Abstract
The ability to avoid predators is crucial to wild prey animals’ survival. Potential danger is signalled, among others, by the presence of predator scents. These odors are used in research both to trigger and to study fear reactions in laboratory animals; they are also employed as repellents against pest rodent species. In our study, we assessed nine predator-derived odors for their effectiveness in eliciting avoidance responses in a free-living colony of Norway rats (Rattus norvegicus). The rats were studied in a field setting. Food was put in two compartments inside the experimental pen: in one of them, predator scent was introduced on experimental days. The rats did not avoid boxes with predator odor and did not display an increased latency of food-carrying behavior or any other fear-related behavior, such as freezing or increased grooming. The results confirm the hypothesis that the foraging of rodents in a well-known territory and in relative proximity to burrows and other shelters is not affected by indirect cues of predation risk, such as the presence of predator urine or feces. We have also concluded that in a well-established colony living in a familiar territory, predator scent holds little promise as rodent repellent.
Similar content being viewed by others
Introduction
In the course of evolution, prey animal species have developed specific adaptations which are essential to survival by enabling them to recognize, avoid and defend themselves against predators. Many species are highly sensitive to the presence of predator odors1 and reactions to carnivore scents have been observed in many mammal species i.a., Norway rats (Rattus norvegicus), brown rats (Rattus rattus), house mice (Mus musculus), field mice (Apodemus sylvaticus), field voles (Microtus agrestis), black tailed deer (Odocoileus hemionus columbianus), European rabbits (Oryctolagus cuniculus), European hedgehogs (Erinaceus europaeus) and sheep (Ovis ares)2, as well as in birds (house finches, Carpodacus mexicanus3,). This effect does not seem to result from novelty avoidance, as Holtzman strain of Norway rats (Rattus norvegicus) have been observed to respond to a scent from an already present predator (cat, Felis catus) and at the same time fail to react to an unexpected odor of aerosol deodorant4. Investigations of predator odor avoidance are important because of the value of such data for developing models of anxiety and anxiety disorders5, as well as for designing effective pest repellents, which protect crops and plantations2 and therefore alleviate human-wildlife conflicts6. The popularity of using predator odors as repellents against vermin species is reflected in the wide range of commercially available products developed with the use of predator urine (e.g., PredatorPee Inc.).
According to the predation risk allocation hypothesis7, prey species should trade off foraging effort against vigilance (behaviors aimed at predator detection) in relation to the temporal variation of predation risk, i.e., they tend to meet their energy needs by minimizing the risk of being killed. The ratio of risk levels between high- and low-risk situations is assumed to affect the time allocated for foraging, vigilance and other activities. Therefore, the intensity of foraging is predicted to be highest during short periods of low risk interspersed with long periods of high risk and, conversely, to be lowest during short periods of high risk interspersed with long periods of low risk. However, if the exposure to high risk is prolonged, the animals will be forced to forage despite the high-risk circumstances in order to meet their energy intake needs.
Behavioral reactions to predator threat include the inhibition of exploratory activity and non-defensive behaviors (such as grooming, reproduction, foraging, feeding), as well as retreating to lookout locations and change of habitat for a safer one2,8,9. A decrease in the number of litter has also been observed in laboratory rats after exposure to predator urine in home areas10 and when being housed in close proximity to live predators (Eurasian lynx, Lynx lynx;11), as well as in wild free-ranging grey-sided voles (Clethrionomys rufocanus) after exposure to predator odor12.
Although prey species seem to exhibit an innate reaction to odors from sympatric and allopatric predators alike, they might not always recognize certain carnivore odors as threatening2,13,14. For example, bush rats (Rattus fuscipes) from Australia do not avoid the odor of fox (Vulpes vulpes) feces, likely because foxes are introduced predators in the area inhabited by rats15. No reaction to feral cat odor was also observed in small marsupials and murids (e.g., yellow-footed antechinus, Antechinus flavipes, and ash-grey mouse, Pseudomys albocinereus) even though these species have coexisted for more than 200 years16. Similarly, the Cypriot mouse (Mus cypriacus) did not avoid the odor of its relatively recent main competitor and known predator, the black rat (Rattus rattus), whereas it avoided the scent of a domestic cat17. It is possible that an aversive response towards predator odors might only occur if the predator and prey share a long evolutionary history and, therefore, the prey becomes genetically programmed to avoid the odors of sympatric predators18 (interestingly, a decline in risk-sensitive behavior of prey species seems to be a rapid response to the predator population decline19). For these reasons, the negative results obtained in predator odor avoidance studies might reflect the mismatch of predator and prey configurations. However, other studies demonstrate that even certain pairings of prey and predator species without a shared evolutionary history may result in predator odor avoidance2,20 and that reduced alien predation pressure after the initial impact might result in the selection for discriminating and avoiding danger in otherwise naïve species21.
Typical odor sources are skin, fur, urine, droppings and secretions of the anal glands, such as TMT (2,5-dihydro-2,4,5-trimethylthiazoline) in foxes2. Urine is a carrier of many chemical signals in animals. Its constituents such as PEA (2-phenylethylamine22) and sulphur compounds (products of digesting animal proteins23,24), are present, to varying degrees, in all predators and constitute crucial components generating fear responses in prey species.
Predator odors carry strong effects on the prey animals’ endocrinal systems, by increasing blood pressure and heart rate in rats25, as well as by increasing corticosterone level and decreasing testosterone levels in males2,26. A significant increase in corticosterone levels has also been observed in Sprague-Dawley rats exposed to TMT, although this effect was not accompanied by defensive behaviors in a small familiar environment27. The authors suggested that the observed lack of predator avoidance in a known area may indicate a mechanism which helps the animal react swiftly in case additional stressful stimuli arrive. However, in a study by Storsberg et al.28, both Lister-Hooded and captive wild rats expressed avoidance behavior in response to TMT, while an upsurge in corticosterone level was observed only in the captive wild rats, which may suggest that the domestication process weakens the physiological reaction to stress in rats. In terms of other physiological reactions, rats exposed to cat odor showed greater Fos immunoreactivity expression in many brain regions than controls, thus suggesting patterns of brain activation in response to stressful stimuli29. In addition, rats’ reactions to predator odors provide insights into the innate fear reactions in animals, as well as into the function of the hypothalamic–pituitary–adrenal axis (HPA axis), which coordinates the function of the sympathetic nervous system22.
In terms of behavioral reactions to predator odors, experimental data often show diverse relationships. Although predator odor exposure has been proposed as a paradigm for testing both behavioral and neuroendocrine effects, results of some studies have failed to detect both of these changes30. In addition, a physiological reaction (an increase in the level of corticosteroids, heart rate and c-Fos immunohistochemistry expression), does not necessarily translate into an avoidance reaction. A study involving 2-propylthietane, the main constituent of weasel (Mustela nivalis) anal secretion, suggests a clear distinction between rats’ endocrinal and behavioral responses to predator odors. The results demonstrated significant increases in corticosterone and ACTH (adrenocorticotrophic hormone) levels without any significant accompanying changes in behavioral indices30.
Predator odors seem to deter certain prey species and may decrease foraging behavior. However, the effectiveness of such odors remains unclear, as not all studies demonstrate a strong repellent effect. For example, no influence was observed of predator scent on the amount of time spent feeding and on the frequency of alert postures in captive antelopes (Cape grysbok, Raphicerus melanotis and gray duiker, Sylvicapra gimmia) and on the rates of trapping free-living rodents (Rhabdomys pumilio and Otomys irroratus) when compared to a control odor31. Similarly, in a laboratory study by Bramley and Waas32, wild-caught Norway rats demonstrated no avoidance of predator scents in comparison with herbivore scents. In a subsequent field study32, forest feeders were marked with synthetic predator odors, but, again, no change was reported in the number or duration of feeder visits by rats, mice (Mus musculus) or brushtail possums (Trichosurus vulpecula). By contrast, a study conducted on wild-caught Hawaiian roof rats (Rattus rattus33) in an artificial laboratory setting demonstrated avoidance behaviors in response to predator odors. To conclude, some studies report all predator odors used in the experiments to be ineffective in preventing prey species from approaching odor-marked locations in natural environment, which suggests that further research is necessary before predator odors can be efficiently implemented as commercial pest repellents.
Avoidance reactions, therefore, seem to be modulated by additional external environmental factors (time of day, light intensity, proximity of shelter, familiarity with the territory, etc.34,35). In addition, evidence shows that the results of experiments measuring the hormonal correlates of behavior are particularly susceptible to changes in the study subjects’ environment, such as transfer from wild to captive setting or even switch from natural to captive diet36.
Moreover, susceptibility to predator odors seems to be modulated by internal factors, such as species characteristics, sex, age, and individual differences2,21. A study by Hogg and File37 suggests that laboratory-bred rats can be split into two groups: subjects exhibiting innate behavioral responses to predator odor and subjects showing no such reactions. Importantly, these two groups did not differ in their reactions in the presence of neutral odor, in the elevated plus-maze tests of anxiety or in social interactions, but they behaved differently when exposed to a cat scent.
Interestingly, laboratory studies often point to a higher efficacy of certain predator odors than is later reported from field studies in which the same odors are utilized. For example, one of the most frequently investigated odor components, TMT, extracted from the red fox’ feces, was demonstrated to have a strong effect on predator-naïve rats and mice in laboratory studies, but a much weaker effect in field studies2. Similarly, in another laboratory study by Sullivan et al.38, laboratory-housed gophers (Thomomys talpoides) avoided TMT but not ferret (Mustela putorius), mink (Mustela vison) or fox urine-related chemical compounds. The field part of this study, conducted in infested fruit tree orchards, reported no location avoidance effects in any of the odor conditions. However, significantly more gophers were captured in orchards where predator odors were placed in their burrows than were caught before the experiment, which suggests an influence of the presence of predator scent on gopher behavior. Another possibility is that only certain specific behaviors present in wild populations are being inhibited by the presence of predator odors, as in a study by Sparrow et al.39 where wombats (Lasiorhinus latifrons) remained in the vicinity of introduced dingo (Canis lupus dingo) scents, but did not dig burrows.
Laboratory experiments, beside their multiple advantages (e.g., full control of the study conditions and considerable control of the distorting variables), are burdened with disadvantages which might explain some of the differences in results obtained in lab and field studies. Experiments involving endocrinology and behavior appear to be particularly susceptible to influences from the environment (natural or laboratory) in which they are performed36. The laboratory setting, as an artificial environment, may account for a reinforcement or inhibition of certain behaviors due to the close proximity of humans and to the repeated handling of test subjects27. The most important issue in laboratory rats – as products of the domestication process in an artificial environment – is that some adaptive behaviors are to a large extent extinguished (e.g., neophobia, aggression40,41,42,43). In addition, domestication affects a variety of traits, including morphology, physiology, neural mechanisms and behavior (e.g.,44,45,46).
For these reasons, field studies conducted on wild species, uninfluenced by the domestication process, provide further insights into the mechanisms of animal behavior. Three main behavioral reactions to predator scents have been the focus of such studies: changes in activity patterns, reductions in non-defensive behaviors and habitat shifts2. Although field studies fail to allow for a precise control of multiple variables, the ecological validity of such studies is higher than the validity of laboratory studies47. Conducting field studies is in line with the ethological principle promoting the study of natural forms of behavior, that is, only such forms of behavior which are of biological significance, such as predator avoidance48.
However, available data are inconsistent due to the influences of many potential external and internal factors, such as the predator species used and study setting (laboratory or natural environment). Therefore, the aim of our study was to investigate the capability of nine different kinds of predator odors (sympatric and non-sympatric) to elicit avoidance/fear reaction in a free-living colony of Norway rats. The laboratory strains of the Norway rat are among the most frequently used species in behavioral and biomedical studies42,49. The popularity of the species, the vast number of studies conducted so far, as well as the fact that the species is often used as a model for investigations of behavioral and physiological mechanisms and psychic processes in other animal species (including humans), make the rat a particularly interesting study subject. Most studies on predator scent avoidance in rats have been conducted either on laboratory rats2 or on wild-caught rats kept in laboratory settings in which they were tested33,50, and sometimes even housed, individually50. Only some studies investigated fear responses to predator scent in wild rodents in natural free-ranging conditions31,32,38,51,52 (for an example of other species see20 for a field study on rabbits, Oryctolagus cuniculus). Our study was conducted near the wild rats’ burrows with the aim of investigating predator odor responses in a familiar environment. We hypothesized that rats familiar with the low-risk habitat will not avoid predator scent when collecting food.
Methods and Materials
Ethics Statement
Under Polish law, the below-described study did not require permission of the local ethics committee for animal experimentation, as it was a non-invasive experiment based on behavioral observation of free-ranging animals. The study was carried out on private land with permission of its owners. All applied procedures were conducted in accordance with the Polish Animal Protection Act (21 August 1997).
Animals
The study was conducted on a free-living colony of Norway rats in their natural habitat, on a farm situated on the outskirts of Warsaw, Poland. Based on the observations and data collected from previously conducted studies on this colony53,54, the size of the rat population living on the farm had been estimated at approx. 40–50 individuals. The animals were not marked to distinguish between individuals. No pest control had been conducted on the farm for 5 years prior to the experiment – neither mechanically, nor by means of poison. Predators present at the farm which the rat colony had close contact with included cats and dogs (Canis lupus familiaris). In addition, the nearby area was home to beech marten (Martes foina), weasels, foxes and birds of prey, although these were never encountered on the farm.
Indoor Pen
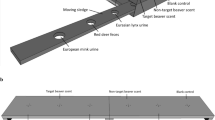
The rats were observed in a barn located on the farm, in a dog- and cat-proof indoor pen fenced with a wire mesh (200 cm/100 cm/100 cm; see Fig. 1). Dog and cat proofing was implemented to prevent predator-induced variability of rat behavior8. We used 4 infrared cameras connected to a digital video recorder, which enabled 24/7 motion detection recording. The rats could easily access the pen through 5 to 8 entrances – the number of entrances (of which 3 to 4 were entrances leading directly from the rats’ underground burrows to the pen) varied in time, as rats typically modify their burrow entrance placement. The nests were situated in the adjacent room. The maximum light intensity in the box and in the surrounding space was 5–25 lx and 10–50 lx, respectively, depending on whether the barn gate was shut or open.
To minimize the risk of influence of human and animal scents, neither the researchers nor other vertebrate animals entered the pen during the study. Food bowls, as well as feed and water, were supplied and put on the ground through a cover placed on top of the pen. Water was provided ad libitum. The staff used disposable nitrile gloves at all times. Due to mice infestation of the barn, which could influence the results of the experiment (both by changing the rats’ behavior and due to food intake by mice), the size of the mouse colony had been reduced significantly by placing mouse live traps in the building one week prior to the start of the experiment. No signs of mice presence in the indoor pen were observed during the experiment.
Apparatus
A box built from OSB (Oriented Strand Board), with external dimensions of 74 × 37.5 cm and wall height of 40 cm (see Fig. 1 and Video 1), was placed at the center of the indoor pen. The box was comprised of two independent compartments with single entrances (10 × 10 cm) located on the same wall of the box. A black rubber bowl (13 cm in diameter and 1.5 cm high) was placed in each of the compartments (A and B – Fig. 1). Pieces of black cardboard were fixed to the bottoms of the bowls by a double-sided adhesive tape; the cardboard pieces were soaked with predator scent (during experimental sessions) or with water (in the control compartment). The bottom of the boxes was covered with earth, which was identical to the ground surface in the remaining part of the experimental arena (clayish sand); the earth layer was ca. 10 mm thick.
Odor Probes
Samples of predator urine: foxes, coyotes (Canis latrans) and mountain lions (Puma concolor), were purchased from PredatorPee Inc. (Bangor, ME, USA). Urine of female Sumatran tigers (Panthera tigris sumatrae) and urine of African lions (Panthera leo) were collected at the Warsaw ZOO by means of a purpose-built sewer system that enabled urine collection from a slanted concrete floor. Lion feces was obtained from the Warsaw ZOO. Urine samples from healthy unneutered male cats and dogs were collected by a local veterinarian by means of bladder catheterization. TMT samples were purchased from Contech Enterprises Inc. (Victoria, Canada). The set of urine samples included odors of both sympatric (cat, dog, fox) and allopatric species (mountain lion, African lion, Sumatran tiger, coyote). In the study, fresh urine samples (i.e., applied several hours after collection and not frozen; lion, cat, dog) were used alongside frozen urine samples (mountain lion, coyote, fox).
Procedure
The procedure applied in the experiment resembled the experimental scheme successfully applied in one of the studies conducted by our team on the same rat colony54 and the observational method used to determine changes in subjects’ behavior after exposure to predator scents is considered an appropriate behavioral test of olfaction55.
The experiment took place in winter 2015/2016 (from 20 January until 18 March – 59 days in total). The average daily temperature in the pen was 6.3 °C (min. −4 °C, max. 10 °C) and the average humidity level was 69% (min. 65%, max. 72%). The temperature during the experimental days was always above 5 °C. The times of sunrises ranged from 8:35 a.m. at the start to 6:44 a.m. at the end of the experiment, whereas the times of sunsets ranged from 5:00 p.m. to 6:46 p.m.
The rats were well habituated to standard laboratory fodder pellets (Labofeed H, WP Morawski, Kcynia, Poland), as well as to the time of feeding - three non-invasive studies53,54 on food and object neophobia had been conducted over the period of twelve months prior to the experiment. The animals were fed at approx. 3 p.m., i.e., before the peak of their circadian activity, which falls immediately after twilight43. To habituate the animals to the experimental procedure, during the first 7 days of the experiment, 15 food pellets (ca. 20 mm long, 12 mm in diameter, 3.5 g +/−0.5 g in weight) were put in the bowls (A and B), located in the two separate compartments of the experimental box (see Fig. 1). Following the habituation period, for 3 subsequent days, the food (15 standard pellets) was served in one of the compartments, in the bowl with the cardboard bottom soaked on a daily basis with predator odor immediately before feeding. Urine samples (1 ml) and lion feces (1 ml of water solution) were smeared on the cardboard bottom with a brush. TMT scent was introduced by fixing 3 10 µl pipette tips, to the bowl by means of an odorless glue; the pipette tips had been used to apply TMT in another study (Storsberg et al.28) and they were subsequently stored in an airtight box, which prevented the disappearance of the scent. Three plastic pieces of similar size were fixed to the bowls in the control box. Fifteen food pellets were also put in the adjacent compartment, which served as control measurement. The procedure with the new predator odor was repeated for 3–5 consecutive days. To prevent habituation to continuous novelty, every experimental period was followed by 2 days during which the animals were given the standard feed in both compartments. To avoid the effect of place, the bowls with the new predator odor were supplied alternately in the right-hand and left-hand compartments. After the period of introducing a given odor was over, to eradicate all traces of smell, the earth from the bottom of the box was replaced in both compartments and new bowls were put in.
After the experiment, three individuals from the colony were captured and tested for Toxoplasma gondii, which may modulate host behavior, decreasing the level of fear of predator odor56,57.
Statistical Analysis
The following variables were calculated based on the video-recordings: the latency to pick individual pellets (calculated from the moment the first rat entered the specific compartment) and the number of instances where rats approached the food without picking the pellet (calculated until the last of the 15 pellets was picked). Only behaviors that were recorded during periods from the moment the pellets were supplied until 8 a.m. on the following day were taken into consideration. The methodological inconvenience of not having individuals marked was partly offset by the number of measurements performed (latency to pick 30 food pellets, over a period of 59 days), by the considerable size of the colony and by a qualitative analysis.
As in a study conducted previously54, two points of reference for the reaction to the introduced novelty (in this case: predator odor) were used: cross-sectional (comparison of the rats’ behavior in the compartment with predator scent and in the compartment with standard bowl and unscented fodder) and longitudinal (analysis of the animals’ behavior when exposed to predator odor at subsequent stages of the experiment). See Video 1 to obtain a general view of the rats’ behavior in the experimental setting.
Results
None of the introduced odors induced avoidance behavior in the rat colony under study (see Figs 2 and 3 and Table 1). The maximum latency to eat the last of the 15 pellets was only 26 minutes 12 seconds in the experimental sessions, and 57 minutes 3 seconds in the initial habituation sessions. A more detailed data analysis was conducted using non-parametric statistical tests. Differences were considered significant for P values of < 0.05.
Cross-sectional Analysis
The statistical analysis commenced with a comparative examination of the rats’ behavior in the experimental compartment (where predator odor was introduced) and in the control compartment, where the cardboard bottom of the bowl was soaked with water instead of predator scent (see Fig. 2 and Table 1). No differences were observed in the behavior of rats between compartments with regard to the pace at which pellets were picked, measured as the length of time that elapsed between instances of picking individual pellets (Mann-Whitney U test – U = 605, P = 0.93). The number of instances where pellets were approached but none of them were picked did not differ either (U = 521, P = 0.113); there was no difference in the latency to pick the first pellet (U = 531.5, P = 0.268), the last pellet (U = 561, P = 0.545) and the number of instances of entering the compartments after picking all the pellets, which served as an exploration indicator (U = 529.5, P = 0.328).
A Kruskal-Wallis test was carried out to evaluate the differences among the nine predator odors with respect to the median latency to pick all food pellets (see Fig. 2). The test revealed no differences [χ2(8, N = 35) = 12.97, P = 0.113].
To assure the robustness of the obtained results, additional tests were undertaken. Firstly, mean latency of picking all food pellets were regressed on a dummy variable taking value of 1 for the experimental compartment and 0 for the control compartment. Under t (0.648), F (0.420) and Wald (0.420) test null hypothesis of equal means cannot be rejected at any conventional level. Secondly, Bayesian Zellner regression58 with the same variable was performed. Regardless of the choice of the g prior – Unit Information Prior59 and Risk Information Criterion60 – the hypothesis of equal means was maintained. The obtained point estimates with standard errors in parentheses under aforementioned prior structure are 0.692 (1.083) are 0.351 (0.773) respectively. This confirms the results obtained with the use of a nonparametric test.
Longitudinal Analysis
Next, we carried out a comparison of the animals’ behavior when confronted with predator odor at subsequent stages of the experiment (see Fig. 3). The factor taken into consideration was the rats’ reaction in the initial phase of novel odor provision (the first 3–5 days during which the novel scent was supplied). The reaction was compared to the rats’ behavior in the preceding 3-day period when no novel scent was provided and when the animals were given the standard (unscented) pellets in both compartments.
No differences at the subsequent stages of the experiment were observed with regard to any of the variables. The median latency to pick first food pellet was not a time-varying parameter [the Friedman test value was χ2(7, N = 4) = 12.303, P = 0.09], nor was the latency to pick the last pellet [χ2(7, N = 4) = 2.95, P = 0.95], or the number of instances of entering the pen without taking the food [χ2(7, N = 4) = 3.71, P = 0.81].
Similarly to the case of cross-sectional data to assure the robustness of the obtained results, additional test were undertaken. Firstly, the latency of picking the first pellet, the latency of picking the last pellet, and the number of instances of entering the pen without picking the food were all regressed on a dummy variable taking value of 0 for habituation days and 1 for experimental days. Secondly, Bayesian Zellner regression58 with the same variables was performed, and with the use of the two different g priors described previously. In all the circumstances, the hypothesis of equal means could not be rejected.
None of the rats consumed the food on the spot. The food which had been picked by rats from the box compartments was always taken away from the box and carried into nearby tunnels. The box was entered by individuals of different ages (assessed by body size) and of both sexes (both males and females were trapped in the pen after the experiment for parasite screening). No regular activity pattern was observed which correlated with the age/size of animals entering the pen. No behavioral signs of stress (i.e., grooming and freezing) or exploration (prolonged sniffing, rearing, unusual locomotor activity) were observed during the tests. The Toxoplasma gondii test conducted on 3 individuals captured after the completion of the study produced a negative result.
Discussion
None of the predator odors used elicited avoidance behavior in the colony of wild rats. Furthermore, rats did not differ in their foraging latency either in the test (predator scented) or in the control compartment; there were no differences in the intensity of exploration of these two locations (entering the pen without collecting food). No difference between the nine predator odors used in the study was detected with respect to foraging, and there were no differences, at the subsequent stages of the experiment, in latency to start foraging and in the intensity of exploration.
The results obtained follow a literature trend where field studies on predator odor responses demonstrate lesser scent effects than laboratory studies2,61,62. Such results indicate that avoidance behavior is not automatic and does not rely on direct cues of predator presence, such as urine odor, but it is rather mediated by various environmental factors, such as type of habitat, temperature and weather34,35,61,63, and social context64,65.
The barn rats were exposed to predator scents in a familiar and safe environment (which enabled them to move along walls and ensured the proximity of burrows and other shelters), which is indicated by the observed lack of behavioral signs of stress (i.e., grooming and freezing) or exploration of the pen. An alternative explanation of the lack of grooming behavior may relate to a possible tendency of animals to minimize the time spent in the enclosure, and thus to restrain from displaying behaviors other than locomotion and food carrying. No predators entered the experimental arena and, therefore, the experimental situation might have been perceived by rats as connected to low risk, despite the presence of predator scent. Similarly, in a study by Koivisto and Pusenius66, the presence of a predator (weasel) caused a rapid decrease in foraging behavior in voles, whereas exposure to predator scent only had a weaker effect. The authors suggested that odor indicates a recent predator presence in a location; however, as weasels frequently move from one place to another, the risk in scent-marked areas might be perceived by voles as not very high. In addition, the costs of movement and thermoregulation in the pen were low, since the tunnel cover/escape routes were close by and foraging occurred indoors during winter (temperatures on experimental days were always above 5 °C). The relatively small distance to cover from the location at which an animal forages decreases the energetic cost of foraging (via low costs of movement and thermoregulation) and, likewise, reduces the risk of predation67. No avoidance behavior was observed (measured either qualitatively or quantitatively), as the anti-predator effort in low-risk circumstances drops to low levels7. Furthermore, the low level of vigilance observed (no sniffing, no freezing) resulted in a higher energetic gain67.
Most laboratory studies on rats and mice use one of the unconditioned tests of anxiety (the plus maze, the light-dark box or the open field test); these paradigms, however, should be approached with caution with regard to the possible interpretations of behavioral data collected68. Aspects worth considering include the questionable measures of anxiety (subjects might exhibit neophobic reactions or natural preferences for enclosed spaces rather than anxiety69) and the intra-individual variation in behavioral responses to anxiety, both of which might diminish the reliability of test measures70. In addition, in an open unfamiliar space, two stress-inducing factors are present, namely the experimental predator odor and a perceived higher risk of predation. Various habitat structures provide different degrees of predation risk and, therefore, modify the spatial behavior of prey species in response to odors71. Such an effect was demonstrated in a study by Cohen et al.72, which showed that exposure to cat odor increased fear responses in rats placed in the elevated plus-maze: they were less likely to enter the open (but not closed) arms of the maze. Another explanation might be the fact that standard experimental protocols used in laboratory tests include animals exposed to long-term low-risk conditions and short-time predator-related stressors during testing7, which are likely to result in an overestimation of the intensity of anti-predator behavior expected in field situations, where perceived long-term predation risk might be considered higher than in a laboratory setting.
Another ecological factor which contrasts our field study with laboratory tests is the social context: subjects lived and were tested in their natural environment and in their social group instead of being tested individually, as is usually the case in a laboratory setting. Rats are highly social animals living in colonies42, which dilutes the predation threat64,65. Moreover, Norway rats have a relatively small home range, and, consequently, when forced to change their habitat, it is unlikely that they will explore an unfamiliar area far away from the nest, which is often the case in laboratory settings73. Although foraging with other group members involves the cost of lower food intake per individual74, benefits include higher food sources detection, lowered predation risk due to the dilution effect64 and an overall increased group vigilance75. Therefore, on the one hand, rats in the company of other colony members might be more likely to forage and explore despite the presence of predator odors. On the other hand, a group setting includes potential opportunities for food sharing/scrounging without the need of some colony members to enter a potentially high-threat area. None of the rats in our study consumed the pellets within the pen, but they took them into the tunnels. Such behavior opens the possibility for food sharing or food theft (either tolerated or non-tolerated) of pellets collected by the pen dwelling individuals76.
Alternative interpretations of the results include a possibility of a parasite infection, methodological aspects, habituation to predator odors, and individual differences among rats.
Toxoplasma gondii causes an infection which might change the behavior of its host by decreasing fear reactions to predator odors56,57. However, medical examination of three members of the barn colony produced a negative test result and, therefore, absence of avoidance reactions to predator scents observed in the barn was not a result of a compromised health of the rat colony.
Multiple aspects of study design can potentially influence the resulting repellent effect of predator odor. Some possible elements include: the incorrect context of predator scent presentation63,77, the use of different sizes of odor-scented stimuli (cloths78), or the age79 and form2,80 of the predator stimulus used. The odor source (either fur, urine, feces or anal glands) influences its effectiveness in eliciting avoidance behavior, with fur seemingly causing the most significant endocrinal and behavioral changes. One possible explanation of this effect is that predator fur may be a stronger signal of predatory threat/presence than feces or urine81. However, fur-derived odors are difficult to control in an experimental situation, as it is unclear which active ingredient in fur gives rise to its potent repellent properties2. Additionally, the molecular complexity of the odor signal is also important, with single compounds being generally less effective in eliciting defensive reactions in prey than odor arrays. Such arrays consist of different volatile compounds which may convey more relevant information about the predation risk, e.g., what prey species are consumed by a given predator80. Age of the predator odor was also linked to its repellent effect, as presented in a study by Hegab et al.79 where Brandt’s voles (Lasiopodomys brandtii) exhibited more defensive responses to freshly defrosted cat feces than to cat feces stored between 2 and 8 days, and in a study by Bytheway et al.51 where free-ranging rats showed a decrease in defensive behavior in response to aged predator odor. Another possibility is that interactions mediated via olfactory cues between predators and prey are more complex than previously assumed82. Instead of consisting of dyadic interactions between a single predator (signaller) and prey (receiver), a complex olfactory web of information may be created by visitations of scent patches by multiple species from various levels of the food chain.
Linked to study design is a possibility that rapid habituation to predator odors may be also a factor limiting their repellent strength2,62. For example, habituation to the repellent effects of cat odor has been observed in laboratory rat studies83. Although rats exhibit an increase in bloodstream corticosterone after being presented with a cloth that had been rubbed on a cat, habituation of this endocrinal response occurs after repeated exposure to the odor stimulus84. However, in our study, habituation to predator scent was unlikely to explain the lack of avoidance reaction, as no changes in subjects’ behavior were observed at the subsequent experimental stages, and our experiment involved 2-day inter-session breaks after each testing session, when the rats were given the standard non-scented pellets in both test compartments.
On an individual level, only very bold animals might have been entering the experimental arena and, therefore, no scent avoidance was observed. In addition, individual differences in pre-experience with predators also modify the behavior of prey species2. In our study, all the rats in the barn colony had had similar exposure to dogs, cats, and humans before the onset of the experiment and, therefore, no large individual variance in terms of pre-experience occurred in the test sample. However, as our study did not aim at investigating the influence of individual differences on antipredator behavior, further studies are needed to provide insights into the link between personality and reactions to predator odors.
Some limitations in the presented study should be acknowledged. Firstly, the exact sample size and composition are unknown due to the absence of individual marking. However, the size of the whole colony was estimated at approx. 40–50 individuals in previous studies53,54 and two complementary kinds of statistical approaches were applied to assure the robustness of the acquired results - both classical and Bayesian analysis showed a strong support for the null hypotheses (no influence of predator scent on the observed rats’ behavior). Additionally, indirect data suggests that the sample included subjects of various ages (estimated by body size) and both sexes (males and females were trapped in the experimental area after the study for parasite analysis). Secondly, the study was conducted on a single wild rat colony which limits the extent of general conclusions. Nevertheless, the investigated context was of high practical value as a cohabitation of rats and humans on a single farm presents ample opportunities for human-wildlife conflicts to occur. Therefore, analysis of the behavior of the whole rat colony, beside shedding a light upon general aspects of behavioral reaction to predator odor in free-living rats, provides valuable insights for the development of pest repellent techniques.
The results of the study and areas for future research can be concisely summarized by quoting Stoddart85: “Clearly they do not always react to the odor of their predators, but do they ever?”. Potential modifications of future studies investigating behavioral aspects of predator odor avoidance in wild rats can be categorized according to two methodological aspects, namely study design and predator odors. Firstly, to gain more insights into predator avoidance behavior in various natural circumstances, experiments could be conducted in habitats with a perceived higher predation risk (such as outdoor areas, plantation fields, and/or areas further away from tunnels and burrows). Secondly, the predator scents utilized could be obtained from different species and from various organic source material. A study by Arnould and Signoret86 suggests that in domestic sheep (Ovis ares), unusual odors from non-sympatric species (lion feces) had a smaller repellent effect than dog feces (however, other studies have supported the use of lion feces as a repellent against deer, Cervus elaphus87,88). A subsequent study89 also included wolf (Canis lupus) feces, which, together with dog feces, produced the strongest repellent effect. Other organic materials include fur and skin, as predator odors obtained from these body areas were suggested to have a more profound effect on prey species than odors derived from urine or feces2. There are also indications that such odors may have longer-lasting effects, as cat fur/skin scent is a highly aversive stimulus and triggers context conditioning when used as an unconditioned stimulus, while other types of predator odors (derived from feces, urine and anal gland secretions) apparently do not. Another direction for future studies is to include a biochemical analysis in order to gain a fuller understanding of wild rats’ reactions to predator odors. A study on Sprague-Dawley rats27 reported that TMT-induced biochemical effects may occur without the expression of defensive behaviors depending on the rats’ environment (safe vs risky).
The high variability of reactions of rat populations and individual animals observed in field studies is a factor that decreases the effectiveness of predator odors as pest management tools. Avoidance is not unconditioned, but it is rather mediated by various environmental factors and rats’ high behavioral plasticity49, which facilitates the changeability of their foraging strategies. The results of our study confirm the hypothesis that foraging of rodents in a well-known territory and in a relative proximity to burrows and other shelters is not affected by indirect cues of predation risk, such as the presence of predator urine or feces. Lack of response to the odors used in the study suggests that animals rely more on the habitat characteristics, such as i.a. closeness of shelter and light intensity. We also conclude that predator scent in the case of a well-established colony in a familiar territory holds little promise as a rodent repellent. Despite methodological limitations of the presented study, it provides valuable insights and directions for future studies, which might involve investigations of odor influence in unknown and, therefore, higher risk areas. Reactions to predator odors seem to depend on a variety of variables, such as odor source, intensity, context of exposure, in addition to the inter-specific variety of prey species and individual characteristics2. Further studies are necessary to shed more light on this complex network of interlinked factors.
References
Fendt, M. Exposure to urine of canids and felids, but not of herbivores, induces defensive behavior in laboratory rats. J. Chem. Ecol. 32, 2617 (2006).
Apfelbach, R., Blanchard, C. D., Blanchard, R. J., Hayes, R. A. & McGregor, I. S. The effects of predator odors in mammalian prey species: a review of field and laboratory studies. Neurosci. Biobehav. Rev. 29, 1123–1144 (2005).
Roth, T. C., Cox, J. G. & Lima, S. L. Can foraging birds assess predation risk by scent? Animal Behav. 76, 2021–2027 (2008).
Courtney, R. J., Reid, L. D. & Wasden, R. E. Suppression of running time by olfactory stimuli. Psychon. Sci. 12, 315–316 (1968).
Staples, L. G. Predator odor avoidance as a rodent model of anxiety: Learning-mediated consequences beyond the initial exposure. Neurobiol. Learn. Mem. 94, 435–445 (2010).
Schulte, B. A. Learning and applications of chemical signals in vertebrates for human–wildlife conflict mitigation in Chemical signals in vertebrates 13 (eds Schulte, B., Goodwin, T. & Ferkin, M.) 499–510 (Springer, 2016).
Lima, S. L. & Bednekoff, P. A. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649–659 (1999).
Blanchard, R. J. & Blanchard, D. C. Antipredator defensive behaviors in a visible burrow system. J. Comp. Psychol. 103, 70–82 (1989).
McGregor, I. S., Schrama, L., Ambermoon, P. & Dielenberg, R. A. Not all ‘predator odors’ are equal: cat odor but not 2,4,5 trimethylthiazoline (TMT; fox odor) elicits specific defensive behaviours in rats. Behav. Brain Res. 129, 1–16 (2002).
Voznessenskaya, V. V. et al. Predator odours as reproductive inhibitors for Norway rats. USDA NWRC Staff Publ. 251, 131–136 (2003).
Naidenko, S. V., Naidenko, S. V., Clark, L. & Voznessenskaya, V. V. Predator presence affects the reproductive success of prey in outdoor conditions in Rats, mice and people: rodent biology and management (eds Singleton, G. R., Hinds, L. A., Krebs, C. J. & Spratt, D. M.) 148–150 (ACIAR, 2003).
Fuelling, O. & Halle, S. Breeding suppression in free-ranging grey-sided voles under the influence of predator odour. Oecologia 138, 151 (2004).
Carthey, A. J. & Banks, P. B. Naïveté in novel ecological interactions: lessons from theory and experimental evidence. Biol. Rev. 89, 932–949 (2014).
Busch, M. & Burroni, N. E. Foraging activity of commensal Mus musculus in semi-captivity conditions. Effect of predator odours, previous experience and moonlight. Pest Manag. Sci. 71, 1599–1604 (2015).
Banks, P. Responses of Australian bush rats, Rattus fuscipes, to the odor of introduced Vulpes vulpes. J. Mammal. 79, 1260–1264 (1998).
Dickman, C. R. Raiders of the last ark: Cats in island Australia. A. Nat. Hist. 24, 44–52 (1993).
Frynta, D., Baladová, M., Eliášová, B., Lišková, S. & Landová, E. Why not to avoid the smell of danger? Unexpected behavior of the Cypriot mouse surviving on the island invaded by black rats. Curr. Zool. 61, 781–791 (2015).
Stoddart, M. The ecology of vertebrate olfaction (Chapman and Hall Ltd, 1980a).
Hollings, T., McCallum, H., Kreger, K., Mooney, N. & Jones, M. Relaxation of risk-sensitive behaviour of prey following disease-induced decline of an apex predator, the Tasmanian devil. Proc. R. Soc. London Ser. B 282, 20150124 (2015).
Tortosa, F. S., Barrio, I. C., Carthey, A. J. & Banks, P. B. No longer naïve? Generalized responses of rabbits to marsupial predators in Australia. Behav. Ecol. Sociobiol. 69, 1649–1655 (2015).
Kovacs, E. K., Crowther, M. S., Webb, J. K. & Dickman, C. R. Population and behavioural responses of native prey to alien predation. Oecologia 168, 947–957 (2012).
Ferrero, D. M. et al. Detection and avoidance of a carnivore odor by prey. Proc. Natl. Acad. Sci. USA. 108, 11235–11240 (2011).
Nolte, D., Mason, J., Epple, G., Aronov, E. & Campbell, D. Why are predator urines aversive to prey? J. Chem. Ecol. 20, 1505–1516 (1994).
Scherer, A. E. & Smee, D. L. A review of predator diet effects on prey defensive responses. Chemoecology 26, 83–100 (2016).
Dielenberg, R. A. & McGregor, I. S. Defensive behavior in rats towards predatory odors: a review. Neurosci. Biobehav. Rev. 25, 597–609 (2001).
Vasilieva, N., Cherepanova, E., von Holst, D. & Apfelbach, R. Predator odour and its impact on male fertility and reproduction in Phodopus campbelli hamsters. Naturwissenschaften 87, 312–314 (2000).
Morrow, B. A., Elsworth, J. D. & Roth, R. H. Fear-like biochemical and behavioral responses in rats to the predator odor, TMT, are dependent on the exposure environment. Synapse 46, 11–18 (2002).
Storsberg, S. et al. Predator odor induced defensive behavior in wild and laboratory rats: A comparative study. Physiol. Behav. https://doi.org/10.1016/j.physbeh.2018.06.009 (2018).
Dielenberg, R. A., Hunt, G. E. & McGregor, I. S. ‘When a rat smells a cat’: The distribution of Fos immunoreactivity in rat brain following exposure to a predatory odor. Neuroscience 104, 1085–1097 (2001).
Perrot-Sinal, T. S., Ossenkopp, K. P. & Kavaliers, M. Brief predator odour exposure activates the HPA axis independent of locomotor changes. Neuroreport 10, 775–780 (1999).
Novellie, P., Bigalke, R. C. & Pepler, D. Can predator urine be used as a buck or rodent repellent? South African Forestry J. 123, 51–55 (1982).
Bramley, G. & Waas, J. Laboratory and field evaluation of predator odors as repellents for kiore (Rattus exulans) and ship rats (Rattus rattus). J. Chem. Ecol. 27, 1029–1047 (2001).
Burwash, M. D., Tobin, M. E., Woolhouse, A. D. & Sullivan, T. P. Laboratory evaluation of predator odors for eliciting an avoidance response in roof rats (Rattus rattus). J. Chem. Ecol. 24, 49–66 (1998).
Orrock, J. L., Danielson, B. J. & Brinkerhoff, R. J. Rodent foraging is affected by indirect, but not by direct, cues of predation risk. Behav. Ecol. 15, 433–437 (2004).
Orrock, J. L. & Danielson, B. J. Temperature and cloud cover, but not predator urine, affect winter foraging of mice. Ethology 115, 641–648 (2009).
Calisi, R. M. & Bentley, G. E. Lab and field experiments: are they the same animal? Horm. Behav. 56, 1–10 (2009).
Hogg, S. & File, S. E. Responders and nonresponders to cat odor do not differ in other tests of anxiety. Pharmacol. Biochem. Behav. 49, 219–222 (1994).
Sullivan, T., Crump, D. & Sullivan, D. Use of predator odors as repellents to reduce feeding damage by herbivores: Northern pocket gophers (Thomomys talipoides). J. Chem. Ecol. 14, 379–390 (1988).
Sparrow, E. E., Parsons, M. H. & Blumstein, D. T. Novel use for a predator scent: preliminary data suggest that wombats avoid recolonising collapsed burrows following application of dingo scent. Aust. J. Zool. 64, 192–197 (2016).
Castle, W. E. The domestication of the rat. Proc. Natl. Acad. Sci. USA 33, 109–117 (1947).
Price, E. O. Behavioral aspects of animal domestication. Q. Rev. Biol. 59, 1–32 (1984).
Barnett, S.A. The rat: A study in behavior (Transaction Publishers, 2007).
Stryjek, R., Modlinska, K., Turlejski, K. & Pisula, W. Circadian rhythm of outside-nest activity in wild (WWCPS), albino and pigmented laboratory rats. PloS One 8, e66055 (2013).
Lockard, R. B. The albino rat: A defensible choice or a bad habit? Amer. Psychol. 23, 734–742 (1968).
Diamond, J. Evolution, consequences and future of plant and animal domestication. Nature 418, 700 (2002).
Pisula, W. et al. Response to novelty in the laboratory Wistar rat, wild-captive WWCPS rat, and the short-tailed opossum. Behav. Process. 91, 145–151 (2012).
Martin, P. & Bateson, P. Measuring behaviour: An introductory guide (Cambridge University Press, 2007).
Tinbergen, N. On aims and methods of ethology. Ethology 20, 410–433 (1963).
Whishaw, I. Q. & Kolb, B. The behavior of the laboratory rat: A handbook with tests. (Oxford University Press, 2004).
Bramley, G. N., Waas, J. R. & Henderson, H. V. Responses of wild Norway rats (Rattus norvegicus) to predator odors. J. Chem. Ecol. 26, 705–719 (2000).
Bytheway, J. P., Carthey, A. J. & Banks, P. B. Risk vs. reward: how predators and prey respond to aging olfactory cues. Behav. Ecol. Sociobiol. 67, 715–725 (2013).
Herman, C. S. & Valone, T. J. The effect of mammalian predator scent on the foraging behavior of Dipodomys merriami. Oikos 91, 139–145 (2000).
Stryjek, R. & Modlinska, K. Neophobia in wild rats is elicited by using bait stations but not bait trays. Int. J. Pest Manag. 62, 158–164 (2016).
Modlinska, K. & Stryjek, R. Food neophobia in wild rats (Rattus norvegicus) inhabiting a changeable environment - a field study. PloS One 11, e0156741 (2016).
Fendt, M., Apfelbach, R. & Slotnick, B. Behavioural tests of olfaction in Olfaction in animal behaviour and welfare (ed. Nielsen, B. L.) 39–60 (INRA, 2017).
Berdoy, M., Webster, J. P. & Macdonald, D. W. Fatal attraction in rats infected with Toxoplasma gondii. Proc. R. Soc. London Ser. B 267, 1591–1594 (2000).
House, P. K., Vyas, A. & Sapolsky, R. Predator cat odors activate sexual arousal pathways in brains of Toxoplasma gondii infected rats. PloS One 6, e23277 (2011).
Zellner, A. On assessing prior distributions and Bayesian regression analysis with g prior distributions in Bayesian inference and decision techniques: Essays in honor of Bruno de Finetti. Studies in Bayesian econometrics 6 (eds Goel, P. K. & Zellner, A.) 233–243 (Elsevier, 1986).
Kass, R. & Wasserman, L. A reference Bayesian test for nested hypotheses and its relationship to the Schwarz criterion. J. Am. Stat. Assoc. 431, 928–934 (1995).
Foster, D. & George, E. The risk inflation criterion for multiple regression. Ann. Stat. 22, 1947–1975 (1994).
Powell, F. & Banks, P. B. Do house mice modify their foraging behaviour in response to predator odours and habitat? Anim. Behav. 67, 753–759 (2004).
Voznessenskaya, V. V. Influence of cat odor on reproductive behavior and physiology in the house mouse Mus musculus in Neurobiology of chemical communication (ed. Mucignat-Caretta, C.) 389–405 (CRC Press, 2014).
Parsons, M. H. et al. Biologically meaningful scents: a framework for understanding predator–prey research across disciplines. Biol. Rev. 93, 98–114 (2018).
Hamilton, W. D. Geometry for the selfish herd. J. Theor. Biol. 31, 295–311 (1971).
Williams, G. C. Natural selection, the costs of reproduction, and a refinement of Lack’s principle. Am. Nat. 100, 687–690 (1966).
Koivisto, E. & Pusenius, J. Effects of temporal variation in the risk of predation by least weasels (Mustela nivalis) on feeding behavior of field voles (Microtus agrestis). Evol. Ecol. 17, 477–489 (2003).
Houston, A. I., McNamara, J. M. & Hutchinson, J. M. General results concerning the trade-off between gaining energy and avoiding predation. Philos. Trans. Royal Soc. B 341, 375–397 (1993).
Denenberg, V. H. Open-field behavior in the rat: What does it mean? Ann. N. Y. Acad. Sci. 159, 852–859 (1969).
Ennaceur, A. Unconditioned tests of anxiety - Pitfalls and disappointments. Physiol. Behav. 135, 55–71 (2014).
Ramos, A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol. Sci. 29, 493–498 (2008).
Borowski, Z. & Owadowska, E. Spatial responses of field (Microtus agrestis) and bank (Clethronomys glareolus) voles to weasel (Mustela nivalis) odour in natural habitat in: Chemical signals in vertebrates (eds Marchlewska-Koj, A., Lepri, J. & Mueller-Schwarze, D.) 289–293 (Kluwer Academic/Plenum Publishers, 2001).
Cohen, H., Benjamin, J., Kaplan, Z. & Kotler, M. Administration of high-dose ketoconazole, an inhibitor of steroid synthesis, prevents posttraumatic anxiety in an animal model. Eur. Neuropsychopharmacol. 10, 429–435 (2000).
Davis, D. E., Emlen, J. T. & Stokes, A. W. Studies on home range in the brown rat. J. Mammal 29, 207–225 (1948).
Magurran, A. E. The adaptive significance of schooling as an anti-predator defence in fish. Ann. Zool. Fennici 27, 51–66 (1990).
McNamara, J. M. & Houston, A. I. Evolutionarily stable levels of vigilance as a function of group size. Anim. Behav. 43, 641–658 (1992).
Galef, B. G. & Giraldeau, L. A. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim. Behav. 61, 3–15 (2001).
Dickman, C. Predation and habitat shift in the house mouse. Mus domesticus. Ecology 73, 313–322 (1992).
Takahashi, L. K., Nakashima, B. R., Hong, H. & Watanabe, K. The smell of danger: A behavioral and neural analysis of predator odor-induced fear. Neurosci. Biobehav. Rev. 29, 1157–1167 (2005).
Hegab, I. M. et al. Defensive responses of Brandt’s voles (Lasiopodomys brandtii) to stored cat feces. Physiol. Behav. 123, 193–199 (2014).
Apfelbach, R., Parsons, M. H., Soini, H. A. & Novotny, M. V. Are single odorous components of a predator sufficient to elicit defensive behaviors in prey species? Front. Neurosci. 9, 263 (2015).
Blanchard, D. C., Griebel, G. & Blanchard, R. J. Conditioning and residual emotionality effects of predator stimuli: some reflections on stress and emotion. Prog. Neuropsychopharmacol. Biol. Psychiatry 27, 1177–1185 (2003).
Banks, P. B., Daly, A. & Bytheway, J. P. Predator odours attract other predators, creating an olfactory web of information. Biol. Lett. 12, 20151053 (2016).
Dielenberg, R. A. & McGregor, I. S. Habituation of the hiding response to predatory odor in rats (Rattus norvegicus). J. Comp. Psychol. 113, 376–387 (1999).
File, S. E., Zangrossi, H., Sanders, F. L. & Mabbutt, P. S. Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol. Behav. 54, 1109–1111 (1993).
Stoddart, D. Some responses of a free-living community of rodents to the odors of predators in Chemical signals: Vertebrates and aquatic invertebrates (eds Mueller-Schwarze, D. & Silverstein, R.) 1–10 (Kluwer Academic/Plenum Publishers, 1980b).
Arnould, C. & Signoret, J. P. Sheep food repellents: Efficacy of various products, habituation, and social facilitation. J. Chem. Ecol. 19, 225–236 (1993).
Abbott, D. et al. A natural deer repellent: chemistry and behavior in Chemical signals in vertebrates (eds. MacDonald, D., Mueller-Schwarze, D. & Natynczuk, S.) 599–609 (Oxford University Press, 1990).
Mueller-Schwarze, D. The responses of young black-tailed deer to predator odors. J. Mammal. 53, 393–394 (1972).
Arnould, C., Malosse, C., Sigoret, J. P. & Descoins, C. What chemical constituents from dog feces are involved in its food repellent effect in sheep? J. Chem. Ecol. 24, 559–576 (1998).
Acknowledgements
This research project was supported by the National Science Centre (Poland) – grant no. UMO-2013/09/B/HS6/03435 and by intramural grant KNOW/IGHZ/RPB/WEW/2016/04 funded by KNOW (Leading National Research Centre) Scientific Consortium “Healthy Animal - Safe Food”, decision of Ministry of Science and Higher Education No. 05–1/KNOW2/2015. The authors would like to express their gratitude to Dr Andrzej Kruszewicz, Dr Anna Jakucińska and Janusz Rak from the Warsaw ZOO (Warszawskie Zoo) for permission and help in obtaining predator odor samples. We would also like to thank the Kawka family for allowing us to conduct experiments on their farm, Dr Izabela Kuźniak-Wajsznor for obtaining the urine samples, Dr David Jańczak for performing medical examinations of the rat colony, Silke Storsberg and Prof. Markus Fendt for sending urine samples, as well as Dr Krzysztof Beck for performing Bayesian analysis.
Author information
Authors and Affiliations
Contributions
R.S. conception and design of research; R.S. performed experiments; R.S. and E.S.G. analyzed data; R.S., B.M. and G.R.J. interpreted results of experiments; R.S. prepared figures; R.S. and B.M. drafted manuscript; R.S., B.M., and G.R.J. edited and revised manuscript; R.S., B.M., G.R.J., and E.S.G. approved final version of manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stryjek, R., Mioduszewska, B., Spaltabaka-Gędek, E. et al. Wild Norway Rats Do Not Avoid Predator Scents When Collecting Food in a Familiar Habitat: A Field Study. Sci Rep 8, 9475 (2018). https://doi.org/10.1038/s41598-018-27054-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27054-4
This article is cited by
-
Identifying the most effective behavioural assays and predator cues for quantifying anti-predator responses in mammals: a systematic review
Environmental Evidence (2023)
-
Of mice and cats: interspecific variation in prey responses to direct and indirect predator cues
Behavioral Ecology and Sociobiology (2023)
-
A newly discovered behavior (‘tail-belting’) among wild rodents in sub zero conditions
Scientific Reports (2021)
-
Using predator feces as a repellent for free-ranging urban capybaras (Hydrochoerus hydrochaeris)
acta ethologica (2021)
-
Are physiological and behavioural responses to stressors displayed concordantly by wild urban rodents?
The Science of Nature (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.