Abstract
Abscisic acid (ABA) is an ancient stress hormone and is detectable in a wide variety of organisms where it regulates innate immunity and inflammation. Previously, we showed that oral supplementation with ABA decreased parasitemia in a mouse model of malaria, decreased liver and spleen pathology and reduced parasite transmission to mosquitoes. Here, we report that higher circulating ABA levels were associated with a reduced risk of symptomatic malaria in a cohort of Plasmodium falciparum-infected Ugandan children. To understand possible mechanisms of ABA protection in malaria, we returned to our mouse model to show that ABA effects on Plasmodium yoelii 17XNL infection were accompanied by minimal effects on complete blood count and blood chemistry analytes, suggesting a benefit to host health. In addition, orally delivered ABA induced patterns of gene expression in mouse liver and spleen that suggested enhancement of host anti-parasite defenses. To test these inferences, we utilized passive immunization and knockout mice to demonstrate that ABA supplementation increases circulating levels of protective, parasite-specific IgG and requires caspase-1 to reduce parasitemia. Collectively, ABA induces host responses that ameliorate infection and disease in an animal model and suggest that further studies of ABA in the context of human malaria are warranted.
Similar content being viewed by others
Introduction
Little is currently known about the effects of abscisic acid (ABA) on innate and adaptive immunity to infection and less yet about the mechanisms underlying these effects. Treatment with ABA has been shown to activate granulocytes, monocytes, and microglial cells in vitro, increasing phagocytosis, production of reactive oxygen species (ROS) and nitric oxide (NO), enhancing nuclear factor (NF)-κB nuclear translocation and release of monocyte chemotactic protein (MCP-1) and tumor necrosis factor α (TNFα)1,2,3,4. Interestingly, ABA decreased TNFα expression in white adipose tissue in a model of inflammatory bowel disease and reduced MCP-1 expression and leukocyte infiltration in the lungs of mice with influenza5,6. Taken together, these data suggest that the effects of ABA on inflammatory signaling are organ- and context-dependent.
The generalized immune response to Plasmodium infection is characterized by a switch from an early pro-inflammatory response to an anti-inflammatory response near peak parasitemia, followed by antibody-mediated parasite clearance. During P. yoelii infection, IgM antibodies are transiently produced, with protective IgG antibodies (e.g., IgG1, IgG2b) becoming predominant later in infection7,8,9. The innate immune response to Plasmodium infection includes clearance of infected red blood cells (iRBCs) by macrophages and dendritic cells in the liver and spleen as well as pro-inflammatory cytokine production following inflammasome activation and signaling through Toll-like receptors and NF-κB10. Detection of hemozoin and parasite genomic DNA within the hepatocyte phagolysosome can activate the inflammasome leading to increased production of interleukin (IL)-18 and IL-1β, which are toxic to the parasite11. Macrophages secrete IL-1β, interferon γ (IFNγ), and TNFα, which enhance intrahepatic killing of parasites and induce natural killer (NK) cell cytotoxicity12,13,14. TNFα is necessary for IFNγ production by NK cells and is associated with resolution of fever during malaria15.
Reciprocal induction of pro-inflammatory cytokine secretion in positive feedback loops can lead to an overactive immune response and immunopathology in malaria16. In this context, peroxisome proliferator-activated receptor gamma (PPARγ) is an important regulator of immunity and inflammation. PPARγ agonists have been proposed as malaria therapeutics and can increase CD36-mediated phagocytosis of iRBCs while reducing malaria-induced TNFα secretion17,18,19. ABA can enhance PPARγ levels, and ABA-associated reductions in inflammation were observed to be PPARγ-dependent in inflammatory bowel disease and influenza5,20. Similarly, in lipopolysaccharide (LPS)-challenged mice, ABA decreased splenic expression of il6, tnfα, and ifnγ in a PPARγ-dependent manner21. We previously demonstrated that ABA supplementation in P. yoelii 17XNL-infected CD-1 mice reduced parasitemia while increasing pparγ expression and decreasing NO synthase (nos) expression in both liver and spleen22.
Here, we report that Ugandan children with asymptomatic falciparum malaria (no fever) had higher circulating plasma ABA levels than did children with symptomatic disease (fever at presentation). Based on these observations, we returned to our genetically tractable model to investigate possible mechanisms of ABA action during Plasmodium infection. We show that orally delivered ABA did not alter complete blood cell count and clinical chemistry analytes in uninfected mice and improved some of these parameters during P. yoelii 17XNL infection. Further, the beneficial effects of elevated plasma ABA on P. yoelii 17XNL infection were dependent on caspase-1 and on increased production of IgG, suggesting that elevated plasma ABA may be clinically relevant and protective in falciparum malaria.
Results
Reduced risk of symptomatic malaria in P. falciparum infected children was associated with higher plasma ABA levels
We quantified ABA levels as previously described22 in 122 plasma samples from P. falciparum infected children in Uganda. Children were selected from the Nagongera and Kanungu surveillance cohorts of the East Africa International Center of Excellence for Malaria Research (ICEMR). These cohorts were established in 2011 for health monitoring, including regular visits every 30–90 days during which blood smears and plasma samples were obtained regardless of symptoms23. Plasma samples from parasitemic children ages 2–4 and 7–9 years were selected randomly with stratification by age and parasite density.
Children with higher plasma ABA levels had asymptomatic infections, not presenting with fever (Fig. 1). These patterns remained evident when samples were stratified by age group (Supplementary Fig. 1), however ABA levels did not correlate with parasitemia (Supplementary Fig. 1). Importantly, plasma levels in children with falciparum malaria were in the same range (<1–100 nM) as detected previously in our mouse model22.
Asymptomatic falciparum malaria in children was associated with higher plasma ABA levels. ABA levels in plasma samples from children, ages 2–9, with detectable P. falciparum parasitemia. Only children without fever (asymptomatic malaria) had plasma ABA concentrations in excess of 20 nM. Each dot represents one individual (n = 122; 39 with fever, 83 without fever). Data were analyzed by Welch two sample t-test (p = 0.003).
ABA supplementation reduced disease and improved blood urea nitrogen levels in P. yoelii 17XNL-infected C57BL/6 mice
Our previous studies utilized outbred mice (CD-1 strain) to examine the effects of ABA on P. yoelii 17XNL parasitemia, pathology and transmission22. Here, we established a model for mechanistic studies with C57BL/6 mice. As in our studies with CD-1 mice, 6–8 week old C57BL/6 mice received water containing 2.56 mM ABA for three days prior to and throughout the course of infection with P. yoelii 17XNL. In four separate cohorts of C57BL/6 mice, we affirmed that ABA supplementation significantly reduced parasitemia (Fig. 2, Supplementary Fig. 2) compared to unsupplemented mice.
ABA supplementation significantly reduced parasitemia in C57BL/6 mice infected with P. yoelii 17XNL. Daily parasitemias of mice with and without oral ABA supplementation. Four replicates were conducted with 3–5 mice per treatment in each replicate. Mouse cohorts were sacrificed for analyses on days 9, 11 and 13, reducing animal numbers after these days. Data were analyzed by unpaired t-test.
At day 9 post-infection (PI), all infected mice exhibited hepatomegaly and splenomegaly typical of malaria (not shown)24. Livers of ABA supplemented mice, however, weighed significantly less than those of unsupplemented mice (Fig. 3A), and there was a trend towards reduced spleen weight with ABA supplementation (Fig. 3B). This was consistent with our previous findings that ABA reduced inflammatory pathology, including microabscesses and leukocyte infiltration in the liver and hyperplasia in the spleen during P. yoelii 17XNL infection22.
ABA supplemented mice have reduced hepatosplenomegaly. Weights of whole liver (A) and whole spleen (B) at day 9 PI from P. yoelii 17XNL-infected mice with and without ABA supplementation. Each dot represents one mouse sacrificed from a single cohort from the data represented in Fig. 2. Data were analyzed by unpaired t-test.
Mouse plasma was analyzed for ABA concentration by liquid chromatography-tandem mass spectrometry (LC-MS/MS, University of California San Francisco Drug Research Unit) and for a variety of analytical markers of overall health (University of California Davis Comparative Pathology Lab). Plasma ABA levels were elevated by daily ad libitum supplementation in the presence and absence of infection (day 10 PI), with variation likely resulting from patterns and timing of water consumption (Fig. 4). Relative to previous observations22, supplemented infected C57BL/6 mice exhibited notably higher levels of plasma ABA than did supplemented CD-1 mice at a similar stage of infection, perhaps due to differences in mouse strain, enhanced sensitivity of LC-MS/MS versus ELISA or both.
Oral ABA supplementation increased circulating levels of ABA in mouse plasma. ABA concentrations, measured by LC-MS/MS, in plasma of uninfected and P. yoelii 17XNL-infected wild type C57BL/6 (WT) and caspase-1 knockout (casp1−/−) mice with and without ABA supplementation. Plasma samples were collected on day 11 PI. Each dot represents one mouse sacrificed from those represented in Fig. 2. Data were analyzed by unpaired t-test.
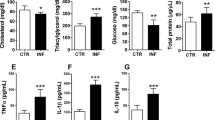
In the presence and absence of infection, ABA supplementation had no effect on levels of plasma albumin, creatine, phosphorus, bilirubin, or total protein (Supplementary Table S1). Infected mice had significantly lower blood urea nitrogen (BUN) levels than uninfected mice (Fig. 5A). ABA supplementation had no effect on BUN levels in uninfected mice but, in the context of infection, ABA-treated mice had plasma BUN levels that were not significantly different from those in uninfected mice (Fig. 5A). Although increased BUN levels have been associated with kidney damage, abnormally low levels can also be indicative of impaired liver function25,26,27, suggesting that the effects of ABA extend beyond resolution of liver histopathology to improved function.
ABA supplementation rescued blood urea nitrogen levels in P. yoelii 17XNL-infected mice but did not alter glucose or insulin levels in plasma. Blood urea nitrogen (A) glucose (B), and insulin levels (C) in plasma of uninfected mice and P. yoelii 17XNL-infected mice (day 11 PI) with and without ABA supplementation. Each dot represents one mouse sacrificed from those represented in Fig. 2. Data were analyzed by unpaired t-test.
ABA supplementation had no effect on baseline blood glucose or insulin levels
ABA supplementation has been shown to increase glucose-potentiated insulin release and has been proposed as an adjunctive treatment for type-2 diabetes28,29. Because hypoglycemia and hyperinsulinemia are pathological symptoms associated with malaria30, we sought to explore the potential for ABA to exacerbate these conditions. Neither baseline glucose nor insulin levels, however, were significantly different from controls after two weeks of oral ABA supplementation in uninfected or P. yoelii 17XNL-infected C57BL/6 mice (day 11 PI, Fig. 5B,C).
ABA increased the proportion of eosinophils and basophils in blood of P. yoelii 17XNL-infected C57BL/6 mice
Complete blood counts (CBC, University of California Davis Comparative Pathology Lab) were performed on whole blood from uninfected and P. yoelii 17XNL-infected mice (day 11 PI) with and without ABA supplementation. In the absence of infection, ABA had no effect on blood cell composition (percentages of neutrophils, lymphocytes, monocytes, eosinophils, basophils, and platelets), hemoglobin levels, or percent hematocrit (Supplementary Table S2). In the presence of infection, the percentages of eosinophils and basophils were reduced and ABA significantly increased these to levels approaching those in uninfected mice (Fig. 6). Though little is known about the role that eosinophils and basophils play in malaria, eosinophilia has been associated with recovery from infection31,32. No other changes in blood cells or hemoglobin values were observed with ABA supplementation (Supplementary Table S2).
ABA supplementation partially rescued the decrease in eosinophils and basophils associated with P. yoelii 17XNL infection. Percentage of eosinophils (A) and basophils (B) in whole blood of uninfected mice and P. yoelii 17XNL-infected mice (day 11 PI) with and without ABA supplementation. Each dot represents one mouse sacrificed from those represented in Fig. 2. Data were analyzed by unpaired t-test.
Gene expression patterns associated with ABA supplementation were suggestive of inflammasome activation and antibody isotype switching in P. yoelii 17XNL-infected mice
We monitored spleen and liver gene expression from days 9–13 PI in C57BL/6 mice, the period corresponding to the greatest reductions in parasitemia following ABA supplementation (Fig. 2, Supplementary Fig. 2). We compared gene expression in these tissues to that in the same tissues in CD-1 mice at days 4–7 PI, reflecting our previous observations that the effects of ABA on parasitemia occurred earlier in this mouse strain22. In spleen of C57BL/6 mice, expression levels of ifnγ, nos2, il6, and tbet were decreased in ABA-supplemented mice at day 10 PI. A trend towards decreased tbet was also evident at day 13 PI (Fig. 7A). No change in tnfα expression was evident at any timepoint. These patterns were consistent with those in spleen of CD-1 mice at days 4–7 PI (Supplementary Fig. S3A)22.
Splenic gene expression patterns following ABA supplementation were suggestive of enhanced IgG production. Relative target gene mRNA levels in spleens of unsupplemented and ABA-supplemented C57BL/6 mice on days 9, 10, 11, and 13 PI (A) and in casp1−/− mice on day 10 PI with P. yoelii 17XNL (B). Each dot represents one mouse with time points in panel (A) collated from three separate cohorts of mice and in panel (B) from one cohort of mice. Data are shown as −Δct, normalized to β-actin, and were analyzed by unpaired t-test. *p < 0.05, #p < 0.1.
In livers of C57BL/6 mice, a significant increase in pparγ expression was evident by day 13 PI (Fig. 8A). Il18 expression was significantly lower in ABA-treated mice on day 9 but increased compared to controls on days 11 and 13 PI. Il1β mRNA levels did not change significantly at any timepoint while il6 expression increased on day 10 PI (Fig. 8A). Increased expression of il18 has been associated with inflammasome activation33,34 and contributes to parasite killing in the liver35. IL-6 can function in concert with IL-1β to induce the synthesis of specific acute phase proteins36. However, IL-6 can also signal through an alternate pathway to promote regenerative and anti-inflammatory signaling in the liver37,38,39, effects that would be consistent with reduced parasitemia in ABA-treated mice as well as reduced hepatomegaly at day 9 PI (Fig. 3A) and recovery of BUN in ABA-treated, infected mice to control levels at day 11 PI (Fig. 5A). ABA-induced gene expression in the liver was similar to patterns in CD-1 mice (Supplementary Fig. S3B)22, although increases in il6 and il1β in the liver were more pronounced in ABA-treated, infected CD-1 mice (Supplementary Fig. S3B). Collectively, the consistency of effects of ABA on parasitemia, hepatosplenomegaly, and gene expression patterns in spleen and liver of ABA-treated, infected C57BL/6 and CD-1 mice suggested that ABA effects were robust across divergent mouse models and provided good confidence for testing hypotheses in the more tractable inbred strain.
Hepatic gene expression patterns following ABA supplementation were suggestive of inflammasome activation and consistent with reduced hepatomegaly. Relative target gene mRNA levels in livers of unsupplemented and ABA-supplemented C57BL/6 mice on days 9, 10, 11, and 13 PI (A) and in casp1−/− mice on day 10 PI with P. yoelii 17XNL (B). Each dot represents one mouse with time points collated from the cohorts of mice as in Fig. 7. Data are shown as −Δct, normalized to β-actin, and were analyzed by unpaired t-test. *p < 0.05, **p < 0.01.
ABA supplementation increased levels of parasite-specific, protective circulating IgG antibodies in P. yoelii 17XNL-infected C57BL/6 mice
Based on gene expression patterns in the spleen (Fig. 7A, Supplementary Fig. S3A), we hypothesized that ABA supplementation could alter antibody synthesis and perhaps isotype patterns in infected mice. Specifically, reduced tbet expression in B cells can increase total IgG synthesis while increased tbet expression correlates with reduced IgG1 production40,41. Additionally, low levels of IFNγ can enhance IgG1 synthesis42, suggesting that ABA-induced repression of tbet and ifnγ in the spleen at day 10 PI (Fig. 7A) could increase total IgG and IgG1 in P. yoelii 17XNL-infected mice.
To determine whether these patterns were evident in our model, we measured antibody levels in plasma from control and ABA-supplemented P. yoelii 17XNL-infected C57BL/6 mice on days 9, 11, and 13 PI. Levels of IgG1 and IgG2c in ABA-supplemented mice were significantly greater than control levels by day 13 PI, with no changes noted in other isotypes (Fig. 9). Parasitemia and antibody titers were significantly negatively correlated, with lower parasitemias and higher levels of IgG1 and IgG2c in ABA-supplemented mice (Fig. 10).
ABA supplementation increased levels of IgG1 and IgG2c in plasma during P. yoelii infection. Data are shown as concentrations of IgM and IgG antibody isotypes in plasma from mice with and without ABA supplementation on days 9, 11 and 13 PI with P. yoelii 17XNL. Each dot represents one mouse with time points collated from the cohorts of mice as in Fig. 7. Data were analyzed by unpaired t-test.
Levels of IgG1 and IgG2c were negatively correlated with parasitemia. IgG1 and IgG2c levels were plotted against parasitemia (days 11 and 13 PI from Fig. 9) from P. yoelii 17XNL-infected mice with and without ABA supplementation. Each dot represents one mouse. Data were analyzed by linear regression.
To determine whether increased circulating total IgG and the protective isotype IgG1, which was elevated to a greater degree than protective IgG2c43 (Fig. 9), were parasite-specific, we examined the cross-reactivity of plasma from infected mice (day 13 PI) and from uninfected mice against proteins from P. yoelii 17XNL-infected and uninfected RBCs. Plasma from ABA-supplemented, infected mice contained higher levels of parasite-specific IgG1 and total IgG compared to unsupplemented, infected mice (Fig. 11A, Supplementary Fig. S4). Specifically, densitometry analysis revealed a 1.67-fold increase in levels of cross-reacting IgG1 and a 1.34-fold increase in levels of cross-reacting total IgG in plasma from ABA-supplemented, infected mice compared to unsupplemented, infected mice (Fig. 11B). Additionally, IgG1 from ABA-treated mice cross-reacted with at least one unique protein (~90 kDa) from infected RBCs that was not bound by IgG1 from unsupplemented, infected mice.
ABA supplementation increased levels of parasite-specific IgG1 and total IgG in mouse plasma. (A) Western blots of protein from uninfected and P. yoelii 17XNL-infected RBCs probed with plasma from uninfected mice, P. yoelii-infected mice or ABA-supplemented P. yoelii-infected mice followed by secondary antibodies to IgG1 and total IgG. The 50 kDa band corresponds to the IgG heavy chain. Asterisk indicates unique protein (~90 kDa) bound by IgG1 from ABA-treated, infected mice. Samples for IgG and IgG1 detection were each run on a single gel. Membranes were cut for incubation with each type of plasma. Total IgG- and IgG1-probed blots were exposed together and images were cropped to remove white space and redundant protein ladders. (B) Densitometry analysis of IgG1- and total IgG-bound proteins from iRBCs. Data are shown as fold change in plasma from ABA-supplemented P. yoelii-infected mice relative to plasma from unsupplemented, infected mice.
To test whether enhance IgG levels were protective, mice were injected (tail vein) with 50 µl of plasma pooled from unsupplemented or ABA-supplemented, infected mice. Pooled plasma from ABA-supplemented mice used for passive immunization had 1.5-fold higher levels of IgG1 than that from control mice (Fig. 12A). At 24 hours after plasma injection, mice were infected with P. yoelii 17XNL. Mice that received plasma from ABA-supplemented, infected mice trended towards lower parasitemia and were significantly different than control-plasma injected mice on days 6 and 8 PI, suggesting that the plasma provided limited protection against infection (Fig. 12B), the duration of which was consistent with the half-life of IgG1 (6–8 days) in serum44.
Plasma from ABA-supplemented mice exhibited limited protection against P. yoelii 17XNL infection. (A) Fold change in antibody isotype in pooled plasma from ABA-supplemented infected mice relative to unsupplemented, infected mice (day 13 PI). (B) Parasitemias of individual mice injected with plasma from unsupplemented, infected mice (control) or ABA supplemented, infected mice (ABA) days 5 through 13 PI. Data were analyzed by unpaired t-test. n = 3–4 mice per group.
The effects of ABA on P. yoelii 17XNL parasitemia were ablated in caspase-1-deficient mice and this pattern was associated with changes in splenic and hepatic gene expression
Inflammasome activation in malaria results in the activation of caspase-1, which cleaves pro-IL-1β and IL-18 into their active forms45. To determine whether the effects of ABA on P. yoelii 17XNL infection were caspase-1-dependent, we examined parasitemia over time and hepatic and splenic gene expression on day 10 PI, when a majority of target mRNA levels were significantly different in ABA-supplemented and unsupplemented C57BL/6 mice (Figs 7A, 8A). In age-matched C57BL/6 mice, ABA supplementation significantly reduced parasitemia on days 8 and 10 PI (Fig. 13). However, while plasma levels of ABA in wild type and casp1−/− mice were comparable (Fig. 4), ABA had no effect on parasitemia in casp1−/− mice from days 7–10 PI (Fig. 13). Intriguingly, ABA supplemented wild type mice had significantly lower parasitemias than ABA-supplemented casp1−/− mice on days 8 and 10 PI, while parasitemias of unsupplemented wild type and casp1−/− mice did not significantly differ (Fig. 13). In the spleen, ABA-dependent decreases in nos2, il6, and tbet expression were ablated in casp1−/− mice, while in casp1−/− mice the ABA-dependent decrease in ifnγ was reversed and ABA treatment increased pparγ expression (Fig. 7B). In the liver, the ABA-dependent increase in il6 was ablated in casp1−/− mice and il1β was significantly reduced in ABA-treated casp1−/− mice (Fig. 8B).
Discussion
In our mouse malaria model, ABA supplementation has minimal to no effects in the absence of malaria and improves host health in the presence of parasite infection. In the absence of supplementation, infected mice do not generate detectable levels of plasma ABA (Fig. 4)22, so supplementation is necessary to study the effects of ABA on infection and pathology. Children with malaria, however, have plasma ABA levels consistent with those in supplemented outbred mice22 and elevated ABA levels were significantly associated with reduced risk of symptomatic malaria in the setting of P. falciparum infection. Given this clinical association, we sought to examine the mechanisms of ABA action in the context of malaria using a supplemented, genetically tractable mouse model.
We infer that no change or, at most, modest beneficial changes in CBC and plasma enzymes in supplemented mice are consistent with the hypothesis that ABA enhances anti-malarial immunity while mitigating inflammatory pathology. In particular, baseline glucose and insulin levels were not altered by ABA supplementation in either infected or uninfected mice, despite the fact that ABA can enhance insulin secretion in vitro and can reduce fasting glucose levels in a mouse model of diabetes6,29. These observations are consistent with the hypothesis that ABA supplementation does not exacerbate malaria-associated hypoglycemia and hyperinsulinemia.
Hepatic immunity clearly controls intrahepatic parasites, but the liver also contributes significantly to the control of erythrocytic asexual stages. These contributions include phagocytosis of iRBCs and secretion of the cytokines IL-6, TNFα, IL-18, and IL-1β46. IL-6 is typically more highly expressed in the liver than spleen47, suggesting that observed ABA-associated patterns in IL-6 expression warrant further study. IL-6 in the liver regulates the synthesis of acute phase proteins and contributes to the resolution of inflammation, the balance of which is dependent on classic and trans signaling by IL-6. In classic signaling, IL-6 binds a membrane bound IL-6 receptor (IL-6R), which interacts with the ubiquitous membrane protein gp130 to initiate JAK/STAT signaling and promote cell proliferation48. This signaling pathway is restricted to cells that express the bound IL-6R, including hepatocytes37,38. In trans signaling, IL-6 binds a soluble IL-6R, allowing it to bind to gp130 and initiate pro-inflammatory processes, most notably the synthesis of acute phase proteins (APP) in the liver. In the context of infection, inflammasome-associated IL-1β shifts IL-6-dependent synthesis of APP to those involved in pathogen defense, including hepcidin, C-reactive protein, serum amyloid P, and serum amyloid A49. Correlations between genetic mutations in il1β and Plasmodium vivax infection suggest that IL-1β is important for controlling parasitemia across Plasmodium species50. The ABA-dependent increase in liver il6 was ablated in casp1−/− mice and il1β was significantly reduced in ABA-treated casp1−/− mice (Fig. 8B), suggesting that the balance of ABA-dependent host immunity and pathology in our model are caspase-1-dependent.
Increased levels of IgG1, IgG2, and IgG3 have been associated with malaria resistance and have been observed to be significantly higher in patients with uncomplicated and asymptomatic malaria compared to those with complicated malaria51,52,53,54. Similarly, T-bet-deficient mice had lower parasitemia, which correlated with increased levels of IgG155. In our studies, plasma from ABA-supplemented mice provided limited protection against P. yoelii 17XNL infection. In a single tail vein injection, ABA levels in 50 µl plasma would be < 0.5 nM, providing confidence that the observed effects were due in part to elevated IgG. The half-life of IgG1 in serum is 6–8 days44, suggesting that a second injection of plasma post-infection could extend protection. IgG1 may enhance parasite clearance via antibody-dependent cell-mediated inhibition (ADCI), in which monocytes are activated by antibody-opsonized merozoites and release a soluble factor that blocks the division of intra-erythrocytic parasites56. Much lower levels of IgG1 are sufficient to trigger ADCI than is necessary for effective direct neutralization. Limited protection, however, also suggests that increased parasite-specific IgG is only one component of the protective response induced by ABA supplementation.
Caspase-1 deficiency abrogated the effect of ABA on parasitemia, suggesting that caspase-1 mediated immunity is critical to the reduction in parasitemia by ABA. Notably, the effects of ABA supplementation on tbet and ifnγ expression, which we hypothesize are driving protective IgG production, were eliminated or reversed in casp1−/− mice (Fig. 7B). Caspase-1 also plays a role in IgG production during influenza infection. Specifically, caspase-1 knockout mice had reduced total IgG levels compared to wild type mice, but this was due mainly to reduced IgG3 levels and reductions in IgG1 levels were not observed57. The lack of effect of ABA supplementation on parasitemia in casp1−/− mice suggests that inflammasome activation is the primary mechanism by which parasitemia is reduced. However, increased IgG may contribute to long-term immunity. Both the reduction in pathology and the enhanced IgG production in our model are consistent with the observations that higher plasma ABA concentrations were significantly associated with non-febrile falciparum malaria. Antibodies against P. falciparum-infected RBCs, as well as higher levels of IgG1, IgG2, and IgG3, have been associated with asymptomatic infection rather than symptomatic, febrile malaria52,58. Future work, however, is needed to determine whether ABA-enhanced antibody production provides protection against or enhances tolerance to infection.
Methods
Human plasma collection and ABA quantification
Plasma samples were selected from 122 P. falciparum infected children presenting for routine visits in the Nagongera and Kanungu surveillance cohorts of the East African International Center of Excellence in Malaria Research (ICEMR). The study protocol was reviewed and approved by the Uganda National Council of Science and Technology and the institutional review boards of the University of California–San Francisco, and Makerere University (DMID Protocol 10-0063). All experiments were performed in accordance with Good Clinical Practice (US Code of Federal Regulations,Ugandan Ethics Committee, and NIH/NIAID). Informed consent was obtained from the parent or guardian of all participating children. Samples were selected based on age stratification (2–4 and 7–9 year-olds), and log parasite density (range: 32 to >100,000 parasites/µL). Parasitemia at routine visits was determined and quantified by microscopy of thick blood smears, as previously described23. Symptomatic subjects were defined as those with fever at the time of the visit or with a reported history of fever. ABA was extracted from plasma as previously described22 and quantified using the Phytodetek Abscisic Acid ELISA kit (Agdia, Elkhart, IN) according to the manufacturer’s instructions. ABA levels were analyzed by unpaired t-test.
ABA was quantified in mouse plasma by LC-MS/MS using an AB Sciex API2000 tandem mass spectrometer coupled with PE 200 series micro LC pumps (calibration range 25–10,000 ng/mL) or API5000 tandem mass spectrometer coupled with Shimadzu Prominence 20ADXR LC pumps (calibration range 0.5–50 ng/mL). ABA was extracted from EDTA mouse plasma samples by solid phase extraction with Oasis® HLB micro-elution plate. Extracted samples were injected onto a Zorbax C8 LC column and eluted with 10 mM NH4FA-acetonitrile containing 0.1% formic acid. Electrospray ionization in negative mode and multiple reaction monitoring were used, and ion pairs m/z 263- >153 was selected for quantification. ABA levels were analyzed by unpaired t-test.
ABA supplementation and infection
Mice received control or 2.56 mM ABA-supplemented water three days prior to intraperitoneal (IP) injection with 1 × 107 P. yoelii 17XNL-iRBCs as described previously22. Parasitemias were determined from thin blood films stained with Giemsa and were defined as the percentage of iRBCs divided by total RBCs. Mice were sacrificed on days 9, 10, 11 and 13 PI. Parasitemia was analyzed by unpaired t-test. Hepatic and splenic gene expression data were obtained from CD-1 mice with previously quantified parasitemias22. Casp1−/− mice were obtained from Jackson Labs. Age-matched (6–8 week old) wild type C57BL/6 mice (Jackson Labs) were used as controls. All experiments were performed in accordance with the recommendations in the Guide for Care and Use of Laboratory Animals of the National Institute of Health and were approved by the Institutional Animal Care and Use Committees at the University of California at Davis under protocol 18948.
Blood analysis
Blood was collected from individual mice via heart puncture with heparinized needles on day 11 PI. Whole blood was collected in heparinized tubes and CBC performed using a HemaVet 950 FS (Drew Scientific). Blood was centrifuged at 5,900 × g for 8 min for plasma collection. Aliquots of plasma were stored at −20 °C and insulin levels determined using the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem #90080) according to the manufacturer’s instructions. Blood cell counts and analytes were analyzed by unpaired t-test.
qRT-PCR
Harvested liver and spleen were flash frozen and stored at −80 °C. RNA was extracted in TriZOL and complementary DNA (cDNA) synthesized according to manufacturer’s instructions (QuantiTect Reverse Transcription Kit, Qiagen). Quantitative real time polymerase chain reaction (qRT-PCR) was performed using validated TaqMan gene expression assays as previously described for mouse β-actin, nos2, tnfα, ifnγ, pparγ, il6, il18, il1β, and tbet (Applied Biosystems)59. Samples were analyzed in triplicate using 250 ng cDNA per reaction. Data were normalized to β-actin and analyzed by unpaired t-test. Outliers were tested by ROUT with Q = 2%.
Antibodies and plasma injection
Antibody levels in mouse plasma were determined using the ProcartaPlex Mouse Antibody Isotyping Panel (Affymetrix eBioscience EPX070-20815-901) according to the manufacturer’s instructions. Day 13 PI plasma from individual mice was pooled. C57BL/6 mice received 50 µL of plasma from control or ABA-supplemented infected mice by tail-vein injection. At 24 hours after plasma injection, mice were infected IP with 1 × 107 P. yoelii 17XNL-infected RBCs.
Western blot
Blood was collected from uninfected and P. yoelii 17XNL-infected mice and centrifuged at 2,000 × g for 10 min at room temperature to isolate RBCs. Equivalent volumes of collected RBCs were washed twice with RPMI medium before being suspended in lysis buffer. RBC proteins were separated by gel by electrophoresis, transferred to nitrocellulose, and incubated overnight at 4 °C in 5% dry milk in Tris-buffered saline, 0.1% Tween 20 (TBST). Gels were stained with Coomassie to quantify total protein. Membranes were incubated for 2 hours at room temperature with 1:100 dilutions of plasma from uninfected mice, from infected mice at day 13 PI, or from ABA-treated infected mice at day 13 PI. Washed membranes were then incubated with 1:1000 goat anti-mouse IgG1 (Abcam #ab97240) or 1:1000 rabbit anti-mouse IgG (Sigma #A9044) HRP-conjugated secondary antibodies for 1 hour at room temperature.
Data availability
All data generated or analyzed during this study are included in this published article and its Supplementary Information files.
References
Bruzzone, S. et al. Abscisic acid is an endogenous cytokine in human granulocytes with cyclic ADP-ribose as second messenger. Proc Natl Acad Sci. 104, 5759–5764 (2007).
Magnone, M. et al. Abscisic acid released by human monocytes activates monocytes and vascular smooth muscle cell responses involved in atherogenesis. J Biol Chem. 284, 17808–17818 (2009).
Bodrato, N. et al. Abscisic acid activates the murine microglial cell line N9 through the second messenger cyclic ADP-ribose. J Biol Chem. 284, 14777–14787 (2009).
Magnone, M. et al. Autocrine abscisic acid plays a key role in quartz-induced macrophage activation. FASEB Journal. 26, 1261–1271 (2012).
Hontecillas, R. et al. Dietary abscisic acid ameliorates influenza-virus-associated disease and pulmonary immunopathology through a PPARγ-dependent mechanism. J Nutr Biochem. 24, 1019–1027 (2013).
Guri, A. J., Hontecillas, R., Si, H., Liu, D. & Bassaganya-Riera, J. Dietary abscisic acid ameliorates glucose tolerance and obesity-related inflammation in db/db mice fed high-fat diets. Clin Nutr. 26, 107–116 (2007).
Langhorne, J., Evans, C. B., Asofsky, R. & Taylor, D. W. Immunoglobulin isotype distribution of malaria-specific antibodies produced during infection with Plasmodium chabaudi adami and Plasmodium yoelii. Cell Immunol. 87, 452–461 (1984).
Azcárate, I. G. et al. Differential immune response associated to malaria outcome is detectable in peripheral blood following Plasmodium yoelii infection in mice. PLOS One. 9, e85664 (2014).
Cohen, S., McGregor, I. A. & Carrington, S. Gamma-globulin and acquired immunity to human malaria. Nature. 193, 733–737 (1961).
Gazzinelli, R. T., Kalantari, P., Fitzgerald, K. A. & Golenbock, D. T. Innate sensing of malaria parasites. Nat Rev Immunol. 14, 744–757 (2014).
Kalantari, P. et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by Plasmodium-derived hemozoin and DNA during malaria. Cell Rep. 6, 196–210 (2014).
Pichyangkul, S., Saengkrai, P. & Webster, H. K. Plasmodium falciparum pigment induces high levels of tumor necrosis factor-alpha and interleukin-1 beta. Am J Trop Med Hyg. 51, 430–435 (1994).
Clark, I. A., al Yaman, F. M. & Jacobson, L. S. The biological basis of malarial disease. Int J Parasitol. 27, 1237–1249 (1997).
Okamura, H. et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 378, 88–91 (1995).
Tripp, C. S., Wolf, S. F. & Unanue, E. R. Interleukin 12 and tumor necrosis factor alpha are costimulators of interferon gamma production by natural killer cells in severe combined immunodeficiency mice with listeriosis, and interleukin 10 is a physiologic antagonist. Proc Natl Acad Sci USA 90, 3725–3729 (1993).
Malaguarnera, L. & Musumeci, S. The immune response to Plasmodium falciparum malaria. Lancet Infect Dis. 2, 472–478 (2002).
Boggild, A. K. et al. Use of peroxisome proliferator-activated receptor gamma agonists as adjunctive treatment for Plasmodium falciparum malaria: a randomized, double-blind, placebo-controlled trial. Clin Infect Dis. 49, 841–849 (2009).
Cabrera, A., Neculai, D. & Kain, K. C. CD36 and malaria: friends or foes? A decade of data provides some answers. Trends Parasitol. 30, 436–444 (2014).
Serghides, L. & Kain, K. C. Peroxisome proliferator receptor gamma-retinoid X receptor agonists increase CD36-dependent phagocytosis of Plasmodium falciparum-parasitized erythrocytes and decrease malaria-induced TNF-alpha secretion by monocytes/macrophages. J Immunol. 166, 6742–6748 (2001).
Guri, A. J., Evans, N. P., Hontecillas, R. & Bassaganya-Riera, J. T cell PPARγ is required for the anti-inflammatory efficacy of abscisic acid against experimental IBD. J Nutr Biochem. 22, 812–819 (2011).
Bassaganya-Riera, J. et al. Abscisic acid regulates inflammation via ligand-binding domain-independent activation of peroxisome proliferator-activated receptor gamma. J Biol Chem. 286, 2504–2516 (2011).
Glennon, E. K. K., Adams, L. G., Hicks, D. R., Dehesh, K. & Luckhart, S. Supplementation with abscisic acid reduces malaria disease severity and parasite transmission. Am J Trop Med Hyg. 94, 1266–1275 (2016).
Kamya, M. R. et al. Malaria transmission, infection, and disease at three sites with varied transmission intensity in Uganda: implications for malaria control. Am J Trop Med Hyg. 92, 903–912 (2015).
Wilson, S. et al. Hepatosplenomegaly associated with chronic malaria exposure: evidence for a pro-inflammatory mechanism exacerbated by schistosomiasis. Parasite Immunol. 31, 64–71 (2009).
Hioki, A. & Ohtomo, H. Significance of blood urea nitrogen as an index of renal function in mice infected with Plasmodium berghei. Parasitol Res. 76, 127–130 (1989).
Hosten, A. O. BUN and creatine in Clinical Methods: the History, Physical, and Laboratory Examinations. (3rd ed. Walker, H. K., Hall, W. D. & Hurst, J. W) 874–878 (Butterworths, 1990).
Nielsen, S. S., Grøfte, T., Tygstrup, N. & Vilstrup, H. Cirrhosis and endotoxin decrease urea synthesis in rats. Hepatol Res. 37, 540–547 (2007).
Magnone, M. et al. Microgram amounts of abscisic acid in fruit extracts improve glucose tolerance and reduce insulinemia in rats and in humans. FASEB J. 29, 4783–4793 (2015).
Bruzzone, S. et al. Abscisic acid is an endogenous stimulator of insulin release from human pancreatic islets with cyclic ADP ribose as second messenger. J Biol Chem. 283, 32188–32197 (2008).
White, N. J. et al. Severe hypoglycemia and hyperinsulinemia in falciparum malaria. N Engl J Med. 309, 61–66 (1983).
Kurtzhals, J. A. et al. Increased eosinophil activity in acute Plasmodium falciparum infection—association with cerebral malaria. Clin Exp Imunol. 112, 303–307 (1998).
Camacho, L. H. et al. The eosinophilic response and haematological recovery after treatment for Plasmodium falciparum malaria. Trop Med Int Health. 4, 471–475 (1999).
Thinwa, J., Segovia, J. A., Bose, S. & Dube, P. H. Integrin-mediated first signal for inflammasome activation in intestinal epithelial cells. J Immunol. 193, 1373–1382 (2014).
Shrivastava, S., Mukherjee, A., Ray, R. & Ray, R. B. Hepatitis C virus induces interleukin-1β (IL-1β)/IL-18 in circulatory and resident liver macrophages. J Virol. 87, 12284–12290 (2013).
Rockett, K. A., Awburn, M. M., Rockett, E. J. & Clark, I. A. Tumor necrosis factor and interleukin-1 synergy in the context of malaria pathology. Am J Trop Med Hyg. 50, 735–742 (1994).
Bode, J. G., Albrecht, U., Häussinger, D., Heinrich, P. C. & Schaper, F. Hepatic acute phase proteins-regulation by IL-6 and Il-1-type cytokines involving STAT3 and its crosstalk with NF-κB-dependent signaling. Eur J Cell Biol. 91, 496–505 (2012).
Scheller, J., Chalaris, A., Schmidt-Arras, D. & Rose-John, S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 1813, 878–888 (2001).
Drucker, C., Gewiese, J., Malchow, S., Scheller, J. & Rose-John, S. Impact of interleukin-6 classic- and trans-signaling on liver damage and regeneration. J Autoimmunity. 34, 29–37 (2010).
Schmidt-Arras, D. & Rose-John, S. IL-6 pathway in the liver: from physiopathology to therapy. J Hepatol. 64, 1403–1415 (2016).
Peng, S. L., Szabo, S. J. & Glimcher, L. H. T-bet regulates IgG class switching and pathogenic autoantibody production. Proc Natl Acad Sci. 99, 5545–5550 (2002).
Liu, N., Ohnishi, N., Ni, L., Akira, S. & Bacon, K. B. CpG directly induces T-bet expression and inhibits IgG1 and IgE switching in B cells. Nat Immunol. 4, 687–693 (2003).
Finkelman, F. D., Katona, I. M., Mosmann, T. R. & Coffman, R. L. IFN-gamma regulates the isotypes of Ig secreted during in vivo humoral immune responses. J Immunol. 140, 1022–1027 (1988).
Sebina, I. et al. IFNAR1-signalling obstructs ICOS-mediated humoral immunity during non-lethal blood-stage Plasmodium infection. PLoS Pathog. 12, e1005999 (2016).
Vieira, P. & Rajewsky, K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. 18, 313–316 (1988).
Kalantari, P. et al. Dual engagement of the NLRP3 and AIM2 inflammasomes by plasmodial-derived hemozoin and DNA during malaria. Cell Rep. 6, 196–210 (2014).
Wunderlich, F., Al-Quraishy, S. & Dkhil, M. A. Liver-inherent immune system: its role in blood-stage malaria. Front Microbiol. 5, 559 (2014).
Takahashi, M., Nishimura, T. & Yokomuro, K. Quantitative analysis of cytokine gene expression in the liver. Immunol Cell Biol. 77, 139–142 (1999).
Fukada, T. et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 5, 449–460 (1996).
Wunderlich, C. M. et al. Cutting edge: inhibition of IL-6 trans-signaling protects from malaria-induced lethality in mice. J Immunol. 188, 4141–4144 (2012).
Santos, M. L. et al. Contribution of inflammasome genetics in Plasmodium vivax malaria. Infect Genet Evol. 40, 162–166 (2016).
Tangteerawatana, P., Krudsood, S., Chalermrut, K., Looareesuwan, S. & Khusmith, S. Natural human IgG subclass antibodies to Plasmodium falciparum blood stage antigens and their relation to malaria resistance in an endemic area of Thailand. Southeast Asian J Trop Med Public Health. 32, 247–254 (2001).
Richards, J. S. et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 51, e50–60 (2010).
Tangteerawatana, P. et al. Differential regulation of IgG subclasses and IgE antimalarial antibody responses in complicated and uncomplicated Plasmodium falciparum malaria. Parasite Immunol. 29, 475–483 (2007).
Leoratti, F. M. S. et al. Pattern of humoral immune response to Plasmodium falciparum blood stages in individuals presenting different clinical expressions of malaria. Malar J. 186, 1–11 (2008).
Oakley, M. S. et al. T-bet modulates the antibody response and immune protection during murine malaria. Eur J Immunol. 44, 2680–2691 (2014).
Mac-Daniel, L. & Ménard, R. Plasmodium and mononuclear phagocytes. Microb Pathog. 78, 43–51 (2015).
Ichinohe, T., Lee, H. K., Ogura, Y., Flavell, R. & Iwasaki, A. Inflammasome recognition of influenza virus is essential for adaptive immune responses. J Exp Med. 206, 79–87 (2009).
Bejon, P. et al. Analysis of immunity to febrile malaria in children that distinguishes immunity from lack of exposure. Infect Immun. 77, 1917–1922 (2009).
Chau, J. Y. et al. Malaria-associated L-arginine deficiency induces mast cell-associated disruption to intestinal barrier defenses against nontyphoidal Salmonella bacteremia. Infect Immun. 81, 3515–3526 (2013).
Acknowledgements
Funding for this study was provided by the UC Davis T32 training grant “Animal Models of Infectious Diseases” and the Floyd and Mary Schwall Dissertation Year Fellowship in Medical Research, awarded to EKKG, and by the National Institute of Allergy and Infectious Diseases (NIAID) as part of the International Centers of Excellence in Malaria Research (ICEMR) program (U19AI089674).
Author information
Authors and Affiliations
Contributions
E.K.K.G. and S.L. designed experiments and wrote the manuscript. E.K.K.G., D.M., B.K.T., L.H. and F.A. conducted the experiments. I.S. and B.G. provided human plasma samples and supporting data. L.G.A. reviewed animal pathology and blood chemistry data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
S.L. and E.K.K.G. are authors on a provisional patent for the use of abscisic acid for the prevention and treatment of malaria (US Patent Application No 15/294,630). All other authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Glennon, E.K.K., Megawati, D., Torrevillas, B.K. et al. Elevated plasma abscisic acid is associated with asymptomatic falciparum malaria and with IgG-/caspase-1-dependent immunity in Plasmodium yoelii-infected mice. Sci Rep 8, 8896 (2018). https://doi.org/10.1038/s41598-018-27073-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27073-1
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.