Abstract
The effects of dimethyl fumarate (DMF) on the immune system in multiple sclerosis (MS) are not completely elucidated. In this study, an extensive immunophenotypic analysis of innate and adaptive immune cells of DMF-treated MS patients was performed. Peripheral blood immune cell phenotypes were determined using flow cytometry in a follow-up study of 12 MS patients before, after 3 and 12 months of DMF treatment and a cross-sectional study of 25 untreated and 64 DMF-treated MS patients. Direct effects of DMF on B cells were analyzed in vitro. After 12 months of DMF treatment, percentages of monocytes, natural killer cells, naive T and B cells and transitional B cells increased. Percentages of (effector) memory T cells, (non) class-switched memory B cells and double negative B cells decreased together with CD4+ T cells expressing interferon-γ (IFN-γ), granulocyte macrophage colony-stimulating factor (GM-CSF) and interleukin-17 (IL-17). DMF treatment was fully effective as of 6 months and directly induced apoptosis and decreased expression of costimulatory CD40, antigen presentation molecule MHCII and B cell activating factor receptor (BAFFR) on B cells. DMF induced a persistent change of the immune system of MS patients, directly induced apoptosis and reduced expression of functional markers on B cells.
Similar content being viewed by others
Introduction
MS is a chronic inflammatory disease of the CNS in which both the innate and adaptive immune system are involved. The immune system of MS patients displays a disrupted balance favoring pro-inflammatory responses. T cells have long been considered as the most important players although the emergence of B cell depleting treatment has emphasized the importance of B cells in MS pathogenesis1,2. Adaptive immune cell subtypes involved in the pathological processes during MS are memory T cells (CD45RO+CD45RA−) and more specifically C-C chemokine receptor type 7 (CCR7)− effector memory T cells, CD8+ T cells and memory (CD27+) B cells3,4,5,6,7,8. CD4+ T helper (Th) 1 and Th17 cells produce pro-inflammatory cytokines including interferon-γ (IFN-γ), interleukin-17 (IL-17) and granulocyte macrophage colony-stimulating factor (GM-CSF) while Th2 and regulatory T (Treg) cells produce anti-inflammatory IL-4 and IL-101,9. Double negative (DN, IgD−CD27−) B cells with a pro-inflammatory cytokine profile are described to be increased in a proportion of MS patients10. For the innate immune system, monocytes (CD14+), neutrophils and natural killer (NK) cells, including CD56dim and CD56bright NK cells, are involved in MS pathogenesis11,12,13,14.
Dimethyl fumarate (DMF, Tecfidera®) was licensed as an oral first-line treatment for MS in 2013. Two phase III clinical trials, DEFINE and CONFIRM, demonstrated clinical efficacy of DMF in relapsing-remitting (RR) MS15,16. DMF reduced the annualized relapse rate and reduced the mean number of new or enlarging MRI lesions throughout the course of the study15,16. Earlier studies have demonstrated that DMF and its active metabolite monomethyl fumarate (MMF) exert neuroprotective and immunomodulatory effects through the activation of transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2) and via the suppression of transcription factor nuclear factor kappa b (NF-kB)17,18.
A number of studies investigated the effect of DMF on certain subtypes of disease-related immune cells19,20. Nevertheless, the working mechanism of DMF is multifactorial and the pathogenesis of MS is directed by interplay between different immune cell subtypes. Previous studies were limited by a cross-sectional design, limited follow-up to 6 months only, a focus on 1 immune cell subtype or the inclusion of patients who were previously treated with second-line therapies. Therefore, we performed an extensive immunophenotypic analysis of the innate and adaptive immune system of RRMS patients under DMF treatment using 3 study designs: a 12 month (m) follow-up study, a cross-sectional study and an in vitro study. In this way we further explored changes of several immune cell types over a longer period in a cause-and-effect way in untreated MS patients or in MS patients previously treated with first-line treatments.
Results
Clinical characteristics
In the follow-up study, 16 RRMS patients were enrolled and peripheral blood was collected before treatment, after 3 m and after 12 m of DMF treatment. Twelve of the 16 MS patients finished the total duration of the study (Table 1), while 4 MS patients dropped out of the study, with 2 due to side effects (gastrointestinal and flushing), 1 due to pregnancy and 1 due to other medication use. Eight of the 12 DMF treated MS patients underwent MRI before and after 12 m of DMF treatment. In all patients, no new or enlarged lesions were detected. Furthermore, 4 of these 8 MS patients showed lesions that were decreased in volume or were less pronounced compared to baseline. Although not significant, EDSS decreased from 2.8 at baseline to 2.3 after 12 m of DMF treatment (p = 0.0547, Table 2). When considering individual MS patients, EDSS improved for 6 patients, remained stable for 4 patients and increased for 2 patients who were clinical responders. Interestingly, a significantly improved cognitive function measured by the PASAT was observed after 3 m of DMF treatment (p < 0.05). Other clinical measures remained stable over the course of the study (Table 2).
Absolute numbers of immune cells
Absolute immune cell numbers of leukocytes, lymphocytes, monocytes and neutrophils were obtained from standard clinical lab tests for 7 DMF-treated MS patients. A significant decrease in the absolute leukocyte (20%) and lymphocyte (46%) numbers was measured after 12 m of DMF treatment (p = 0.0313, Table 2). Grade 3 lymphopenia (absolute lymphocyte numbers <0.5 × 109 cells/L) was not observed in this patient cohort. Absolute monocyte and neutrophil numbers remained stable. Specific cell subtypes of both the innate and adaptive immune system, calculated as described in the methods section, showed significantly reduced numbers after 12 m but not after 3 m of DMF treatment. The absolute number of CD56dim NK cells was significantly reduced (p = 0.0156), while CD56bright NK cell numbers were significantly increased (p = 0.0469). The absolute numbers of T cells (CD3+) and all T cell subtypes were significantly decreased (p < 0.05), except for naive T cells (p = 0.1563 for CD4+CD45RO−CD45RA+ and p = 0.0781 for CD8+CD45RO−CD45RA+) and IL-17+ CD4+ T cells (p = 0.1094). Within the B cell population, the absolute numbers of total B cells (CD19+) and all B cell subtypes were decreased (p < 0.05) except for transitional B cells (p = 0.6875) and naive B cells (p = 0.0781), which were not affected. In conclusion, most immune cell subtypes showed decreased circulating absolute numbers following 12 m of DMF treatment. Therefore, it was important to investigate the remaining peripheral blood immune cell population by determining immune cell subtype percentages.
Percentages of innate immune cell subtypes
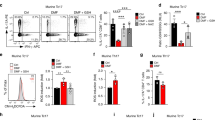
When analyzing innate immune cell subtypes (gated as in Fig. 1a), increased percentages of monocytes (p = 0.0005), CD56+ (p = 0.0005) and CD56dim (p = 0.0269) NK cells were detected after 12 m (Fig. 1b), but not after 3 m (Supplementary Fig. 1) of DMF treatment. Thus, DMF treatment increased the percentages of all analyzed innate immune cell subtypes.
DMF treatment increased frequencies of monocytes and NK cells. (a) A representative analysis of innate immune cell subtypes is shown. Single cells were selected using forward scatter (FSC) area and width. Lymphocytes were gated in the singlet gate using FSC and side scatter (SSC) parameters. CD56dim NK cells (CD3−CD16+CD56dim) and CD56+ NK cells (CD3−CD16+CD56+) were gated from lymphocytes and the CD3- cell population. Monocytes (CD14+) were gated from monocyte gate. (b) Frequency of monocytes and NK cells at baseline and after 12 m of DMF treatment in RRMS patients (n = 12). Each line represents an individual patient and average values are depicted as histograms. Wilcoxon matched-pairs signed rank test was used to determine p values. *p < 0.05, **p < 0.01, ***p < 0.001. NK = natural killer, m = months.
Percentages of adaptive immune cell subtypes
After 12 m of DMF treatment, T cell percentages decreased, although not significantly (data not shown). Within the T cell population (gated as in Fig. 2a), CD4+ T cells remained stable, while CD8+ T cells decreased (p = 0.0034, Fig. 2b). Most importantly, a decrease in the percentages of memory and effector memory CD4+ and CD8+ T cells was evident (p < 0.01) while naive CD4+ and CD8+ T cell percentages increased (p < 0.01). No effect of DMF treatment was observed on Treg percentages (Supplementary Fig. 1). When analyzing cytokine-expressing CD4+ T cells (gating shown in Supplementary Fig. 2), decreased percentages of pro-inflammatory IFN-γ+ (p = 0.0015), GM-CSF+ (p = 0.0005) and IL-17+ (p = 0.0488) CD4+ T cells (Fig. 3a) were detected and anti-inflammatory IL-4+ and IL-10+ CD4+ T cells were unchanged (Fig. 3b, Supplementary Fig. 2).
DMF treatment reduced (effector) memory T cell frequencies after 12 m of treatment in MS patients. (a) A representative analysis of T cell subtypes is shown. Single cells were selected using FSC area and width. Lymphocytes were gated within the singlet gate using FSC and SSC parameters. Next, CD4+ T cells and CD8+ T cells were gated from lymphocytes. Naive T cells (CD45RA+CD45RO−), memory T cells (CD45RA−CD45RO+) and effector memory T cells (CD45RA−CCR7−) were gated from the CD4+ or CD8+ T cell population. (b) Frequency of T cell subtypes at baseline and after 12 m of DMF treatment in RRMS patients (n = 12). Each line represents an individual patient and average values are depicted as histograms. Wilcoxon matched-pairs signed rank test was used to determine p values. *p < 0.05, **p < 0.01, ***p < 0.001. m = months.
Reduction in the frequency of T cells expressing pro-inflammatory cytokines after 12 m of DMF treatment. Frequency of CD4+ T cells expressing pro-inflammatory cytokines (a), including IFN-γ, GM-CSF, IL-17 or anti-inflammatory cytokines (b), including IL-4 and IL-10, in RRMS patients at baseline and after 12 m of DMF treatment (n = 12). Each line represents an individual patient and average values are depicted as histograms. Wilcoxon matched-pairs signed rank test was used to determine p values. *p < 0.05, **p < 0.01, ***p < 0.001. IFN-γ = interferon γ, GM-CSF = granulocyte macrophage colony-stimulating factor, IL-17 = interleukin-17, IL-4 = interleukin-4, IL-10 = interleukin-10, m = months.
Furthermore, B cell percentages were not affected by DMF treatment (Fig. 4). Within the B cell population (gated as in Fig. 4a), percentages of transitional and naive B cells increased (Fig. 4b). Further, DMF treatment resulted in decreased percentages of DN (p = 0.0161), class-switched memory (p = 0.0034) and non class-switched memory (p = 0.0161) B cells. After 3 m of DMF treatment, no significant differences were observed in the percentages of all adaptive immune cell subtypes (Supplementary Fig. 1).
DMF treatment reduced memory and DN B cell subtypes after 12 m of treatment in MS patients. (a) A representative analysis of B cell subtypes is shown. Single cells were selected using FSC area and width. Lymphocytes were gated in the singlet gate using FSC and SSC parameters. B cells (CD19+) were gated from lymphocytes followed by analysis of transitional B cells (CD27−CD38high), naive B cells (CD27−IgD+), non class-switched memory B cells (CD27+IgD+), class-switched memory B cells (CD27+IgD−) and DN B cells (CD27−IgD−). (b) B cell subtypes at baseline and after 12 m of DMF treatment in RRMS patients (n = 12). Each line represents an individual patient and average values are depicted as histograms. Wilcoxon matched-pairs signed rank test was used to determine p values. *p < 0.05, **p < 0.01, ***p < 0.001. DN = double negative, m = months.
In vitro treatment of B cells from 5 untreated RRMS patients with DMF or MMF indicated that DMF induced a trend towards an increased regulatory B cell (Breg) percentage (p = 0.06, Supplementary Fig. 3). MMF decreased the percentage of TNF-α+ B cells, although not significantly (p = 0.06). Together, these results indicate that 12 m DMF treatment reduced percentages of pro-inflammatory and memory T and B cell subtypes and increased percentages of naive T and B cells and transitional B cells.
T cell subtypes in a cross-sectional study
Since 3 m DMF treatment only partly reflected changes reported at 12 m, additional time points were included in a cross-sectional study to identify how soon the reported effect was found after treatment (Table 1). Memory CD4+ and CD8+ T cell percentages were reduced, while naive CD4+ and CD8+ T cell percentages were increased after 6 m of DMF treatment compared to untreated MS patients (Fig. 5). Furthermore, percentages of memory CD8+ T cells were decreased, while naive CD8+ T cells were increased after 6–12 m compared with 1–5 m of DMF treatment. After prolonged treatment (>12 m), memory and naive CD4+ and CD8+ T cell percentages remained stable. Thus, DMF is fully effective after 6 m of treatment.
DMF treatment is fully effective on immune cells after 6 m of treatment. Frequencies of naive and memory CD4+ and CD8+ T cells in HC (n = 10), untreated RRMS patients (n = 25), 1–5 m DMF-treated RRMS patients (n = 23), 6–12 m DMF-treated RRMS patients (n = 23), >12 m DMF-treated MS patients (n = 18). A Kruskal-Wallis one-way ANOVA was used to compare the different groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. HC = healthy control, DMF = dimethyl fumarate, m = months.
Direct effect of DMF on B cell apoptosis
We next investigated induction of apoptosis as one of the underlying mechanisms of the drop in absolute lymphocyte numbers. Previously, in vitro studies showed that DMF induced T cell apoptosis with a preferential effect on memory T cells21. Since DMF treatment decreased the percentage of memory B cells while increasing naive B cells, we investigated whether DMF induced apoptosis of B cells and whether naive B cells showed a lower vulnerability to DMF-induced apoptosis. Here, the direct effect of DMF and MMF on B cell apoptosis was investigated (Table 1). In HC, DMF induced B cell apoptosis at 25 µM (p < 0.05) and 50 µM (p < 0.001) compared to baseline (Fig. 6). In untreated MS patients, apoptosis was induced with 50 µM DMF (p < 0.01), although late B cell apoptosis was already induced at 25 µM (p < 0.05). In HC, late apoptosis was only induced at 50 µM DMF (p < 0.001). MMF treatment did not induce B cell apoptosis (Supplementary Fig. 4) and no difference was detected between memory and naive B cells (data not shown). In summary, DMF induced concentration-dependent apoptosis of B cells from HC and MS patients with B cells of MS patients appearing to be more vulnerable.
DMF induces apoptosis in B cells in a concentration-dependent manner. Purified B cells of HC and untreated RRMS patients were treated in vitro with the following concentrations of DMF: 10 µM (HC: n = 12, MS: n = 5), 16 µM (HC: n = 10, MS: n = 4), 25 µM (HC: n = 8, MS: n = 6) and 50 µM (HC: n = 7, MS: n = 5) or left untreated (HC: n = 12, MS: n = 6). (a) Frequency of live B cells (Annexin V−7-AAD−), (b) apoptotic B cells (Annexin V+7-AAD−), (c) late apoptotic B cells (Annexin V+7-AAD+) and (d) necrotic B cells (Annexin V−7-AAD+) of HC and MS patients. A Kruskal-Wallis one-way ANOVA was used to compare the different groups. *p < 0.05, **p < 0.01, ***p < 0.001. HC = healthy control, MS = multiple sclerosis, DMF = dimethyl fumarate.
Direct effect of DMF on functional markers of B cells
We previously demonstrated the potential of MS B cells to induce autoreactive T cell responses via antigen presentation and increased costimulatory molecule expression on MS B cells4. As immunomodulatory treatment abrogated these effects4, we investigated whether in vitro treatment with DMF directly decreased the expression of antigen presentation and costimulatory molecules (Table 1). DMF, but not MMF, directly decreased B cell expression of HLA-DR/DP/DQ and CD40 in HC (p = 0.0234 and p = 0.0078, respectively) and untreated MS patients (p = 0.0313 and p = 0.0313, respectively, Fig. 7A, Supplementary Fig. 5). In contrast, DMF increased CD80 expression on B cells of untreated MS patients (p = 0.0469), while no effect was detected in HC or on CD86 expression. However, DMF had no effect on the percentage of CD80+ B cells while it decreased the percentage of CD86+ B cells in HC.
DMF downregulates the expression of antigen-presentation molecules, costimulatory molecule CD40 and survival marker BAFFR on B cells. (A) Purified B cells of HC and untreated RRMS patients were treated in vitro with 10 µM DMF or were left untreated. Expression (MFI) of HLA-DR/DP/DQ (HC: n = 8, MS: n = 7), CD40 (HC: n = 8, MS: n = 7), CD80 (HC: n = 9, MS: n = 7) and CD86 (HC: n = 9, MS: n = 7) on B cells and % CD80+ (HC: n = 8, MS: n = 7) and % CD86+ (HC: n = 9 MS: n = 7) B cells is depicted for HC and MS patients. (B) Purified B cells of HC and untreated RRMS patients were treated in vitro with the following concentrations of DMF: 10 µM (HC: n = 14, MS: n = 5), 16 µM (HC: n = 11, MS: n = 4), 25 µM (HC: n = 9, MS: n = 6) and 50 µM (HC: n = 7, MS: n = 5), or with MMF: 50 µM (HC: n = 9, MS: n = 4) or were left untreated (HC: n = 14 and MS: n = 5). (C) Plasma BAFF levels of RRMS patients at baseline (n = 11), 3 m of DMF treatment (n = 9) and 12 m of DMF treatment (n = 11) and expression (MFI) of BAFFR on B cells of RRMS patients at baseline and 12 m of DMF treatment (n = 12). A Kruskal-Wallis one-way ANOVA was used to compare the different groups and a Wilcoxon matched-pairs signed rank test was used to determine p values between two groups. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. HLA-DR/DP/DQ = human leukocyte antigen, HC = healthy control, MS = multiple sclerosis, DMF = dimethyl fumarate, MFI = mean fluorescence intensity, BAFF(R) = B cell activating factor (receptor), pos = positive.
B cell survival was further investigated via BAFFR expression. DMF, but not MMF, directly decreased BAFFR expression on B cells in vitro, already at 10 µM (HC: p < 0.05; MS: p = 0.0625) with stronger effects using higher DMF concentrations (p < 0.01, Fig. 7B). An increased baseline BAFFR expression level was observed on MS B cells compared to HC (p < 0.0001). In our follow-up study, an increased BAFFR expression was detected after 12 m of DMF treatment and no effect was measured on BAFF plasma levels (Fig. 7C). This suggests that DMF treatment has a direct effect on B cell survival by decreasing BAFFR expression and an indirect effect ex vivo due to a shift in B cell subtypes.
Discussion
In 2013, DMF (Tecfidera®), was licensed as an oral first-line treatment for RRMS15,16. However, its mechanism of action is still not fully elucidated. Here, we used 3 study designs and demonstrated that DMF treatment induced a persistent change of both the innate and adaptive immune system of MS patients. Furthermore, DMF directly induced concentration-dependent apoptosis and reduced expression of functional markers of B cells.
Next to the immunological findings, clinical parameters were analyzed in the 12 m follow-up study. DMF treatment improved cognitive function (PASAT) and showed a trend towards improved EDSS of MS patients. Cognitive decline is a serious complication in MS that is characterized by deficits in (non)verbal memory, information processing speed and sustained attention22. However, validation in a larger cohort of DMF-treated MS patients is needed.
We further showed that DMF treatment selectively targets or has a stronger effect on particular immune cell subtypes, since it decreased total leukocyte and lymphocyte numbers without affecting monocytes, neutrophils and anti-inflammatory subtypes such as CD56+ NK cells and transitional B cells. Absolute numbers in this study are comparable to others21,23,24,25. The B cell subtype numbers are in line with Li et al. where DMF preferentially decreased mature B cells without affecting transitional B cells after at least 3 m of treatment20. However, our study reinforced this by showing a persistent change after 12 m. We further confirmed the preferential loss of CD8+ T cells (58%) versus CD4+ T cells (41%) and additionally indicated that this loss was persistent after 12 m of treatment. However, in our study none of the DMF-treated MS patients showed a decline of CD4+ T cells below the lower limit of normal (LLN, 400/µl) and only 14% of the patients showed a decline of CD8+ T cells below the LLN (200/µl), which is in contrast with other studies24,25. This could be due to the difference in patient numbers and inclusion criteria. After 3 m of DMF treatment, no significant changes were observed in the number of innate and adaptive immune cell subtypes, which is in agreement with Spencer et al.25.
As the absolute numbers of almost all immune cell subtypes decreased, it was important to consider their percentages to elucidate which subtypes were preferentially affected by DMF treatment. We showed that innate immune cell percentages (monocytes and NK cells) increased, implying that DMF prevails normal innate immune function. The relative increase in monocytes could be the result of the decreased absolute lymphocyte number, as the absolute monocyte number did not change. CD56+ NK cells were increased after DMF treatment, while CD56dimNK cells increased to a lesser extent. Medina et al. demonstrated an increase of CD56+ NK cells after 6 m of DMF treatment, but we reinforced this result by showing its persistence after 12 m of DMF treatment26. Furthermore, Chaves et al. and Medina et al. did not find a change in total NK cell percentages which indicates that it is important to investigate NK cell subtypes since CD56+ and CD56dim NK cells were described to have different functions26,27. CD56+ NK cells were first considered regulatory NK cells by Cooper et al. due to a reduced cytotoxicity and an increased production of cytokines compared to cytotoxic CD56dim NK cells28. Further, NK cells have become increasingly important as players in MS pathogenesis, as the expansion of CD56+ NK cells was previously correlated with the efficacy of daclizumab in MS patients29. Regulatory NK cells cytolyze activated T cells in a comparable way as cytotoxic NK cells, whereas they have no cytotoxicity towards resting T cells29. Since regulatory NK cells cytolyze activated T cells and their expansion is linked with the disease activity of MS patients, regulatory NK cells are clearly important in MS. As with daclizumab, the increase in regulatory NK cells is one of the beneficial mechanisms of DMF. Other mechanisms of DMF, including T cell apoptosis and inhibition of dendritic cell maturation, might indirectly be a consequence of this finding21,30.
Within the adaptive immune system, DMF treatment reduced percentages of pro-inflammatory immune cell subtypes such as (effector) memory (CD4+ and CD8+) T cells and (non) class-switched memory and DN B cells in the follow-up study. Furthermore, DMF treatment increased naive T and B cells and transitional B cells. This switch from a pro-inflammatory profile into an anti-inflammatory profile is in line with other studies19,26,31,32. Since the same results are obtained in different study populations of different countries (Spain, Germany, USA and Belgium) it appears that DMF has a rather stable immunological effect, although it has many mechanisms of action. In addition, our cross-sectional study emphasizes the long-lasting effect of DMF.
Medina et al. and Smith et al. reported a reduction of memory B cells and the latter also reported an increase in naive B cells at 6 m of treatment26,33. Our study additionally indicated a persistent effect of DMF after 12 m treatment on naive and memory B cells. In contrast to others, who reported no effect of DMF on transitional and Breg cells after 6 m of treatment26,33, we detected an increase in transitional B cells. This could indicate that the effect of DMF on transitional B cells is only detectable after a longer treatment period, which was confirmed by Lundy et al., who showed increased Breg cells in 4 of the 8 MS patients after 12 m of DMF treatment compared to 4–6 m of DMF treatment34. Furthermore, we can speculate that transitional and naive B cells are more resistant to DMF treatment than memory B cells, although we demonstrated that DMF directly induced B cell apoptosis without a preferential effect on memory or naive B cells. Indirect effects, such as the interplay between immune cells or the output of new B cells from the bone marrow, could be responsible for the findings of the follow-up study. Transitional B cells are enriched for Breg which could have beneficial effects in MS3. Furthermore, results from in vitro treatment of B cells with DMF induced increased Breg percentages, although not significantly. In addition, MMF induced a trend towards a decreased percentage of TNF-α+ B cells. Thus, the increased Breg percentage34 and decreased percentage of TNF-α+ B cells20,33 that were previously described after DMF treatment could be regulated in a direct manner. As Breg are known to suppress Th1 and Th17 differentiation while inducing Treg generation35, the decreased IFN-γ+ and IL-17+ CD4+ T cells after DMF treatment in the follow-up study could be an indirect consequence of an increase in Breg.
When considering CD4+ T cells expressing the anti-inflammatory cytokines IL-4 and IL-10, we could not find a difference after treatment with DMF. One explanation could be the great patient diversity in IL-4+ and IL-10+ CD4+ T cells. Because of this variety a population-wide conclusion about the effect of DMF on IL-4+ and IL-10+ CD4+ T cells cannot be made. Other studies also did not detect a difference in the percentage of IL-4+ or IL-10+ CD4+ T cells19,26,36. In contrast, Wu et al. detected an increase in IL-4+ T cells, although the percentage of IL-4+ T cells was much lower than detected in other studies32.
One of the limitations of our study is that we did not include memory CD4+ cytokine expressing cells. Since we observed a decrease in pro-inflammatory cytokine expressing CD4+ cells it is still possible that this reduction is due to the reduction of memory CD4+ cells observed in the follow-up study. Furthermore, it would have been interesting to assess CD8+ cytokine expressing T cells.
The direct effect of DMF on B cells was studied in more detail. Gillard et al. demonstrated that DMF and MMF are active between 5.5 and 50 µM and 50 and 150 µM, respectively. Therefore, DMF was used at different concentrations between 10 µM and 50 µM, while 50 µM of MMF was used37. DMF induced concentration-dependent B cell apoptosis in HC and untreated RRMS patients starting from a concentration of 25 µM DMF. As MMF did not have an effect on B cell apoptosis, DMF is responsible for the full effect on the reduction of B cell numbers. Although DMF could not be detected in serum of HC, a degree of systemic penetrance of DMF has been reported with DMF-glutathione conjugates found in urine of DMF-treated psoriasis patients38. Furthermore, Litjens et al. demonstrated that DMF could be taken up by lymphocytes and monocytes39.
Due to the fact that the exact concentration of DMF in the circulation or in immune cells of RRMS patients is not known and cannot be determined exactly, it is important to investigate the direct effects of DMF on B cells in low concentrations in vitro. Since Blewett et al. demonstrated that DMF directly interacts with cysteine residues of T cells in concentrations between 10 and 50 µM and that this is mechanistically relevant to the immunosuppressive activity of the drug and since we have demonstrated that no apoptosis is induced in concentrations below 25 µM, we examined whether DMF also exerted a direct effect on functional markers of B cells at a concentration of 10 µM40. In this study, we demonstrated that DMF directly decreased B cell expression of antigen presentation HLA-DR/DP/DQ and costimulatory molecule CD40. This could lead to reduced T cell activation, since B cells are critical antigen presenting cells in MS that can stimulate T cell proliferation to foreign or neuro-antigens4. Previous reports demonstrated increased CD40 ligand on MS T cells and hyper-responsiveness of MS memory B cells to CD40 stimulation41,42. Therefore, we suggest that the decreased B cell CD40 expression induced by DMF will result in disrupted B cell activation and thus B cell mediated pathology. Furthermore, these results could explain the decrease in memory T cells and reduced T cell proliferation and activation following DMF treatment23,27,31,32,43.
DMF directly reduced B cell expression of the survival marker BAFFR. However, BAFF serum levels were unchanged and B cell BAFFR expression was increased in the follow-up study. The latter effect could be due to increased transitional and naive B cells after DMF treatment. BAFF has been described to play a dual role in MS pathology including both pathological effects44 and positive effects on treatment outcome and detrimental effects of BAFF antagonists45. Interestingly, BAFFR expression was increased on B cells of untreated MS patients compared to HC. An increased BAFFR mRNA level was previously demonstrated in MS patients46. BAFF is important for maintaining antibody-producing plasma cells and the direct effect of DMF on BAFFR expression could be beneficial for MS pathogenesis.
In conclusion, this study shows that DMF treatment persistently changes the immune balance of MS patients and illustrates the multifactorial working mechanism of DMF. The effects of DMF elucidated here could help explain its therapeutic efficacy in MS.
Methods
Study subjects
RRMS patients and healthy controls (HC) were recruited at the Zuyderland Medical Center (Sittard, The Netherlands), Rehabilitation & MS-Center (Overpelt, Belgium) and Biomedical Research Institute (Diepenbeek, Belgium). All experimental protocols and methods were conducted in accordance with institutional guidelines and regulations and was approved by the institutes’ ethics committees (METC14-T-96, 15.143/neuro15.12) and all study subjects gave written informed consent. For the follow-up study, peripheral blood was collected before, after 3 m and 12 m of DMF treatment. Clinical outcome measures including the Expanded Disability Status Scale (EDSS) and the Fatigue Scale for Motor and Cognitive Functions (FSMC) were analyzed. Furthermore, the timed 25-foot walk (T25FW), the Paced Auditory Serial Addition Test (PASAT-3″) and the Nine Hole Peg test (NHPT) were analyzed according to the Multiple Sclerosis Functional Composite (MSFC). All samples were stored in the University Biobank Limburg. Further clinical details and numbers of MS patients and HC are provided in Table 1. In all analyses, HC were matched to MS patients as closely as possible with regard to age and sex.
Cell isolation
Peripheral blood mononuclear cells (PBMC) were isolated from whole blood by Ficoll density gradient centrifugation (Lympholyte, Cedarlane Laboratories, Uden, The Netherlands). PBMC of MS patients and HC were cryopreserved and thawed before use, unless stated otherwise. PBMC collected at different time points during the follow-up study were simultaneously thawed to exclude inter-assay variation. PBMC were cryopreserved in 10% dimethyl sulfoxide (DMSO, Sigma Aldrich, Overijse, Belgium) and fetal bovine serum (FBS, Life Technologies, Gent, Belgium) using a slow temperature-lowering method (Coolcell®, VWR, Haasrode, Belgium). After 24 h, cryovials were transferred to liquid N2 until analysis. PBMC were thawed by bringing the temperature of the cryovials to 0 °C in a water bath (37 °C). Hereafter, cold thawing medium consisting of 20% FBS in RPMI 1640 (Lonza, Basel, Switzerland) was added and PBMC were centrifuged at 4 °C. PBMC were suspended in thawing medium in a concentration of 10 × 106 cells/1000 µl containing DNAse (1/100) and incubated at 37 °C for 10 minutes. Subsequently, cells were washed twice. Median cell recovery was 76 ± 18%. B cells were purified from freshly isolated PBMC by negative magnetic selection (STEMCELL Technologies SARL, Grenoble, France). Purity of B cells was routinely ≥98.4%.
Flow cytometry
In the follow-up study, PBMC were stained with anti-human CD14 PerCP, CD16 FITC, CD56 PE-Cy7 (BD Biosciences, Erembodegem, Belgium) and CD3 APC-Cy7 (BioLegend, Antwerp, Belgium). B cell subtypes were analyzed using anti-human CD19 PE-Cy7, immunoglobulin (Ig)M PerCP-Cy5.5 (BioLegend), CD27 APC, IgD PE-CF594, IgG FITC (BD Biosciences), CD38 APC-ef780 (eBioscience, San Diego, USA) and IgA PE (Miltenyi Biotec, Leiden, The Netherlands). T cell subtypes were analyzed with anti-human CD4 APC (BioLegend), CD45RA APC-ef780, CD127 PE, CD25 PE-Cy7 (eBioscience), CD45RO PE-CF594, CD8 FITC and CCR7 PerCP-Cy5.5 (BD Biosciences). Expression of B-cell activating factor receptor (BAFFR) on B cells was assessed using CD19 PE-Cy7, CD268 APC-Cy7 (BAFFR) (BioLegend).
For intracellular T cell cytokine analysis, PBMC were stimulated with phorbol 12-myristate 13-acetate (PMA, 50 ng/ml) and calcium ionomycin (1 µg/ml, Sigma-Aldrich, Overijse Belgium) in the presence of Golgistop (BD Biosciences) for 4 h. PBMC were fixed and permeabilized using Cytofix/Cytoperm (BD Biosciences) and stained with anti-human IL-10 PE-Cy7, GM-CSF APC, CD4 PE-Cy5 (BioLegend), IL-4 PE, IL-17 FITC (eBioscience) and IFN-γ PE-CF594 (BD Biosciences). Freshly isolated PBMC were analyzed in the cross-sectional study using anti-human CD4 APC, CD8 PE, CD45RO (BD Biosciences). Apoptosis and BAFFR expression of in vitro cultured B cells was measured using anti-human CD19 PE-Cy7, CD268 APC-Cy7 (BioLegend), CD27 APC, IgG PE, IgD PE-CF594 (BD Biosciences), annexin V FITC and 7-AAD (eBioscience). Expression of costimulatory and antigen presentation molecules was assessed using anti-human CD19 PerCP-Cy5.5, CD27 APC, CD80 PE, CD86 PE-CF594, HLA-DR/DP/DQ FITC (BD Biosciences) and CD40 PE-Cy7 (eBioscience). When analyzing frequency of Breg and pro-inflammatory B cells, B cells were restimulated during the last 4 h with PMA (50 ng/ml) and calcium ionomycin (1 µg/ml, both Sigma-Aldrich) in the presence of GolgiStop (BD Biosciences). Fixation and permeabilization were done using Cytofix/Cytoperm (BD Biosciences). The antibodies used were anti-human CD19 BV421, CD24 PE-Cy7, IL-10 Brilliant Violet 711 (all from BD Biosciences), CD38 APC-ef780, Fixable Viability Dye-eFluor506 (all from eBioscience) and TNF-α PerCP-Cy5.5 (BioLegend). All flow cytometry was performed on a FACSAria II or LSRFortessa flow cytometer using FACSDiva software (BD Biosciences). Absolute numbers of leukocytes, lymphocytes, monocytes and neutrophils were obtained from standard clinical lab tests for 7 DMF-treated MS patients. Absolute numbers of lymphocyte subtype populations were calculated as follows: absolute lymphocyte number (109/l) × percentage of lymphocyte subtype population × 10.
In vitro B cell cultures
B cells were cultured in 96-well round-bottom plates (Greiner Bio-One B.V., Alphen aan den Rijn, The Netherlands) at 3 × 105 cells in RPMI 1640 (Lonza, Basel, Switzerland) with 10% fetal bovine serum (FBS, Life Technologies, Gent, Belgium), 50 U/ml penicillin, 50 mg/ml streptomycin (Invitrogen, Carlsbad, CA), 0.1 mM nonessential amino acids and 1 mM sodium pyruvate (Sigma-Aldrich). For analysis of B cell apoptosis, survival marker and expression of costimulatory and antigen presentation molecules, B cells were treated during 24 h with DMF (10 µM–50 µM), MMF (50 µM) or were left untreated (DMSO). After 1 h, B cells were stimulated with 2 µg/ml CpG2006 (ODN2006, Invivogen, Toulouse, France) for the following 23 h. When measuring the frequency of Breg and pro-inflammatory B cells, purified B cells were treated during 48 h with DMF (10 µM), MMF (50 µM) or were left untreated in the presence of 1 µg/ml soluble CD40 ligand together with 2 µg/ml CpG2006.
ELISA
BAFF plasma levels were quantified before and after 12 m of DMF treatment using a commercial ELISA kit according to the manufacturer’s protocol (R&D systems, Abingdon, United Kingdom).
Statistics
Statistical analyses were performed using Graphpad Prism 6. Wilcoxon matched-pairs signed rank test was used for comparison of variables at baseline and follow-up and for comparing cells with or without DMF treatment (in vitro). When comparing multiple groups, one-way ANOVA (Kruskal-Wallis) was used. A p value less than 0.05 was considered statistically significant.
References
Dittel, B. N. CD4 T cells: Balancing the coming and going of autoimmune-mediated inflammation in the CNS. Brain, behavior, and immunity 22, 421–430, https://doi.org/10.1016/j.bbi.2007.11.010 (2008).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. The New England journal of medicine 358, 676–688, https://doi.org/10.1056/NEJMoa0706383 (2008).
Claes, N., Fraussen, J., Stinissen, P., Hupperts, R. & Somers, V. B. Cells Are Multifunctional Players in Multiple Sclerosis Pathogenesis: Insights from Therapeutic Interventions. Frontiers in immunology 6, 642, https://doi.org/10.3389/fimmu.2015.00642 (2015).
Fraussen, J. et al. B cells of multiple sclerosis patients induce autoreactive proinflammatory T cell responses. Clinical immunology (Orlando, Fla.) 173, 124–132, https://doi.org/10.1016/j.clim.2016.10.001 (2016).
Kivisakk, P. et al. Expression of CCR7 in multiple sclerosis: implications for CNS immunity. Annals of neurology 55, 627–638, https://doi.org/10.1002/ana.20049 (2004).
MacLeod, M. K., Kappler, J. W. & Marrack, P. Memory CD4 T cells: generation, reactivation and re-assignment. Immunology 130, 10–15, https://doi.org/10.1111/j.1365-2567.2010.03260.x (2010).
Neumann, H., Medana, I. M., Bauer, J. & Lassmann, H. Cytotoxic T lymphocytes in autoimmune and degenerative CNS diseases. Trends in neurosciences 25, 313–319 (2002).
Wulff, H. et al. The voltage-gated Kv1.3 K(+) channel in effector memory T cells as new target for MS. The Journal of clinical investigation 111, 1703–1713, https://doi.org/10.1172/jci16921 (2003).
Noster, R. et al. IL-17 and GM-CSF expression are antagonistically regulated by human T helper cells. Science translational medicine 6, 241ra280, https://doi.org/10.1126/scitranslmed.3008706 (2014).
Claes, N. et al. Age-Associated B Cells with Proinflammatory Characteristics Are Expanded in a Proportion of Multiple Sclerosis Patients. Journal of immunology (Baltimore, Md.: 1950) 197, 4576–4583, https://doi.org/10.4049/jimmunol.1502448 (2016).
Gjelstrup, M. C. et al. Subsets of activated monocytes and markers of inflammation in incipient and progressed multiple sclerosis. Immunology and cell biology 96, 160–174, https://doi.org/10.1111/imcb.1025 (2018).
Glennon-Alty, L., Hackett, A. P., Chapman, E. A. & Wright, H. L. Neutrophils and redox stress in the pathogenesis of autoimmune disease. Free radical biology & medicine, https://doi.org/10.1016/j.freeradbiomed.2018.03.049 (2018).
Kastrukoff, L. F. et al. Clinical relapses of multiple sclerosis are associated with ‘novel’ valleys in natural killer cell functional activity. Journal of neuroimmunology 145, 103–114 (2003).
Naegele, M. et al. Neutrophils in multiple sclerosis are characterized by a primed phenotype. Journal of neuroimmunology 242, 60–71, https://doi.org/10.1016/j.jneuroim.2011.11.009 (2012).
Fox, R. J. et al. Placebo-controlled phase 3 study of oral BG-12 or glatiramer in multiple sclerosis. The New England journal of medicine 367, 1087–1097, https://doi.org/10.1056/NEJMoa1206328 (2012).
Gold, R. et al. Placebo-controlled phase 3 study of oral BG-12 for relapsing multiple sclerosis. The New England journal of medicine 367, 1098–1107, https://doi.org/10.1056/NEJMoa1114287 (2012).
Gerdes, S., Shakery, K. & Mrowietz, U. Dimethylfumarate inhibits nuclear binding of nuclear factor kappaB but not of nuclear factor of activated T cells and CCAAT/enhancer binding protein beta in activated human T cells. The British journal of dermatology 156, 838–842, https://doi.org/10.1111/j.1365-2133.2007.07779.x (2007).
Linker, R. A. et al. Fumaric acid esters exert neuroprotective effects in neuroinflammation via activation of the Nrf2 antioxidant pathway. Brain: a journal of neurology 134, 678–692, https://doi.org/10.1093/brain/awq386 (2011).
Gross, C. C. et al. Dimethyl fumarate treatment alters circulating T helper cell subsets in multiple sclerosis. Neurology(R) neuroimmunology & neuroinflammation 3, e183, https://doi.org/10.1212/nxi.0000000000000183 (2016).
Li, R. et al. Dimethyl Fumarate Treatment Mediates an Anti-Inflammatory Shift in B Cell Subsets of Patients with Multiple Sclerosis. Journal of immunology (Baltimore, Md.: 1950) 198, 691–698, https://doi.org/10.4049/jimmunol.1601649 (2017).
Ghadiri, M. et al. Dimethyl fumarate-induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurology(R) neuroimmunology & neuroinflammation 4, e340, https://doi.org/10.1212/nxi.0000000000000340 (2017).
Amato, M. P., Zipoli, V. & Portaccio, E. Multiple sclerosis-related cognitive changes: a review of cross-sectional and longitudinal studies. Journal of the neurological sciences 245, 41–46, https://doi.org/10.1016/j.jns.2005.08.019 (2006).
Fleischer, V. et al. Treatment response to dimethyl fumarate is characterized by disproportionate CD8+ T cell reduction in MS. Multiple sclerosis (Houndmills, Basingstoke, England), 1352458517703799, https://doi.org/10.1177/1352458517703799 (2017).
Khatri, B. O. et al. The effect of dimethyl fumarate (Tecfidera) on lymphocyte counts: A potential contributor to progressive multifocal leukoencephalopathy risk. Multiple sclerosis and related disorders 4, 377–379, https://doi.org/10.1016/j.msard.2015.05.003 (2015).
Spencer, C. M., Crabtree-Hartman, E. C., Lehmann-Horn, K., Cree, B. A. & Zamvil, S. S. Reduction of CD8(+) T lymphocytes in multiple sclerosis patients treated with dimethyl fumarate. Neurology(R) neuroimmunology & neuroinflammation 2, e76, https://doi.org/10.1212/nxi.0000000000000076 (2015).
Medina, S. et al. Optimal response to dimethyl fumarate associates in MS with a shift from an inflammatory to a tolerogenic blood cell profile. Multiple sclerosis (Houndmills, Basingstoke, England), 1352458517717088, https://doi.org/10.1177/1352458517717088 (2017).
Chaves, C., Ganguly, R., Ceresia, C. & Camac, A. Lymphocyte subtypes in relapsing-remitting multiple sclerosis patients treated with dimethyl fumarate. Multiple sclerosis journal - experimental, translational and clinical 3, 2055217317702933, https://doi.org/10.1177/2055217317702933 (2017).
Cooper, M. A. et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood 97, 3146–3151 (2001).
Bielekova, B. et al. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proceedings of the National Academy of Sciences of the United States of America 103, 5941–5946, https://doi.org/10.1073/pnas.0601335103 (2006).
Peng, H. et al. Dimethyl fumarate inhibits dendritic cell maturation via nuclear factor kappaB (NF-kappaB) and extracellular signal-regulated kinase 1 and 2 (ERK1/2) and mitogen stress-activated kinase 1 (MSK1) signaling. The Journal of biological chemistry 287, 28017–28026, https://doi.org/10.1074/jbc.M112.383380 (2012).
Longbrake, E. E. et al. Dimethyl fumarate selectively reduces memory T cells in multiple sclerosis patients. Multiple sclerosis (Houndmills, Basingstoke, England) 22, 1061–1070, https://doi.org/10.1177/1352458515608961 (2016).
Wu, Q. et al. Dimethyl Fumarate Selectively Reduces Memory T Cells and Shifts the Balance between Th1/Th17 and Th2 in Multiple Sclerosis Patients. Journal of immunology (Baltimore, Md.: 1950) 198, 3069–3080, https://doi.org/10.4049/jimmunol.1601532 (2017).
Smith, M. D., Martin, K. A., Calabresi, P. A. & Bhargava, P. Dimethyl fumarate alters B-cell memory and cytokine production in MS patients. Annals of clinical and translational neurology 4, 351–355, https://doi.org/10.1002/acn3.411 (2017).
Lundy, S. K. et al. Dimethyl fumarate treatment of relapsing-remitting multiple sclerosis influences B-cell subsets. Neurology(R) neuroimmunology & neuroinflammation 3, e211, https://doi.org/10.1212/nxi.0000000000000211 (2016).
Flores-Borja, F. et al. CD19 + CD24hiCD38hi B cells maintain regulatory T cells while limiting TH1 and TH17 differentiation. Science translational medicine 5, 173ra123, https://doi.org/10.1126/scitranslmed.3005407 (2013).
Longbrake, E. E. et al. Dimethyl fumarate induces changes in B- and T-lymphocyte function independent of the effects on absolute lymphocyte count. Multiple sclerosis (Houndmills, Basingstoke, England), 1352458517707069, https://doi.org/10.1177/1352458517707069 (2017).
Gillard, G. O. et al. DMF, but not other fumarates, inhibits NF-kappaB activity in vitro in an Nrf2-independent manner. Journal of neuroimmunology 283, 74–85, https://doi.org/10.1016/j.jneuroim.2015.04.006 (2015).
Rostami-Yazdi, M., Clement, B., Schmidt, T. J., Schinor, D. & Mrowietz, U. Detection of metabolites of fumaric acid esters in human urine: implications for their mode of action. The Journal of investigative dermatology 129, 231–234, https://doi.org/10.1038/jid.2008.197 (2009).
Litjens, N. H. et al. In vitro pharmacokinetics of anti-psoriatic fumaric acid esters. BMC pharmacology 4, 22, https://doi.org/10.1186/1471-2210-4-22 (2004).
Blewett, M. M. et al. Chemical proteomic map of dimethyl fumarate-sensitive cysteines in primary human T cells. Science signaling 9, rs10, https://doi.org/10.1126/scisignal.aaf7694 (2016).
Ireland, S. J. et al. Antibody-independent B cell effector functions in relapsing remitting multiple sclerosis: clues to increased inflammatory and reduced regulatory B cell capacity. Autoimmunity 45, 400–414, https://doi.org/10.3109/08916934.2012.665529 (2012).
Kosmaczewska, A. et al. Different patterns of activation markers expression and CD4+ T-cell responses to ex vivo stimulation in patients with clinically quiescent multiple sclerosis (MS). Journal of neuroimmunology 189, 137–146, https://doi.org/10.1016/j.jneuroim.2007.06.021 (2007).
Schloder, J., Berges, C., Luessi, F. & Jonuleit, H. Dimethyl Fumarate Therapy Significantly Improves the Responsiveness of T Cells in Multiple Sclerosis Patients for Immunoregulation by Regulatory T Cells. International journal of molecular sciences 18, https://doi.org/10.3390/ijms18020271 (2017).
Huntington, N. D. et al. A BAFF antagonist suppresses experimental autoimmune encephalomyelitis by targeting cell-mediated and humoral immune responses. International immunology 18, 1473–1485, https://doi.org/10.1093/intimm/dxl080 (2006).
Hartung, H. P. & Kieseier, B. C. Atacicept: targeting B cells in multiple sclerosis. Therapeutic advances in neurological disorders 3, 205–216, https://doi.org/10.1177/1756285610371146 (2010).
Thangarajh, M., Gomes, A., Masterman, T., Hillert, J. & Hjelmstrom, P. Expression of B-cell-activating factor of the TNF family (BAFF) and its receptors in multiple sclerosis. Journal of neuroimmunology 152, 183–190, https://doi.org/10.1016/j.jneuroim.2004.03.017 (2004).
Acknowledgements
We thank Igna Rutten and Kim Ulenaers (Hasselt University, Biomedical Research Institute and UBilim), Rehabilitation & MS Center (Overpelt), Sandra Liedekerken, Tiny Kempkens, Judith Poeth, Lianne van Gennip and all MS nurses at Zuyderland Medisch Centrum (Sittard) for patient recruitment and sample collection. We thank prof. dr. Niels Hellings for critical reading. This work was supported by Hasselt University and Maastricht University. Funding was provided via an investigator-initiated trial grant from Biogen. J. Fraussen is a postdoctoral fellow of the Fund for Scientific Research, Flanders.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: G.M.D., J.F., V.S. Performed the experiments and analyzed the data: G.M.D. Contributed reagents/materials/analysis tools: R.H., B.V.W. Wrote the paper: G.M.D., J.F., B.V.W., R.H., V.S.
Corresponding author
Ethics declarations
Competing Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: G.M.D., J.F., V.S.: nothing to disclose; B.V.W.: received Research and Travel Grants, Honoraria for MS-Expert Advise and Speakers Fees from Bayer-Schering, Biogen, Sanofi Genzyme, Merck-Serono, Novartis, Roche and TEVA; R.H.: received research grants and honoraria for advisory boards from BIOGEN, Merck and Sanofi-Genzyme.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Montes Diaz, G., Fraussen, J., Van Wijmeersch, B. et al. Dimethyl fumarate induces a persistent change in the composition of the innate and adaptive immune system in multiple sclerosis patients. Sci Rep 8, 8194 (2018). https://doi.org/10.1038/s41598-018-26519-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26519-w
This article is cited by
-
Effect of siponimod on lymphocyte subsets in active secondary progressive multiple sclerosis and clinical implications
Journal of Neurology (2024)
-
Dimethyl fumarate decreases short-term but not long-term inflammation in a focal EAE model of neuroinflammation
EJNMMI Research (2022)
-
Fumarate suppresses B-cell activation and function through direct inactivation of LYN
Nature Chemical Biology (2022)
-
The immunology of multiple sclerosis
Nature Reviews Immunology (2022)
-
Targeting immunometabolism as an anti-inflammatory strategy
Cell Research (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.