponry in a fighting cricket. Plos One 3, e3980 (2008).
Mowles, S. L., Cotton, P. A. & Briffa, M. Flexing the abdominals: do bigger muscles make better fighters? Biol. Lett. 7, 358–360 (2011).
O’Dea, R. E., Jennions, M. D. & Head, M. L. Male body size and condition affects sperm number and production rates in mosquitofish, Gambusia holbrooki. J. Evo. Biol. 27, 2739–2744 (2014).
Sturm, R. Comparison of sperm number, spermatophore size, and body size in four cricket species. J. Orthop. Res. 23, 39–47 (2014).
Harris, W. E. & Moore, P. J. Sperm competition and male ejaculate investment in Nauphoeta cinerea: effects of social environment during development. J. Evo. Biol. 18, 474–480 (2005).
Mallard, S. T. & Barnard, C. J. Competition, fluctuating asymmetry and sperm transfer in male gryllid crickets (Gryllus bimaculatus and Gryllodes sigillatus). Behav. Ecol. Sociobiol. 53, 190–197 (2003).
Zeng, Y. & Zhu, D. H. Trade-off between flight capability and reproduction in male Velarifictorus aspersus crickets. Ecol. Entomol. 37, 244–251 (2012).
Zeng, Y., Zhou, F. H., Kang, W. N. & Zhu, D. H. Availability of unfertilised eggs increases the fitness of nymphal crickets (Gryllidae). Ecol. Entomol. 42, 500–505 (2017).
Acknowledgements
This work is funded by the Hunan Provincial Education Department Project (Grant No. 17B281) and by the National Nature Science Foundation of China (Grant No. 31070586).
Author information
Authors and Affiliations
Contributions
Y. Z. and D.Z. conceived and designed the experiments. F.Z. performed the experiments and analyzed the data. Y.Z. wrote the manuscript. D.Z. made significant contribution to the revision.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zeng, Y., Zhou, FH. & Zhu, DH. Fight outcome briefly affects the reproductive fitness of male crickets. Sci Rep 8, 9695 (2018). https://doi.org/10.1038/s41598-018-27866-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27866-4
This article is cited by
-
Fight outcome influences male mating success in Drosophila prolongata
Journal of Ethology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.