Abstract
Meniscal allograft transplantation yields good and excellent results but is limited by donor availability. The purpose of the study was to evaluate the effectiveness of radiated deep-frozen xenogenic meniscal tissue (RDF-X) as an alternative graft choice in meniscal transplantation. The xenogenic meniscal tissues were harvested from the inner 1/3 part of the porcine meniscus and then irradiated and deeply frozen. The medial menisci of rabbits were replaced by the RDF-X. Meniscal allograft transplantation, meniscectomy and sham operation served as controls. Only a particular kind of rabbit-anti-pig antibody (molecular ranging 60–80 kD) was detected in the blood serum at week 2. The menisci of the group RDF-X grossly resembled the native tissue and the allograft meniscus with fibrocartilage regeneration at postoperative 1 year. Cell incorporation and the extracellular matrix were mostly observed at the surface and the inner 1/3 part of the newly regenerated RDF-X, which was different from the allograft. The biomechanical properties of the group RDF-X were also approximate to those of the native meniscus except for the compressive creep. In addition, chondroprotection was achieved after the RDF-X transplantation although the joint degeneration was not completely prevented. To conclude, the RDF-X could be a promising alternative for meniscal transplantation with similar tissue regeneration capacity to allograft transplantation and superior chondroprotection. The potential minor immunological rejection should be further studied before its clinical application.
Similar content being viewed by others
Introduction
Meniscus plays an important role in stabilization and lubrication of the knee joint. Meniscus replacement aims to re-establish normal joint load transmission to prevent the degeneration of the articular cartilage after meniscectomy1,2. Meniscus allograft transplantation has been proven promising for pain relief3 and Noyes et al. evaluated the long-term results of meniscus transplantation with successful outcome. However, joint degeneration could not be completely prevented by meniscus transplantation4,5. Moreover, the availability of allograft is very limited and there are concerns over the possibility of disease transmission6,7.
The Collagen Meniscus Implants (CMI) is a structure fabricated by collagen type I isolated and purified from bovine Achilles tendon. This normal human meniscus sized implant is enriched with glycosaminoglycans (GAGs). Rodkey et al.8 reported the results of implantation of CMI at 2-year follow-up. All patients have exhibited clinical symptoms improvement and the regenerated tissue showed new fibrocartilage tissue formation. Steadman9 and Zaffagnini et al.10 reported the results of CMI implantations with more than five years follow-up, respectively. Both studies shed light on its promising clinical evolution and assumed chondroprotection. However, the transplanted CMI appeared shrinkage in the study with larger population11.
Xenografts, such as porcine small intestinal submucosa (SIS), have been used to replace meniscal tissue in a dog model12. However, the biochemical and biomechanical properties of SIS could not perfectly match those of the native meniscus, thus the clinical translation have not been reported13. In this study, porcine menisci was treated with irradiation and deep freezing, and then implanted into rabbit knee joints. Compared with other methods of decellularization14, irradiation and deep freezing was more simple and efficient, which have been applied for meniscus allograft processing. The purpose of the present study was to evaluate the potential of the radiated deep-frozen xenogenic meniscal tissue (RDF-X) as alternative for meniscal transplantation. Tissue regeneration and chondroprotective effects of RDF-X (Xeno group) were evaluated at one year follow-up, compared with those of the allograft (Allo group), meniscectomy (Meni group), or the sham surgery (Sham group). We hypothesized that the RDF-X could bring about certain benefits in meniscal regeneration and long-term protection of the articular cartilage. There might be difference between the xenograft group and the allograft group in terms of cell infiltration and tissue regeneration.
Results
Characterization of the RDF-X meniscal tissue
As shown in Fig. 1, the collagen network collapsed and the dense internal structure were damaged. A large amount of gaps were found between the loose collagen fibers. The originally existed fibrocartilage cells were extinct and appeared karyopyknosis. According to the toluidine blue (TB) staining results, the GAGs obviously reduced after the treatment and the original neatly arranged fiber became messy. The significantly lighter color was shown in the immunohistochemistry (IHC) for the collagen type I (Col I) and the collagen type II (Col II).
Characterization of the radiated deep-frozen xenogenic meniscal tissue (RDF-X) meniscal tissue. (A) The collagen network collapsed and originally exsisted fibroacartilage cells appeared karyopyknosis (black arrow). The glycosaminoglycans and collagen type I and II (Col I and II) were markedly reduced after the process throughout the toluidine blue (TB) and immunohistochemistry (IHC) for Col I and II staining (B–D). Scale bar = 100 μm.
Compared to the native meniscus in scanning electron microscope (SEM), collagen bundles in the RDF-X appeared to be looser. Gaps and micropores were found within the destructive collagen network (approximately 20–150 μm).
Assessment of immunological rejection
According to the result of the Western-blot (Fig. 2), no antibody was detected in the joint fluid from postoperative week 2 to week 6. In the blood serum, a rabbit-anti-pig antibody with the molecular ranging 60–80 kD was detected at week 2. However, this antibody was not detected at week 4 and week 6.
Gross evaluation of the meniscus
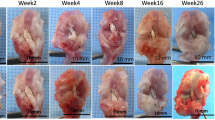
One rabbit of the Allo group died of diarrhea at week 30 and was excluded. Ten menisci of the Xeno group and 9 menisci of the Allo group were obtained at postoperative 1 year for evaluation (Fig. 3). All the grafts healed to their normal attachment sites, with no sign of disruption or gap formation. Seven menisci in the Xeno group seemed approximately normal at gross glance with a shiny-white color and a smooth surface. No obvious synovium hypertrophy was detected. Meniscus partial tear with rough surface was found in three menisci of the Xeno group. In the Allo group, all the menisci remained normal except for one with mild shrinkage. Calcification was found at the anterior horn of 2 menisci in each group. As shown in Table 1, the scores of the Xeno group in terms of the shape, tears, surface and tissue were significantly lower than those of the Allo group (P < 0.05). However, no significant difference was found in the total scores.
Gross and historical (general view and high-power magnification) images of meniscus tissue. Macroscopic view of implants or native menisci are show on the left. Insert box identifies the region analyzed by histology as listed below. General view of outer, intermediate, and inner zone of implants or native menisci (hematoxylin and eosin (H&E) staining) are show in the middle (Scale bar = 100 μm). High-power magnification images of meniscus tissue are shown on the right (Scale bar = 25 μm). Integrated cells (black arrow) were observed in the implants of Xeno group and Allo group.
Histology of the meniscus
The synovial cells infiltrated into the graft over time in most of the transplanted menisci. The semi-quantitative histological analysis of both groups were shown in Table 2. The results of the overall parameters were similar between the two groups except for the lymphocyte infiltration. However, for the cell infiltration and the GAG distribution (Table 3), difference existed between the Xeno group and the Allo group. The relatively uniform distribution was found for the outer, intermediate and the inner 1/3 zone of the Xeno graft but most of the cells concentrated in the outer 1/3 zone of the allograft.
In the Xeno group, blood vessels and fibrous tissue were observed surrounding the graft. However, full cell incorporation and fibrocartilage regeneration were observed only in 2 menisci, which revealed no blank zone. For the other 8 menisci, cell incorporation were seen only at the upper and lower surface and the inner 1/3 part of the newly regenerated menisci. Furthermore, the phenotype of those cells was more approximate to hyaline chondrocyte surrounded by more TB staining and Col II (Fig. 4) but with few Col I and collagen type III (Col III) (Fig. 5).
In the Allo group, 2 menisci achieved full cell incorporation and demonstrated normal meniscus histology. Most of the cells concentrated in the outer 1/3 part near the synovial edge with fibrochondrocyte phenotype for 7 menisci. There was only a small amount of cells infiltration in the intermediate and the inner part of the allograft.
Ultrastructure evaluation
For the RDF-X (Fig. 6E,F) and the allograft (Fig. 6G,H) at postoperative 1 year, the reconstruction of the normal structure was incomplete. However, in the areas with fibrocartilaginous tissue regeneration, the loose collagen bundle network had been replaced by the dense extracellular matrix with fibrochondrocyte in the lacunas (Fig. 6E–H), which was approximate to the native meniscus (Fig. 6A–D).
Ultrastructure evaluation of meniscus tissue. The whole (A), outer (B) and inner (C) regions of native rabbit meniscus. The fibrochondrocyte (white arrow) was seen in some lacunas (D). Menisci of the Xeno group (E,F) and the Allo group (G,H) at postoperative 1 year, and some integrated cells (white arrow) were also seen. The cell incorporation were observed only at the upper and lower surface and the inner 1/3 part of the new regenerated meniscus in the Xeno group while most of the cell infiltration concentrated in the outer 1/3 part of the graft in the Allo group.
Biomechanical evaluation
In Fig. 7, tensile modulus were 121.0 ± 82.2 MPa for the Xeno group, 110.9 ± 29.2 MPa for the Allo group and 109.9 ± 18.0 MPa for the Native group, respectively. No significant difference was found between the three groups (P > 0.05). The ultimate tensile strength of the Xeno group (27.0 ± 14.7 MPa) and the Allo group (26.39 ± 18.3 MPa) was less than that of the Native group (38.65 ± 8.7 MPa) but with no significant difference (0.1 > P > 0.05). Furthermore, there was no significant difference of the Young’s moduli in compression between the three groups (109.5 ± 53.7 MPa for the Xeno group, 80.9 ± 52.9 MPa for the Allo group and 123.4 ± 98.0 MPa for the Native group, P > 0.05).
Biomechanical properties. Tensile modulus (A), ultimate tensile strength (B) and compressive modulus (C) of the menisci from the Xeno group and the Allo group had no significant difference compared with the native menisci. The tensile (D) and compressive (E) creep compared with the native menisci (*P < 0.05, n = 6 per group).
In terms of the creep test, the compressive creep at 1st minute of the Xeno group was significantly less than that of the Native group (P = 0.03033). The tensile creep at 1st minute (P = 0.043) and 10th minute (P = 0.07) of the Allo group was significantly greater than those of the Native group. No significant difference was found in any of the creep test results between the Allo group and the Xeno group.
Chondroprotection evaluation
According to the macroscopic evaluation of joints (Fig. 8), various degrees of cartilage damage were observed in all the transplantation and meniscectomy joints while no degradation was shown in Sham group.
As shown in hematoxylin and eosin (H&E) and TB staining of articular cartilage surfaces in the femoral condyles (FC) and tibial plateau (TP) (Fig. 9A,B), complete disorganization of the cartilage and severe reduction of TB staining were revealed in the Meni group. The sections of both the Xeno and the Allo groups revealed clefts to the transitional or radial zone with slighter reduction of TB staining in FC, and the surface irregularities were also displayed in TP. According to the International Cartilage Repair Society (ICRS) and Mankin scores (Fig. 9C,D), the meniscus covered (MC) regions of the Xeno group showed significantly lower scores than those of the Meni group at postoperative 1 year (P < 0.05). The Mankin score at the FC-M1/3 of the Xeno group was significantly lower than that of the Meni group (P = 0.001). Although both of the Xeno and the Allo groups showed more serious joint degeneration compared to the Sham group (P < 0.05), there was no significant difference between the two groups.
H&E and TB staining of articular cartilage surfaces in the femoral condyles (FC) (A) and tibial plateau (TP) (B). Scale bars = 100 μm. As indicated by International Cartilage Repair Society (ICRS) (C) and Mankin scores (D), the Xeno group presented lower cartilage degeneration in both the femur and tibia compared with Meni group (*P < 0.05, ***P < 0.001).
Discussion
The purpose of the present study was to evaluate the RDF-X as a substitute for the meniscus allograft. To our knowledge, this is the first study to examine the viability and the chondroprotective effects of xenogenic menisci in a one-year rabbit model. Throughout the relative long-term evaluation, similar tissue regeneration and cartilage protection were confirmed in the Xeno group compared to the Allo group. So far, no biologically derived or synthetic graft could completely prevent the potential damage of the post-meniscectomy joints. As the tissue closest to the native meniscus other than the allograft, the treated xenogeneic meniscus provided the extracellular matrix promoting cell incorporation and biomechanical support after transplantation. The positive results indicated that the RDF-X might be an alternative for meniscal replacement.
As in our previous report of the porcine RDF-X transplantation for 24 weeks, the treated xenogeneic meniscal tissue could heal the synovium with tissue regeneration and delay the degeneration of articular cartilage in the short-term15. However, some authors have noted that those grafts transplanted longer than 24 weeks usually led to construct ruptures and joint deterioration16. The main factor for those failures is the inadequate strength of the implants leading to long-term degeneration. Thus the rabbit model with a 1-year follow-up of the present study is useful for evaluating articular changes after meniscal transplantation in a longer term. In addition, the degenerative changes of articular cartilage in rabbit knees progress at a faster rate than in larger animals or humans17 and the present results could be a reference for the long-term outcome in the future clinical use.
In the present study, the RDF-X showed similar mechanical properties to the native meniscus in the range of 75–150 Mpa18. In spite of no significant difference in the tensile modulus between the three groups, a higher value of tension strength was observed in native menisci. This might be due to the fact that the regenerated menisci of the Xeno group contained fewer Col I, which directly contributed to resistance in tension loading19. These results might also explain the higher rate of meniscus tears in the Xeno group as showed in Table 1 and the ultimate tensile stress of both the Xeno and the Allo groups were significantly less than that of the Native group. The relatively poor tensile strength of the graft might be due to the treatment including radiation and deep freezing, which have been confirmed to destroy the collagen structure and lead to the reduction of the mechanical strength. Furthermore, the compressive modulus of the Xeno group was significantly more than that of the Allo group and the source location of the xenograft might be the cause. According to the previous study, the meniscus is structurally and functionally heterogeneous20. The xenograft of the present study was extracted from the inner 1/3 part of the pig meniscus consisting more collagen II and GAG, which showed higher compressive modulus compared to the outer part. It should be also noted that the compression testing was performed to the transected part, which only presented the mechanical strength of the tissue itself, instead of the biomechanical performance of the total meniscus. As for the meniscus works through tensile strength pulling from the anterior and posterior horns, the biomechanical testing with the total meniscus in situ should be studied in the future.
An early rejection was detected at week 2 in the blood serum and the result was similar to the previous xenogenic chondrocytes transplantation21. Despite that the radiation and the deep freezing weakened the immunogenicity of the xenogeneic meniscal tissue, the residual fragments of the dead cells still present in the xenograft. Thus the application of the xenograft caused the synovitis due to the release of the xenogeneic collagen and cell particles in the knee joint and the blood. The molecular ranged 60–80 kD but the antigen was still unknown, which might need further studies including immunoprecipitation. In addition, as we have reported previously, a large number of lymphocytes infiltration were observed around the xenograft at week 6. Those results indicated that both of the humoral immunity and the cellular immunity played a certain role in the early stage after the transplantation of the xenograft. At week 12, most of the xenografts were still surrounded by lymphocytes but with a much smaller number at week 2415. At postoperative 1 year, normal synovial cells surrounded all the xenograft except for two menisci with a small amount of lymphocyte infiltration. The immunological rejection seemed to have no significant effect on the long-term viability of the xenograft. However, there were three menisci of the group RDF-X with partial tears and rough surface and collagen collapse was detected in histological evaluation. Those might be the effect of the chronic rejection, which could not be detected by our presented methods. In spite of the relatively low immunogenity of the cartilage, the meniscus or the ligament, decellularization would be still necessary before the xenogenic extracellular matrix was used before transplantation.
Another interesting finding is that there was difference in the mode of the cell infiltration and tissue regeneration between the Xeno group and the Allo group. The cell incorporation were observed only at the upper and lower surface and the inner 1/3 part of the new regenerated meniscus in the Xeno group while most of the cell infiltration concentrated in the outer 1/3 part of the graft in the Allo group. Those results indicated that the regeneration of the RDF-X was realized by synovium enfolding other than gradually cell incorporation from the peripheral synovium. The regeneration mode of the RDF-X might be mostly related to the immune rejection to the tissue with heterogeneous origin. In addition, the phenotype of the regenerated cells of the Xeno group demonstrated more approximated hyaline chondrocyte surrounded by a little more TB staining and Col II but with fewer Col I and III, which could be the effect of the compressive stimulus from the femur and the tibia differentiating the synovial cells or the stem cells in the enfolding synovium into the chondrocyte22.
The main limitation in this study is that the implant was only obtained from the inner region of meniscal tissue without peripheral synovium, which contains more immunogens. Due to the heterogeneous characters of meniscus, the inner region contained large amounts of Col II and glycosaminoglycans with little immunogenicity. Therefore the initial biomechanical properties was not equivalent to those of native menisci. Thus further studies might research different regions of menisci for xenogenic grafts. Secondly, the rabbit-anti-pig antibody detection was only performed in the early 6 weeks. Although the antibody was not detected in the 4–6 week, reappearence is possible in the later stages. Thirdly, surgeries were performed for both knees for all the rabbits for the sake of reducing animal use. There might due to the impact of sham and total meniscectomy on the xenograft and allograft, respectively.
Conclusions
The RDF-X showed similar results to allogenic meniscus at 1-year follow-up. However, some problems such as the potential immunological rejection and the shape matching remain to be unsolved before clinical application.
Materials and Methods
All animal experiments were complied with the “Guide for the Care and Use of Laboratory Animals” published by the National Academy Press (NIH Publication No. 85–23, revised 1996) and approved by the Animal Care and Use Committee of Peking University.
Harvest and preparation of the xenogenic meniscal tissues
10 large white pigs were obtained from other unrelated studies within 24 hours of animal sacrifice, and their menisci were removed for use as the source of xenografts. The pig meniscus was washed in phosphate-buffered saline (PBS, Sigma Diagnostics, St Louis, MO) to remove excess blood and the inner one-third part of the meniscus tissue was shaped into wedge-shaped semilunar discs using the removed rabbit medial meniscus as template. The allograft menisci were also harvested from the xenograft group after total medial meniscectomy. Then the shaped xenogenic and allogenic meniscal tissues were packaged separately and treated with 25 k Gray gamma irradiation and deeply frozen (−80 °C) for 6 to 14 days.
In vitro assessment of the xenogenic meniscal tissues
Light Microscopy
The treated xenogenic meniscal tissues was fixed in 10% (v/v) neutral buffered formalin (Sigma Diagnostics, St Louis, MO) and then dehydrated in alcohol and embedded in paraffin. Five-micrometer-thick sections were cut and then stained with H&E and TB (as a label for proteoglycans). Besides, sections were treated with IHC procedure labeling Col I and II as previous described23,24.
Scanning Electron Microscopy
Specimens were fixed in a solution of 1% (w/v) osmium tetroxide and 1.5% (w/v) potassium ferrocyanide for 3 h. Slices were washed in pH 7.2 PBS, dehydrated in ascending grades of ethanol and subjected to critical-point drying in CO2. Dried slices were mounted on standard stubs, gold-coated in a sputter coater and then observed on a SEM microscope (SEM100-CX II, Japan).
In vivo assessment of the xenogenic meniscal tissues
Study design
20 skeletally mature male New Zealand White rabbits weighing between 2.0 and 2.5 kg were included in this study. Total medial meniscectomy were performed for the right knees of all the rabbits. To ensure the uniform time interval and avoid secondary operation, all the allografts were from an unrelated study with the same treatment procedure as the xenograft. Both of the treated allograft and the xenograft were prepared before the operations and were transplanted immediately after meniscectomy. Ten right knees received the treated xenogenic meniscal tissues (Xeno group) and 10 others allograft menisci (Allo group). The left knees of the Allo group underwent total medial meniscectomy without implantation of a graft (Meni group). As a control, the left knees of the Xeno group underwent sham operation involving exposure of the medial joint and close in layers (Sham group). All the rabbits were sacrificed at postoperative 1 year.
Surgical procedures
No immunosuppressive agents were used in this study. Intramuscular penicillin (400 thousand units) was administered preoperatively as antibiotic prophylaxis. The rabbits were anesthetized with intravenous urethane (1 g/kg). The surgical procedure was performed as previously reported24. Briefly, the right knee was approached through a medial parapatellar incision. Medial meniscus was resected sharply along the periphery and detached from their anterior and posterior junction. Care was taken not to injure the medial collateral ligament which was important for the postoperative stability of knee joints. The treated xenogenic meniscal tissue or the meniscus allograft was thawed by immersion in sterile saline and then sutured to the adjacent synovium with non-resorbable 5-0 sutures (Ethicon, Johnson & Johnson, Amersfoort, Netherlands). The extracapsular knotting technique was used for the posterior horn. The anterior and posterior horns of the graft were reattached to the appropriate ligamentous structures. The capsule, periarticular tissues, and skin were closed with Vicryl 3-0 sutures (Ethicon, Johnson & Johnson). For the Meni group, only total resection of the medial meniscus was performed. For the Sham group, the operation was performed on the medial compartment using the same approach required for the meniscectomy procedure without operating on the meniscus. After the operation, the animals were immediately allowed to have movement and weight-bearing activity and were not restricted in any way. The antibiotic prophylaxis was continued for 3 days.
Evaluation of initial immunological rejection
At postoperative week 2, week 4 and week 6, 1 ml blood serum was extracted from the rabbit of each group. Meanwhile, 1 ml PBS was infiltrated into the transplanted knee joint and was then extracted as the dilution of synovial fluid. Both of the blood serum and the dilution of synovial fluid was treated with Western-blot program to detect the Rabbit-anti-Pig antibody.
The shredded pig meniscal tissue was lysed in buffer for 30 min. Samples of the lysate were resolved on SDS-PAGE 12% (v/v) gels. For Western blot assays, the separated protein bands were electro-blotted onto nitrocellulose membranes, at a constant current of 250 mA in transbuffer (50 mM Tris, pH 8.0, containing 0.192 M glycine and 20% (v/v) methanol), using a Bio-Rad Trans-Blot Cell. The strips were incubated for 1 hr at room temperature in blocking buffer (TBS containing 5% (w/v) nonfat milk), followed by an overnight incubation at 4 °C with constant agitation in 1:40 dilution of serum and dilution of synovial fluid in blocking buffer. After 3 washes with TBS containing 0.05% (v/v) Tween 20, strips were incubated for 1 hr with appropriate HRP-conjugated anti-immunoglobulin reagents and the antigen-antibody complexes visualized using the ECL detection system as recommended by the manufacturer (Applygen Technologies Inc., Beijing, China). Data was recorded with Bio-Rad GelDoc2000.
Evaluation of meniscus
Gross evaluation
The joints were dissected with the femur separated from the tibia and the meniscus left attached to the tibia plateaus. Photographs were taken (Nikon 4600, Nikon Photo Products Inc, Tokyo, Japan) of the tibial plateau and of the exposed femoral condyles. The menisci were grossly evaluated by the integration, implant position, horn position, shape, tears, surface, size, tissue and the synovium reaction25. Each parameter was scored 1 to 3 based on the situation of the implants.
Histological evaluation
The menisci were separated from joints and treated with the above-mentioned routine procedure. Then, five-micrometer-thick sections were sliced and then stained with H&E, TB and Col II. Besides, sections were treated with PR staining to observe collagen type I and III. Meniscus was cut to produce blocks showing their wedge-shaped profile, which were divided into outer, intermediate and inner regions of the regenerated menisci in histological sections (Fig. 10A). The sections were evaluated according to the meniscus histology scoring system16. The number of integrated cells and the TB metachromasia in each zone were semi-quantitatively evaluated separately. Cell number: 0-no cell ingrowth, 1-very small amount of cell ingrowth, 2- moderate amout of cell ingrowth, 3-large number cell ingrowth, 4- normal amount of cell growth. TB metachromasia: 0- no, 1-mild, 2-obvious. All the sections were evaluated by 3 experienced researchers and the mean score was attained as the final results.
Regional division of meniscal tissue and osteochondral specimens. Meniscus was devided into outer, intermediate and inner regions (A). Medial FC were divided evenly into anterior (FC-A1/3), middle (FC-M1/3) and posterior (FC-P1/3) third regions (B). The medial TP was divided into weight bearing (WB) regions and meniscus covered (MC) regions (C).
Ultrastructure evaluation
The menisci of the Allo group and the Xeno group and the normal rabbit tissue of the Sham group were treated with the same SEM procedure as mentioned above.
Biomechanical properties evaluation
The tensile and the compressive samples were cut into rectangular shapes. The tensile testing samples were about 3 × 6 × 1 mm3 and the compressive samples were about 2 × 2 × 1 mm3. The biomechanical properties of specimens were tested by a material testing machine (AG-IS, Shimadzu, Japan) with a 50 N load23. The working temperature is about 23 °C and the test specimens were kept hydrated using 0.9% (w/v) NaCl solution. For the tensile testing, the tissue was preconditioned to a maximum displacement of 0.5 mm at a displacement rate of 0.5 mm/min for 8 cycles. The sample was tested to ultimate failure at a rate of 0.5 mm/min26. For the compressive testing, the tissue was loaded at a displacement rate of 0.1 mm/min with a maximum force of 10 N for 8 cycles. The elastic modulus was analyzed from the linear portion of the stress-strain curve, and only used the date of 5 cycles after 3 preconditioned cycles27.
Evaluation of chondroprotection
Three researchers who were blinded to the experimental groups independently evaluated the cartilage macroscopically of the femur and tibia according to the criteria of the ICRS cartilage injury classification28. The cartilage of the medial femoral condyle and the tibial plateau were analyzed and the medial tibial plateau was divided into the WB and MC regions (Fig. 10C). Each region was evaluated once by one researcher. The mean score of the researchers was then taken for the final evaluation.
The osteochondral specimens were decalcified in 10% (w/v) EDTA (Titriplex III, Merck, Darmstadt, Germany) for 3 weeks. When the decalcification was completed, the osteochondral specimens were sectioned in the coronal plane at the midpoint of the tibial plateau in the axial plane at the midpoint of the medial femoral condyle (Fig. 10B,C). Histological sections of medial FC were divided evenly into anterior (FC-A1/3), middle (FC-M1/3) and posterior (FC-P1/3) third regions. The sections of the medial tibial plateaus were divided into WB and MC regions. Each region of the histologic sections was graded with the Mankin grading system for hyaline cartilage degeneration29. All histological sections were scored by three attending pathologists blinded to the procedures performed. The mean score of both investigators was used for the final evaluation.
Statistical analysis
The nonparametric test was used for the skewness data, unknown distribution data or data with heterogeneity of variance. The Mann-Whitney rank-sum test was used to compare macroscopic and histological scores between different groups and regions. The T-test was used for the mechanical evaluation. All statistical analyses were completed with SPSS 11.5 software program. Statistical significance was set at P < 0.05.
References
Holzer, L. A., Leithner, A. & Holzer, G. Surgery versus physical therapy for meniscal tear and osteoarthritis. N. Engl. J. Med. 369, 677, https://doi.org/10.1056/NEJMc1307177#SA1 (2013).
Krause, W., Pope, M., Johnson, R. & Wilder, D. Mechanical changes in the knee after meniscectomy. The Journal of Bone & Joint Surgery 58, 599–604 (1976).
Jiang, D. et al. Comparative study on immediate versus delayed meniscus allograft transplantation: 4- to 6-year follow-up. Am. J. Sports Med. 42, 2329–2337, https://doi.org/10.1177/0363546514541653 (2014).
Ahn, J. H., Kang, H. W., Yang, T. Y. & Lee, J. Y. Risk Factors for Radiographic Progression of Osteoarthritis After Meniscus Allograft Transplantation. Arthroscopy 32, 2539–2546, https://doi.org/10.1016/j.arthro.2016.04.023 (2016).
Noyes, F. R. & Barber-Westin, S. D. Long-term Survivorship and Function of Meniscus Transplantation. Am. J. Sports Med. 44, 2330–2338, https://doi.org/10.1177/0363546516646375 (2016).
Rodeo, S. A. et al. Histological analysis of human meniscal allografts. A preliminary report. J. Bone Joint Surg. Am. 82-a, 1071–1082 (2000).
Rodeo, S. A. Meniscal allografts–where do we stand? Am. J. Sports Med. 29, 246–261 (2001).
Rodkey, W. G., Steadman, J. R. & Li, S. T. A clinical study of collagen meniscus implants to restore the injured meniscus. Clin. Orthop. Relat. Res., S281–292 (1999).
Steadman, J. R. & Rodkey, W. G. Tissue-engineered collagen meniscus implants: 5- to 6-year feasibility study results. Arthroscopy 21, 515–525, https://doi.org/10.1016/j.arthro.2005.01.006 (2005).
Zaffagnini, S. et al. Arthroscopic collagen meniscus implant results at 6 to 8 years follow up. Knee Surg. Sports Traumatol. Arthrosc. 15, 175–183, https://doi.org/10.1007/s00167-006-0144-4 (2007).
Bulgheroni, P. et al. Follow-up of collagen meniscus implant patients: clinical, radiological, and magnetic resonance imaging results at 5 years. Knee 17, 224–229, https://doi.org/10.1016/j.knee.2009.08.011 (2010).
Cook, J. L. et al. Kinetic study of the replacement of porcine small intestinal submucosa grafts and the regeneration of meniscal-like tissue in large avascular meniscal defects in dogs. Tissue Eng. 7, 321–334, https://doi.org/10.1089/10763270152044189 (2001).
Zhang, Z.-Z. et al. Scaffolds drive meniscus tissue engineering. RSC Advances 5, 77851–77859, https://doi.org/10.1039/C5RA13859K (2015).
Stabile, K. J. et al. An acellular, allograft-derived meniscus scaffold in an ovine model. Arthroscopy 26, 936–948, https://doi.org/10.1016/j.arthro.2009.11.024 (2010).
Jiang, D., Zhao, L. H., Tian, M., Zhang, J. Y. & Yu, J. K. Meniscus transplantation using treated xenogeneic meniscal tissue: viability and chondroprotection study in rabbits. Arthroscopy 28, 1147–1159, https://doi.org/10.1016/j.arthro.2012.01.001 (2012).
Kon, E. et al. Tissue engineering for total meniscal substitution: animal study in sheep model–results at 12 months. Tissue Eng Part A 18, 1573–1582, https://doi.org/10.1089/ten.TEA.2011.0572 (2012).
Moskowitz, R. W. et al. Experimentally induced degenerative joint lesions following partial meniscectomy in the rabbit. Arthritis Rheum. 16, 397–405 (1973).
Sweigart, M. A. & Athanasiou, K. A. Tensile and compressive properties of the medial rabbit meniscus. Proc. Inst. Mech. Eng. H 219, 337–347 (2005).
Cheung, H. S. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect. Tissue Res. 16, 343–356 (1987).
Makris, E. A., Hadidi, P. & Athanasiou, K. A. The knee meniscus: structure-function, pathophysiology, current repair techniques, and prospects for regeneration. Biomaterials 32, 7411–7431, https://doi.org/10.1016/j.biomaterials.2011.06.037 (2011).
Osiecka-Iwan, A. et al. Transplants of rat chondrocytes evoke strong humoral response against chondrocyte-associated antigen in rabbits. Cell Transplant. 12, 389–398 (2003).
O’Conor, C. J., Case, N. & Guilak, F. Mechanical regulation of chondrogenesis. Stem Cell. Res. Ther. 4, 61, https://doi.org/10.1186/scrt211 (2013).
Zhang, Z. Z. et al. Role of scaffold mean pore size in meniscus regeneration. Acta Biomater. 43, 314–326, https://doi.org/10.1016/j.actbio.2016.07.050 (2016).
Zhang, Z. Z. et al. 3D-Printed Poly(epsilon-caprolactone) Scaffold Augmented With Mesenchymal Stem Cells for Total Meniscal Substitution. Am. J. Sports Med., 363546517691513, https://doi.org/10.1177/0363546517691513 (2017).
Kon, E. et al. Tissue engineering for total meniscal substitution: animal study in sheep model. Tissue Eng Part A 14, 1067–1080, https://doi.org/10.1089/ten.tea.2007.0193 (2008).
Proctor, C. S., Schmidt, M. B., Whipple, R. R., Kelly, M. A. & Mow, V. C. Material properties of the normal medial bovine meniscus. J Orthop Res 7, 771–782, https://doi.org/10.1002/jor.1100070602 (1989).
Drury, J. L., Dennis, R. G. & Mooney, D. J. The tensile properties of alginate hydrogels. Biomaterials 25, 3187–3199, https://doi.org/10.1016/j.biomaterials.2003.10.002 (2004).
Brittberg, M. & Winalski, C. S. Evaluation of cartilage injuries and repair. J. Bone Joint Surg. Am. 85-A(Suppl 2), 58–69 (2003).
Mankin, H. J., Dorfman, H., Lippiello, L. & Zarins, A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J. Bone Joint Surg. Am. 53, 523–537 (1971).
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant Nos 51273004, 31200725, 31670982, 81630056).
Author information
Authors and Affiliations
Contributions
D.J. and Z.-Z.Z. and F.Z. equally contributed to this study: conception and design, collection and assembly of data, analysis and interpretation of data, drafting of the article; S.-J.W. and Y.-S.Q.: assistance in analysis and interpretation of data; L.-H.Z. and J.-Y.Z.: technical support on data analysis and interpretation. J.-K.Y.: conception and design, critical revision of the article, final approval of the article.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jiang, D., Zhang, ZZ., Zhao, F. et al. The Radiated Deep-frozen Xenogenic Meniscal Tissue Regenerated the Total Meniscus with Chondroprotection. Sci Rep 8, 9041 (2018). https://doi.org/10.1038/s41598-018-27016-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-27016-w
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.