Abstract
Ocean acidification (OA), the dissolution of excess anthropogenic carbon dioxide in ocean waters, is a potential stressor to many marine fish species. Whether species have the potential to acclimate and adapt to changes in the seawater carbonate chemistry is still largely unanswered. Simulation experiments across several generations are challenging for large commercially exploited species because of their long generation times. For Atlantic cod (Gadus morhua), we present first data on the effects of parental acclimation to elevated aquatic CO2 on larval survival, a fundamental parameter determining population recruitment. The parental generation in this study was exposed to either ambient or elevated aquatic CO2 levels simulating end-of-century OA levels (~1100 µatm CO2) for six weeks prior to spawning. Upon fully reciprocal exposure of the F1 generation, we quantified larval survival, combined with two larval feeding regimes in order to investigate the potential effect of energy limitation. We found a significant reduction in larval survival at elevated CO2 that was partly compensated by parental acclimation to the same CO2 exposure. Such compensation was only observed in the treatment with high food availability. This complex 3-way interaction indicates that surplus metabolic resources need to be available to allow a transgenerational alleviation response to ocean acidification.
Similar content being viewed by others
Introduction
Atlantic cod (Gadus morhua) supports large, commercial fisheries in many areas of the Northern Atlantic. Different stocks are adapted to a wide range of temperatures and are predicted to react differently to changing temperatures, depending on where they already exist in relation to their thermal optimum1. The most Northern stocks are currently benefiting from ocean warming2 through range expansion and through direct positive effects of increasing temperatures on recruitment and growth1. Along with global warming, however, another inevitable and direct effect of CO2 emissions through human activity is ocean acidification, the dissolution of excess CO2 in ocean waters. The rate of ocean acidification is predicted to be highest in the Arctic ocean3, while the Arctic is also warming faster than the global average4, which puts Arctic species at a higher risk to be negatively affected by climate change than more southerly species. Given the mounting evidence for negative CO2 effects on fish early life stages5,6,7,8,9,10,11,12 any expectations that global warming might have positive effects on the fisheries in these areas2 is therefore premature. While some progress has been made in research on the potential for acclimation and adaptation in other taxa in response to acidification13,14, the role of transfer of information via transgenerational effects other than changing the heritable information of DNA sequence as a mechanism to accommodate climate change at the individual level (also called transgenerational plasticity or acclimation) is still under debate.
Only a handful of studies have recently addressed whether and how much transgenerational acclimation might affect fitness-relevant traits or population limiting rates of offspring, such as survival or growth, in response to ocean acidification. Most evidence of positive transgenerational effects comes from tropical reef fish, namely the Spiny Chromis (Acanthochromis polyacanthus)15,16 and the Fire Clownfish (Amphiprion melanopus)17, although Welch et al.16 showed no capacity for transgenerational capacity for olfactory responses to ocean acidification in spiny damselfish. However, in summary Rummer and Munday (2017)18 conclude that there is evidence of transgenerational acclimation and adaptation in response to ocean acidification and temperature. For temperate fish species, physiological responses to parental acclimation in stickleback exist19,20,21. Schade et al.19 demonstrated reduced survival and body size at 30dph and enlarged otoliths when fathers or both parents were acclimated to the high CO2 level. Shama & Wegner (2014)20 demonstrated that the reproductive output was mainly determined by the temperature the mother experienced with a carry-over effect from the grandmother. In older stages, maternal and maternal grandmother environments influenced stickleback’s body size, but in opposing directions, indicating that the mechanisms in the transfer of environmental information differed between the generations20. Acclimation of mothers to higher temperatures led to a more efficient offspring mitochondrial respiratory capacity, also reflected in the expression of the relevant genes (Shama et al.)21. Exploring the potential of fish populations to adapt to ocean acidification through multi-generational experiments similar to work in coral reef fishes22 and in stickleback, is unfeasible for most commercial species because of their long generation times (e.g. Atlantic cod 3–5 years) and larger body size. Assessing the effect of parental acclimation is therefore the closest current research can come to assess transgenerational effects. This research impasse is all the more troubling, because temperate to boreal species with commercial importance can be remarkably sensitive to high CO25,6. For example, our group has previously shown in separate experiments that Atlantic cod stocks from the Western Baltic and the Barents Sea consistently suffered from higher mortality rates under realistic, end-of-century levels of ocean acidification, with the salient finding that daily mortality rates doubled in both stocks8.
Here, we exposed adult cod to either ambient seawater or seawater with increased aquatic CO2 concentrations, and therefore pH changes, for six weeks prior to spawning. We used ~1100 µatm as the high CO2 treatment, which is expected globally around the year 2100 following the scenario IPCC RCP 8.54. However, local predictions show that this level of acidification will likely be reached in the Arctic under the RCP 4.5 or at even lower emissions23. The exposure of the adult generation to CO2 coincides with the last stages of gonadal development and egg maturation24. Resulting eggs and larvae were reared either in the parental CO2 concentrations or the opposite treatment (i.e. high CO2 treatment in low CO2 and vice versa). Larval survival and growth were measured and histological samples of certain organs, including the eyes and the liver, were processed and analyzed. The tested hypothesis was that larvae, which came from parents, who already experienced exposure to high CO2 during gonadal development, might cope better with these conditions due to possible acclimation of the parents or because developing eggs in the mother experienced the high CO2 conditions.
Additionally, we tested the effect of energy limitation by including two different feeding regimes as additional treatments, fully crossed with the CO2 treatments. Acid-Base balance is a costly process in marine fishes. Hydrogen ions are expelled at the gills by an H+/Na+ exchanger. While this is a passive process, it is fuelled by the concentration gradient of sodium ions between the gill cell and the seawater, which is maintained by the K+/Na+ ATPase25,26. During continuous exposure to a high CO2 aquatic environment, this process needs to be constantly upregulated, resulting in an additional energetic cost to the organism27. This aspect of ocean acidification and its effect on acid-base balance has so far been largely ignored when studying fishes, though it is reasonable to assume that organisms, which are energy limited already, may respond differently to CO2 stress than those, which are not limited. We therefore hypothesized that larvae, which are fed ad libitum may be more resilient to the exposure to high CO2 than those on a lower feeding regime and that this might interact with the effect of parental acclimation to high CO2.
Results
In this study, the parental exposure to high CO2 modified the physiological reaction of larvae in the subsequent generation. This furthermore depended on the food availability, as shown by the significant three-way interaction of larval CO2, parental CO2 and food treatment (Table 1). Offspring in the high CO2 treatment of parents that were exposed to high CO2 survived better under high food availability (24.5% on day 16) compared to offspring from non-acclimated parents (10.5%), but worse under low food availability (on average 13.2% on day 16, compared to 21.3%) (Fig. 1). In the high food treatment, larvae of parents acclimated to high CO2 under high CO2 showed survival intermediate between the ambient CO2 treatment (49.4% on the final sampling day) and those without prior exposure in the parental generation to increased acidification (10.5%). This compensation was completely missing in the low food treatment. Here, larvae exposed to high CO2 coming from CO2 acclimated parents showed even lower survival (13.2%) than those from non-acclimated parents (21.3%). The survival of larvae in ambient CO2 conditions, but from parents acclimated to high CO2 (27.2%), was lower than that of larvae under ambient conditions from parents under ambient conditions (35.6%), and slightly higher than that of larvae, who were exposed to high CO2, but whose parents came from ambient CO2 conditions (21.2%).
Survival of cod larvae from hatching to 16 days post-hatching in the high food treatment (left) and the low food treatment (right) depending on parental CO2, larval CO2 treatment and food. Shown are mean values and standard error across three replicates per treatment. The first letter of the legend refers to the parental CO2 treatment, the second to the larval CO2 treatment and the third to the food treatment. (A-Ambient, C-High CO2, H-High Food, L-Low Food).
Larval growth in terms of dry weight and standard length at day 36 post-hatching was not affected by the CO2 treatment of parents nor the offspring, but larvae in the low food treatment were smaller (Fig. 2), indicating that offspring growth was energy limited. Since the parental generation is a F3 aquaculture stock, bred for optimal growth, it is unlikely that this absence of an effect of exposure to high CO2 is directly transferable in wild populations.
Dry weight (in mg, on the left) and Standard length (in mm, on the right) in 36 days post-hatching cod larvae depending on parental CO2 and larval CO2 treatment and food availability. Shown are mean values and standard deviation of ten larvae per three replicates. The first letter of the legend refers to the parental CO2 treatment and the second to the larval CO2 treatment. (A-Ambient, C-High CO2) High food is shown in dark circles and low food in lighter triangles.
Under CO2 concentrations corresponding to realistic end-of century ocean acidification levels, we found histological damage suggesting impairments of major organ functioning. Particularly the larvae in the high CO2 treatment, which came from acclimated parents, showed strong impairments more frequently independently of the food treatment (Figs 3 and 4). Vacuoles in the pigment layer of the retina of the 35 days old larvae were registered in all treatments but were more frequent in larvae from tanks with elevated CO2 concentrations (Fig. 3). Gill structure looked similar in all investigated larvae. Similar heart morphology was also noted in all larvae. The kidney tissue sections showed apparently normal tubuli and glomeruli in all groups. Liver morphology varied between individual samples and CO2 regimes (Fig. 4c,d). Glycogen granules were noted in all livers sectioned (Fig. 4c), while numerous empty vacuoles (representing lipid inclusions) of variable sizes were characteristic of some of the CO2 treated larvae (Fig. 4d). Such abnormal vacuolation will impair liver function5,6. In contrast, larvae from the ambient treatment had smaller and more regular vacuoles (Fig. 4c). Hepatocyte vacuolation did not occur more frequently in the larval group in the high food compared to the low food treatment.
Examples of histological sections; (a) Transverse of eye with few vacuoles in the pigmented layer of the retina from AAL larva; (b) Transverse section of eye with many vacuoles in the pigmented layer of the retina from CCH larva; (c) Transverse section of liver and oesophagus of AAL larva. Note few vacuolizations in the liver; (d) Transverse section of liver and oesophagus of CCH larva. Note numerous vacuolizations in the liver.
Discussion
In this study, we analyzed whether or not parental acclimation together with the corresponding exposure of gamete development in the parents to high aquatic CO2 conditions would result in improved performance of the next generation in a high CO2 environment. Our data were collected for a commercially exploited fish species, the Atlantic cod. In response to elevated CO2, in this independent new data set, cod larval mortality increased in accordance to observations previously described by Stiasny et al.8. Additionally, this study found that parental acclimation alleviated negative effects of high CO2 exposure on larval mortality to some degree, suggesting that such effects may change first order effects that only address physiological effects within the same generation5,6,7,8. However, this alleviation was only observed under high food availability, demonstrating the complexity of ocean acidification with other interacting factors, here food availability on fish physiology and survival. Therefore, knowledge of the food web interactions in response to ocean acidification has to be taken into consideration to predict how acclimation effects can potentially alleviate the so far observed direct negative effects of ocean acidification on larval fish survival and the long-term effects on stocks.
The potential energetic impact of ocean acidification on marine fish species has often been ignored in previous studies, except for a recent study by Bignami et al.28 showing a negative effect of elevated CO2 at complete feeding cessation on the starvation potential in larval cobia and a study of Hurst et al.29, which found no interaction between CO2 and nutritional stress in the northern rock sole29. Since it is well known that adult fish, juveniles and later larval stages are efficient at acid base regulation, they have often been assumed to be robust to ocean acidification, ignoring the fact that this comes at a high energetic cost and a changed acid base balance of the blood25,27. We were able to show for the first time that food limitation in fact has a large impact on the effect of high CO2 exposure and can significantly affect the fitness of cod larvae.
Considering that our simulated low food treatment is likely to be above common prey availabilities in the field, it is unlikely that fish larvae in the wild will have the necessary energy available to regulate efficiently throughout their development and, as also shown in this study, to benefit from parental acclimation to similar conditions as experienced by them. The study shows the importance of including energy availability in experimental studies of ocean acidification in the future.
Since the exposure time of the parental generation to high CO2 was only six weeks in duration, we are unable to distinguish between transgenerational effects in the strictest sense and an effect of exposure of the early zygotic development in the mother or father to CO2, a point well raised by Torda et al.14. However, our main aim was to explore whether the exposure of multiple life stages to increased CO2 environments would benefit the overall survival and therefore reproductive potential or not.
Our results show that parental exposure to high CO2 conditions results in reduced survival, when the larvae were raised under ambient CO2 concentrations, compared to those, where neither parents nor larvae experienced any level of acidification. Possibly, there is already a negative impact of high CO2 exposure on the eggs mediated via the mother during early zygotic development, which does result in lower fitness during later life stages.
The histological organ impairments found here are consistent with those found by Frommel et al.5,6,11. The degree of damage is lower, probably due to the more realistic, lower levels of carbon dioxide used in this experiment (1100 µatm in this study compared to 1800 and 4200 µatm by Frommel). The histological sections show that even in the high food treatment, where larval survival was partly compensated by the parental acclimation, larvae still suffered developmental impairments and organ damage in response to high CO2. This might suggest, that even though survival is slightly improved at this point by parental exposure to CO2, the long-term fitness of the larvae may very well suffer, since the impairments of the organs may result in later functional problems.
In conclusion, we found an effect of parental acclimation to CO2 exposure, however only under ideal conditions concerning prey availability, a situation unlikely to be expected in the wild. Our results highlight the importance of energy availability. This is adding to the uncertainty of effects of ocean acidification on marine fish, since acidification may well change ecosystems and the food web structures30,31,32, therefore altering prey availabilities.
Methods
The experiments were performed at the Centre for Marine Aquaculture (Senter for marin akvakultur, formerly the Norwegian Cod Breeding Centre Nasjonal avlsstasjon for torsk) of Nofima outside of Tromsø, Norway during the spring of 2014. We used a full factorial experimental design combining high and low CO2 treatments to simulate ocean acidification and two different feeding treatments during the larval stage development.
Water treatment
Deep-water from 40 to 60 m depths was pumped from the Grøtsundet directly into the Centre for Marine Aquaculture. The water is aerated with oxygen before it enters the parental tanks in the brood fish hall. The seawater used for the egg incubators and larval tanks is furthermore filtered by a 90 µm drum filter, passes through a protein skimmer and a sand filter and is then UV treated before being used. Carbon dioxide concentrations in the acidified treatment were controlled by the semi-automated pH-Stat IKS Aquastar Systems, which activates magnetic valves to allow CO2 influx from a CO2 bottle. A pH sensor is attached to the outflow of the header tanks and if the pH is above a threshold, the system opens the valves in order to allow an inflow of CO2 in short pulses in order to maintain the seawater at the target value of 7.75. The pH was additionally checked daily with a WTW pH 3310 hand probe with a SenTix® H pH-electrode. Water samples for carbonate chemistry were taken and analyzed at the University of Tromsø based on the Best Practices Guide (See Stiasny et al.8, for more details on the carbonate chemistry in the experiment).
Parental treatment
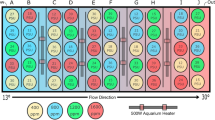
Adult cod from the aquaculture stock of the company Nofima AS at the Centre for Marine Aquaculture in Tromsø, Norway were transferred from the sea cages to the centre on 16th January 2014 to start the incubation. These aquaculture stocks were a F3 generation mixture of two wild stocks, the Norwegian coastal cod and the North-East-Arctic cod from the Barents Sea. They were kept in net cages in the fjord and were transferred using a well boat and transfer tanks. They were split into the two separate treatment tanks each of 4 m diameter filled with 18 m3 seawater containing about 80 fish each. The tanks had a constant seawater flow through of 225 l/minute. The light regime was matched weekly to outside conditions.
The adult cod were regularly checked for running eggs and sperm. When mature and running, they were strip-spawned and eggs were fertilized in vitro. Five non-siblings’ families from the ambient CO2 parental treatment and seven non-siblings’ families from the high CO2 parental treatment were used. Additionally, eggs from a natural spawning event were added to the ambient parental treatment, contributing 57% of the larvae in the ambient parent/ambient larvae treatment and 70% in the ambient parental/high CO2 larvae treatment. All eggs that were used were spawned on the same day.
Egg and larval treatment
Fertilized eggs were transferred to incubators, which were kept at 6 °C and were constantly aerated. Families were kept in separate incubators, so that a balanced number of hatched larvae could be transferred at the start of the larval experiment. After hatching the larval density was assessed in the incubators by counting the number of larvae in five aliquots each of 100 ml and extrapolating the average number per ml to the volume of the incubator. 11 000 larvae were transferred into each rearing tank and the larval experiment was commenced. This day was set as 0 days post-hatching (dph), even though larvae had hatched over several days before. Each treatment combination was replicated in three separate larval tanks, which were randomly distributed within the larval rearing setup.
The larval tanks were initially maintained at 6 °C, but the temperature was later raised to 10 °C in all tanks to assist growth rates and survival33. The larvae were kept in light 24 hours a day. Larvae were fed with Nannochloropsis and Brachionus at different time intervals for the different food treatments (seven in the high compared to three times daily in the low food treatment). The prey concentrations given per feeding remained constant and the same for both treatments.
For more information on feeding concentrations, please consult the article and the SI of Stiasny et al.8.
Survival measurements
Survival was measured three times in the larval tanks, on days 8, 12 and 16 post-hatching, by measuring the numbers of remaining larvae. Five subsamples of 0.8 l were taken across the whole water column using a pipe, which could be closed at the bottom, and the number of living larvae in the subsamples was counted. An increased aeration during the sampling process ensured an even distribution of larvae in the rearing tanks. The accuracy and precision of the method was repeatedly checked in separate tanks with a known number of larvae. After day 16 post-hatching of the experiment, the method became inaccurate and imprecise. This is likely due to the increased swimming ability of the larvae, combined with improving sensory abilities, which probably resulted in an uneven distribution of larvae in the tanks due to avoidance behaviour towards the pipe and the increased aeration. Survival data was therefore disregarded after 16 dph, but larvae were sampled for growth and histology measurements until 36 and 35 dph respectively.
Growth
Ten larvae per tank were sampled at day 36 post-hatching, euthanized using Tricaine methanesulfonate (MS222), then frozen at −20 °C and later measured for Standard Length (mm) using calibrated digital images. In order to measure dry weight, larvae were freeze dried before being weighed.
Statistical analysis
All statistical analyses were run in the program R (Version 3.3.2) and RStudio (Version 1.0.136). For growth measurements ten larvae per tank were sampled in order to get an accurate assessment of the variance in the tanks. To include the possibility of tank effects a linear mixed effects model (lme) was run to test for differences and interactions between the treatments, but also including the tank as a random factor. The dry weight was log transformed in order to achieve normality of residuals. Survival in percent was logit transformed before being assessed using a repeated-measures multi-factorial ANOVA including interactions between all treatments and across the three sampling days.
Histology
Euthanized larvae were fixed in 4% buffered formaldehyde at 35 dph, embedded in Technovit® or paraffin, sectioned transversely or longitudinally respectively at 3 µm, followed by staining with methylene blue (Technovit sections) or haematoxylin and eosin (paraffin sections)34. Technovit-sections from head region (with eyes, gills and heart), front part of gut (with liver, pancreatic tissue, kidney tissue) as well as paraffin sections were studied and photographed in the microscope (Leitz Aristoplan with a Leica DFC295 camera). Moderate or numerous amounts of vacuoles in the pigment layer of the retina were noted and given a subjective score from 0 to +++ (some-several-many) (see also Frommel et al.6). A similar score was used for registrations of lipid vacuoles in the cod larvae livers. The scoring was done by a single person (Inger-Britt Falk-Petersen) using repeated assessments.
The experiments were conducted at the Centre for Marine Aquaculture (formerly the National Cod Breeding Centre), NOFIMA, Tromsø, Norway in accordance to the national rules and regulations and all efforts where undertaken to minimize stress and suffering of the fish. The Norwegian Animal Research Authority (Forsøksdyrutvalget) approved the experiments (ethics permit number is FOTS ID 6382).
Data availability
The datasets generated during and/or analysed during the current study will be available in the PANGEA repository.
References
Drinkwater, K. F. The response of Atlantic cod (Gadus morhua) to future climate change. ICES J. Mar. Sci. 62, 1327–1337 (2005).
Kjesbu, O. S. et al. Synergies between climate and management for Atlantic cod fisheries at high latitudes. Proc. Natl. Acad. Sci. USA 111, 3478–83 (2014).
Steinacher, M., Joos, F., Frölicher, T. L., Plattner, G.-K. & Doney, S. C. Imminent ocean acidification projected with the NCAR global coupled carbon cycle-climate model. Biogeosciences Discuss. 5, 4353–4393 (2009).
IPCC. Climate Change 2013 - The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel of Climate Change. (Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, https://doi.org/10.1017/CBO9781107415324 (2013).
Frommel, A. Y. et al. Organ damage in Atlantic herring larvae as a result of ocean acidification. Ecol. Appl. 24, 1131–1143 (2014).
Frommel, A. Y. et al. Severe tissue damage in Atlantic cod larvae under increasing ocean acidification. Nat. Clim. Chang. 2, 42–46 (2012).
Baumann, H., Talmage, S. C. & Gobler, C. J. Reduced early life growth and survival in a fish in direct response to increased carbon dioxide. Nat. Clim. Chang. 2, 38–41 (2012).
Stiasny, M. H. et al. Ocean Acidification Effects on Atlantic Cod Larval Survival and Recruitment to the Fished Population. PLoS One 11, e0155448 (2016).
Munday, P. L. et al. Replenishment of fish populations is threatened by ocean acidification. Proc. Natl. Acad. Sci. USA 107, 12930–4 (2010).
Dixson, D. L., Munday, P. L. & Jones, G. P. Ocean acidification disrupts the innate ability of fish to detect predator olfactory cues. Ecol. Lett. 13, 68–75 (2010).
Frommel, A. Y. et al. Ocean acidification has lethal and sub-lethal effects on larval development of yellowfin tuna, Thunnus albacares. J. Exp. Mar. Bio. Ecol. 482, 18–24 (2016).
Chambers, R. C. et al. Ocean acidification effects in the early life-stages of summer flounder, Paralichthys dentatus. Biogeosciences Discuss. 10, 13897–13929 (2013).
Sunday, J. M. et al. Evolution in an acidifying ocean. Trends Ecol. Evol. 29, 117–125 (2014).
Torda, G. et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 7, 627–636 (2017).
Miller, G. M., Watson, S.-A., Donelson, J. M., McCormick, M. I. & Munday, P. L. Parental environment mediates impacts of increased carbon dioxide on a coral reef fish. Nat. Clim. Chang. 2, 858–861 (2012).
Welch, M. J., Watson, S., Welsh, J. Q., McCormick, M. I. & Munday, P. L. Effects of elevated CO2 on fish behaviour undiminished by transgenerational acclimation. Nat. Clim. Chang. 4, 1086–1089 (2014).
Allan, B. J. M., Miller, G. M., McCormick, M. I., Domenici, P. & Munday, P. L. Parental effects improve escape performance of juvenile reef fish in a high-CO2 world. Proc. R. Soc. B Biol. Sci. 281, 20132179 (2014).
Rummer, J. L. & Munday, P. L. Climate change and the evolution of reef fishes: Past and future. Fish Fish. 22–39, https://doi.org/10.1111/faf.12164 (2016).
Schade, F. M., Clemmesen, C. & Wegner, K. M. Within- and transgenerational effects of ocean acidification on life history of marine three-spined stickleback (Gasterosteus aculeatus). Mar. Biol. 161, 1667–1676 (2014).
Shama, L. N. S. & Wegner, K. M. Grandparental effects in marine sticklebacks: transgenerational plasticity across multiple generations. J. Evol. Biol. 27, 2297–2307 (2014).
Shama, L. N. S. et al. Transgenerational effects persist down the maternal line in marine sticklebacks: gene expression matches physiology in a warming ocean. Evol. Appl. 9, 1096–1111 (2016).
Donelson, J. M., Munday, P. L., McCormick, M. I. & Pitcher, C. R. Rapid transgenerational acclimation of a tropical reef fish to climate change. Nat. Clim. Chang. 2, 30–32 (2011).
AMAP. AMAP Assessment 2013: Arctic Ocean Acidification. (2013).
Craik, J. C. A. & Harvey, S. M. Biochemical changes occuring during final maturation of eggs of some marine and freshwater teleosts. J. Fish Biol. 24, 599–610 (1984).
Melzner, F. et al. Physiological basis for high CO2 tolerance in marine ectothermic animals: pre-adaptation through lifestyle and ontogeny? Biogeosciences Discuss. 6, 4693–4738 (2009).
Perry, S. F. & Gilmour, K. M. Acid – base balance and CO2 excretion in fish: Unanswered questions and emerging models. Respir. Physiol. Neurobiol. 154, 199–215 (2006).
Heuer, R. M. & Grosell, M. Elevated CO2 increases energetic cost and ion movement in the marine fish intestine. Sci. Rep. 6, 34480 (2016).
Bignami, S., Sponaugle, S., Hauff, M. & Cowen, R. K. Combined effects of elevated pCO2, temperature, and starvation stress on larvae of a large tropical marine fish. ICES J. Mar. Sci. J. du Cons. 74, fsw216 (2016).
Hurst, T. P., Laurel, B. J., Hanneman, E., Haines, S. A. & Ottmar, M. L. Elevated CO2 does not exacerbate nutritional stress in larvae of a Pacific flatfish. Fish. Oceanogr. 26, 336–349 (2017).
Nagelkerken, I. & Connell, S. D. Global alteration of ocean ecosystem functioning due to increasing human CO 2 emissions. Proc. Natl. Acad. Sci. 112, 13272–13277 (2015).
Nagelkerken, I., Goldenberg, S. U., Ferreira, C. M., Russell, B. D. & Connell, S. D. Species Interactions Drive Fish Biodiversity Loss in a High-CO 2 World. Curr. Biol. 27, 2177–2184.e4 (2017).
Sswat, M. et al. Food web changes under ocean acidification promote herring larvae survival. Nat. Ecol. Evol. https://doi.org/10.1038/s41559-018-0514-6 (2018).
Puvanendran, V. et al. Effects of different step-wise temperature increment regimes during egg incubation of Atlantic cod (Gadus morhua L.) on egg viability and newly hatched larval quality. Aquac. Res. 46, 226–235 (2015).
Pedersen, T. & Falk-Petersen, I. B. Morphological changes during metamorphosis in cod (Gadus morhua L.), with particular reference to the development of the stomach and pyloric caeca. J. Fish Biol. 41, 449–461 (1992).
Acknowledgements
Funding was provided through the BIOAcid project (Biological Impacts of Ocean ACIDification) funded by the German Ministry for Education and Research (BMBF), the AquaExcel transnational access grant for aquaculture infrastructures funded by the EU FP7 programme and the Bonus Baltic Sea research and development programme (Art 185) BIO-C3 project, funded jointly by the EU and the BMBF (Grant No. 03F0682A). The experiment was conducted at the Norwegian Cod Breeding Centre, NOFIMA, Tromsø, Norway. The authors are grateful to the whole staff at the centre for their help and support.
Author information
Authors and Affiliations
Contributions
M.H.S., G.G., C.R.B. and C.C. designed the experiment; M.H.S., F.H.M., G.G., C.R.B., V.P., A.M. and C.C. performed the experiment; I.B.F.P. performed the histological analysis and wrote the section on that topic; M.H.S., F.H.M., T.B.H.R. and C.C. analysed data; M.H.S., C.C. and T.B.H.R. wrote the main paper; All authors discussed the results and implications and commented on the manuscript at all stages.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Stiasny, M.H., Mittermayer, F.H., Göttler, G. et al. Effects of parental acclimation and energy limitation in response to high CO2 exposure in Atlantic cod. Sci Rep 8, 8348 (2018). https://doi.org/10.1038/s41598-018-26711-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26711-y
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.