Abstract
To ensure the reliability of the planned multi-center clinical trial, we assessed the consistence and comparability of the quantitative parameters of the eight PET/CT units that will be used in this trial. PET/CT images were scanned using a PET NEMA image quality phantom (Biodex) on the eight units of Discovery PET/CT 690 from GE Healthcare. The scanning parameters were the same with the ones to be used in the planned trial. The 18F-NaF concentration in the background was 5.3 kBq/ml, while the ones in the spheres of diameter 37 mm, 22 mm, 17 mm and 10 mm were 8:1 as to that of the background and the ones in the spheres of diameter 28 mm and 13 mm were 0 kBq/ml. The consistency of hot sphere recovery coefficient (HRC), cold sphere recovery coefficient (CRC), hot sphere contrast (QH) and cold sphere contrast (Qc) among these 8 PET/CTs was analyzed. The variation of the main quantitative parameters of the eight PET/CT systems was within 10%, which is acceptable for the clinical trial.
Similar content being viewed by others
Introduction
Multicenter study is a commonly used research method in clinical trial. Researchers can collect sufficient clinical data efficiently in this approach. However, to ensure the reliability of the research, the quality control (QC) process is usually crucial in these studies.
Quantitation of Standard Uptake Value (SUV) and related parameters play an important role in the diagnosis and treatment response evaluation using Positron Emission Tomography/Computed Tomography (PET/CT). However, it is well known that SUVs from different PET/CT units are of great discrepancy. The SUVs from different manufacturers are usually un-comparable. Fortunately, we (eight hospitals in China, installed Discovery PET/CT 690 from GE Healthcare within two years) agreed to initiate a multicenter clinical trial regarding the solid tumor treatment response evaluation using PET/CT.
Although we use the same PET/CT model, the discrepancy among different units tends to be larger than that in a single center, because of different experiment conditions, such as temperature, geography, and cross calibration. The assessment of the consistence and comparability of the quantitative data from these eight PET/CT units was the first step of our QC process. To the best of our knowledge, few researches on this have been done. Therefore we assessed the consistence and comparability of the quantitative data on these eight PET/CT units, based on NEMA NU2 20121 standard, as the first step of the QC process of our multicenter clinical trial.
Results
Recovery coefficient
The HRC (hot sphere recovery coefficient) and CRC (cold sphere recovery coefficient), as well as the mean value, standard deviation (SD) value and variability (variability = SD/mean x 100%, generally named coefficient of variability, CV) of the eight PET/CT systems are presented in Table 1. The HRC and CRC distributions with different diameter of spheres are graphically shown in Figs 1–3. The variation of HRCmean (HRC of mean uptake) and CRCmean (CRC of mean uptake) were both within 10%. The corresponding value of HRCmax (HRC of maximum uptake) was less than 10% in hot lesions with diameter ≥17 mm, but more than 10% in hot lesions with diameter of 10 mm in the eight PET/CT systems. Along with the reduction of the sphere diameter, HRCmean decreased, while CRCmean increased. HRCmax increased along with the increasing diameter until 17 mm, and reached the plateau at 1 thereafter.
Contrast
QH (hot sphere contrast) and Qc (cold sphere contrast) were calculated by employing equations 4–5. The QH, Qc, as well as the mean value, SD value and variability for the eight PET/CT systems are presented in Table 2. The QH and Qc distributions with different diameter of spheres are graphically shown in Figs 4 and 5. The variation of QH was less than 10% in hot lesions with diameter ≥17 mm, but more than 10% in hot lesions with diameter of 10 mm. The variation of Qc in 13 mm diameter sphere was also more than 10% in the eight PET/CT systems.
Background variability
Background variability (N) was calculated by employing equation 6. The result, as well as the mean value, SD value and variability in the eight PET/CT systems are presented in Table 3. The N distributions with different diameter of spheres are graphically shown in Fig. 6. The variation of N was less than 10% in hot lesions with diameter ≥17 mm, but more than 10% in hot lesions with diameter of 10 mm and in cold lesions with diameter of 13 mm.
Discussion
NEMA NU2 image quality phantom simulates the clinical hot and cold lesions and reflects the clinical image quality of PET/CT system. NEMA NU2 specifies two largest spheres (diameter of 37 mm and 28 mm) as the cold lesions and the other spheres (diameter of 22 mm, 17 mm, 13 mm and 10 mm) as the hot lesions. Considering the features of our planned clinical trial, to simulate the performances of hot tumor lesions with a variety of sizes, we used the two spheres with diameter of 28 mm and 13 mm for cold lesions and the others for hot lesions.
The recovery coefficient is a simple and intuitive indicator of partial volume effects. Due to the inherent limitations in theory and detection, the spatial resolution of PET is worse than CT (Computed Tomography) or MRI (Magnetic Resonance Imaging) system. This results in severe partial volume effect on PET images2,3. Ideally, recovery coefficient is 1 for hot lesions (most malignant tumors in PET) and 0 for cold lesions. However, the measured activity of hot lesion is usually lower than the actual value due to the partial volume effect. The cold lesion will lead to the opposite result. The smaller the size of the lesion is, the greater the partial volume effect will be2,3.
A study showed that when the sphere diameter is equal to three times the spatial resolution of the system expressed as the Full Width at Half Maximum (FWHM), the maximum pixel value of the image is theoretically 99.4% of the true value2. However, when the image is superimposed by noise, the measured activity can be higher than the actual one3,4. For a hot lesion whose size is close to the FWHM of the imaging system, the maximum activity concentration measured on the image can be reduced to 29% of the actual one2. This is consistent with our results. As the data showed in Table 1, in most cases, HRCmax results were larger than 1, except in the sphere with diameter of 10 mm. In addition, the variation of HRCmax was much larger than that of HRCmean. Therefore, the quantitative data related to the average value in the region-of-interest, such as SUVmean or SUVpeak5 will be favorable in the following clinical trial, instead of SUVmax6,7,8,9. In our study, HRCmax increased along with the increasing diameter until 17 mm probably because of decreasing partial volume effect. When diameter became larger than 17 mm, HRCmax almost reached its theoretical value, i.e. 1. The fluctuation thereafter is mainly due to the noise in the image. A longer scanning time may help to mitigate the fluctuation.
Theoretically, there can be a platform area in the center of large lesions with no “activity loss”. The impacts of partial volume effect mainly on the edge of the lesion in this case and causes “activity loss”2,3. Our result showed that the HRCmean of all hot spheres with diameter of 37 mm, 22 mm, 17 mm and 10 mm were smaller than 1. In addition, the smaller the hot lesion was, the lower the HRCmean would be.
Partial volume effect, caused by the finite spatial resolution, is the main cause of error in PET quantification. As Figs 1 and 2 show, the lines of the eight units are almost parallel. This indicates that the variation among them was not from random error and statistical fluctuation of counts. This probably is caused by the differences in the accuracy of cross calibration between PET/CT system and the accessories used as well as the differences in spatial resolution in these units. Above all, the variation of the main quantitative data was within 10%, which is acceptable in the following multicenter clinical trial.
Our results showed also that the recovery capacity in small lesions was lower than the one in large lesions. In addition, the variation in small lesions among the eight units was larger. This will be an important cause of error in the following clinical trial. In this case, partial volume effect correction is necessary10,11.
With an ideal mean value of 100%, the contrast of the lesion in PET images reflects the relationship between the lesion and the surrounding background. This represents the overall image quality and the ability to detect small lesions of a PET system. As Table 2 and Figs 4 and 5 show, the contrast of hot and cold lesions decreased as the size of the lesion became smaller. The contrast of the biggest hot lesion (diameter 37 mm), and the smallest hot lesion (diameter 10 mm) were only 79.08% and 32.31% respectively. The smaller the lesion, the worse the detection ability of the device. This is consistent with other studies12. The contrasts were different among the eight devices. For hot spheres with diameter of 10 mm, the variation was up to 26.3%. This indicates that the small lesion detectability among these eight units are different. Therefore, more attention should be taken in the data analysis related to small lesion detectability in the following clinical trials.
Background variability reflects the noise level of the image. The greater the variability value, the higher the image noise. Our results showed that the background variability increased as the ROIs became smaller. The background variability is equal to the ratio of the SD to the mean value in the background ROI, and SD is equal to the square root of the mean value. In other words, the background variability is equal to the reciprocal of the square root of the mean value in the ROI13. When diameter of ROI was not more than 13 mm, the background variability among these eight devices was greater than 10%, suggesting that, in order to ensure the consistence in the following clinical trial, the background ROI should be as large as possible (diameter greater than or equal to 17 mm) when target-to-background ratio related parameters are investigated.
In summary, the variation of major quantitative performances was within 10% among the eight PET/CT units, which is acceptable for the following multicenter clinical trial. However, there are still some aspects that need to pay more attention in the setup of QC protocol before the clinical trial. Firstly, the quantitative data related to the average voxel value, such as SUVmean or SUVpeak will be favorable, instead of SUVmax. Secondly, for lesions with diameter ≤13 mm, the analysis of quantitative data needs to be careful. Thirdly, more attention should be taken in the data analyses related to small lesion detectability. Lastly, the background ROI should be drawn as large as possible when target-to-background ratio related parameters are investigated.
Methods
PET/CT facilities
Eight Discovery PET/CT 690 (GE Healthcare) scanners, installed within two years in China, were enrolled in this trial. Facilities are distributed in eight cities in China, from the northernmost one (Harbin, latitude 45.75 degrees) to the southernmost one (Guangzhou, latitude 23.16 degrees). Eight PET/CT scanners were numbered randomly from 1 to 8.
Imaging
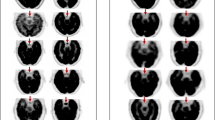
One NEMA image quality phantom (Biodex) of PET1 was used in this trial. The phantom internal length is 180 mm, and the cross section is in the shape of the human thorax. The longest inner diameters in the horizontal and vertical directions in the cross section are 294 mm and 224 mm, respectively. At the axial center of the phantom, a cylindrical insert with an outer diameter of 50 mm and an inner length of 180 mm was placed. Low density (0.3 g/ml) substance was filled into the cylinder insert to simulate the lung tissue. Six hollow spheres with thickness of 1 mm were evenly distributed around the lung insert. The inner diameters of these spheres were 37 mm, 28 mm, 22 mm, 17 mm, 13 mm and 10 mm, respectively.
To mitigate the operating discrepancy, one operator performed all the phantom preparation, imaging and data analysis procedures. The 18F-NaF concentration in the background was 5.3 kBq/ml, while the ones in the spheres of diameter 37 mm, 22 mm, 17 mm and 10 mm were 8:1 as to that of the background to simulate the hot lesion, and the ones in the spheres of diameter 28 mm and 13 mm were 0 kBq/ml to simulate the cold lesion.
The image acquisition and reconstruction protocols of the planned clinical trial will be the same with this investigation. PET images were acquired (2 beds, 2 min/bed), then reconstructed with Ordered Subset Expectation Maximization (OSEM) algorithm, 24 subsets, 2 iterations, and Gaussian post filter with full width at half maximum 6.4 mm, and with Time-of-Flight (TOF) and Point Spread Function (PSF) technologies. A z-axis 1:2:1 filter was used. The slice thickness of the reconstructed PET image was 3.27 mm. CT images were acquired with 140 kVp tube voltage and automatic tube current (15–180 mA), rotation speed 0.5 s and pitch of 0.984. Adaptive Statistical Iterative Reconstruction (ASiR, GE Healthcare) reconstruction algorithm was used for the reconstruction of CT data. The CT images were used for attenuation correction of PET images and image fusion with PET.
Image analysis
The image analyses were performed using Xeleris Workstation (GE Healthcare). For recovery coefficient analysis, the sphere Volumes-of-Interest (VOIs) were delineated in the center of the sphere on PET images. The radioactive uptake concentration (kBq/ml), maximum pixel counts in hot lesions (Cmax,H,j), average pixel counts in hot lesions (Cmean,H,j), as well as in cold lesions (Cmean,C,j) and their SDwere assessed, where j represents for the number of the sphere.
For contrast and background variation analyses, we used the method of NEMA NU21 standard. The Regions-of-Interest (ROIs) were delineated on the transaxial CT images in the center of the spheres, and then projected on the PET images. The mean counts (CB,j) and SDB, jof ROIs with the same diameter were recorded.
HRC and CRC were calculated by employing Equations 1–3 14,15. While QH, Qcand background variability (N) were assessed by employing Equations 4–6 1.
Ethics Statement
Only phantom study is included in this study, so basically the ethics statement is not necessary.
References
National Electrical Manufacturers Association. Performance Measurements of Positron Emission Tomographs. NEMA Standards Publication NU 2-2012. Rosslyn, USA(2012).
Chen, Y. et al. Partial Volume Effect and Its Influence Factors for Sphere Lesion PET Images. Atomic Energy Science and Technology. 48(6), 1136–1142 (2014).
Chen, Y. et al. Simulation study and experiment verify of partial volume effect for positron emission tomography. Atomic Energy Science and Technology. 38(5), 467–473 (2004).
Chen, Y., Geng, J., Tian, J., He, Y. & Chen, S. Partial volume effect in PET images: phantom study. Chin J Med Imaging Technol. 20(1), 99–102 (2004).
Wahl, R., Jacene, H., Kassamon, Y. & Lodge, M. From RECIST to PERCIST: Evolving considerations for PET response criteria in solid tumors. J Nucl Med. 50(5), 122s–150s (2009).
Geworski, L., Knoop, B. O., de Cabrejas, M. L., Knapp, W. H. & Munz, D. L. Recovery correction for quantitation in emission tomography: a feasibility study. Eur J Nucl Med. 27, 161–169 (2000).
International Standard IEC 61675-1:Radionuclide imaging devices-characteristics and test conditions - Part1: Positron emission tomographs. International Electrotechnical Commission. Geneva,(1998).
Geng, J. et al. Influence of partial volume effect on dual-head coincidence FDG images. Chin J Nucl Med. 23(4), 55–57 (2003).
Lin, C. et al. Repeatability of quantitative 18f-naf pet: a multicenter study. Journal of Nuclear Medicine Official Publication Society of Nuclear Medicine 57(12), 1872 (2016).
Chen, Y. et al. A simple method for partial volume effect correction in PET images. Nuclear Electronics & Detection. Technology. 27(6), 1231–1238 (2007).
Geng, J., Chen, Y., Tian, J., Yao, S. & Chen, S. Lesion’s standard uptake value correction in PET images. Nuclear Electronics & Detection Techonlogy. 29(4), 925–930 (2009).
Reynés-Llompart, G. et al. Performance Characteristics of the Whole-Body Discovery IQ PET/CT System. J Nucl Med. 58(7), 1155–1161 (2017).
Geng, J., Chen, Y., Yin, D., Tian, J. & Chen, S. Noise components on positron emission tomography images. Biomed Mater Eng. 13(2), 181–186 (2003).
Hoffman, E. J., Huang, S. C. & Phelps, M. E. Quantification in positron emission computed tomography: 1. Effect of object size. J Comput Assist Tomogr. 3, 299–308 (1979).
Kessler, R. M., Ellis, J. R. & Eden, M. Analysis of emission tomographic scan data: limitations imposed by resolution and background. J Comput Assist Tomogr. 8, 514–522 (1984).
Author information
Authors and Affiliations
Contributions
J.G. programmed the experiment. Y.Z. wrote the manuscript. C.G. and J.H. analyzed the data. J.L. prepared the figures. N.W., J.X. and H.X. conceived the experiment. L.Y. performed the experiment. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhu, Y., Geng, C., Huang, J. et al. Measurement and Evaluation of Quantitative Performance of PET/CT Images before a Multicenter Clinical Trial. Sci Rep 8, 9035 (2018). https://doi.org/10.1038/s41598-018-27143-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27143-4
This article is cited by
-
Influence of reconstruction techniques on PET/CT image quality and quantitative accuracy: a phantom study
Radiation Detection Technology and Methods (2023)
-
An optimized imaging protocol for [99mTc]Tc-DPD scintigraphy and SPECT/CT quantification in cardiac transthyretin (ATTR) amyloidosis
Journal of Nuclear Cardiology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.