Abstract
Little is known about advanced glycation end products (AGEs) participation in glucose homeostasis, a process in which skeletal muscle glucose transporter GLUT4 (Scl2a4 gene) plays a key role. This study investigated (1) the in vivo and in vitro effects of AGEs on Slc2a4/GLUT4 expression in skeletal muscle of healthy rats, and (2) the potential involvement of endoplasmic reticulum and inflammatory stress in the observed regulations. For in vivo analysis, rats were treated with advanced glycated rat albumin (AGE-albumin) for 12 weeks; for in vitro analysis, soleus muscles from normal rats were incubated with bovine AGE-albumin for 2.5 to 7.5 hours. In vivo, AGE-albumin induced whole-body insulin resistance; decreased (~30%) Slc2a4 mRNA and GLUT4 protein content; and increased (~30%) the nuclear content of nuclear factor NF-kappa-B p50 subunit (NFKB1), and cellular content of 78 kDa glucose-regulated protein (GRP78). In vitro, incubation with AGE-albumin decreased (~50%) the Slc2a4/GLUT4 content; and increased cellular content of GRP78/94, phosphorylated-IKK-alpha/beta, nuclear content of NFKB1 and RELA, and the nuclear protein binding into Slc2a4 promoter NFKB-binding site. The data reveal that AGEs impair glucose homeostasis in non-diabetic states of increased AGEs concentration; an effect that involves activation of endoplasmic reticulum- and inflammatory-stress and repression of Slc2a4/GLUT4 expression.
Similar content being viewed by others
Introduction
Advanced glycation end products (AGEs) have been extensively implicated in the genesis and progression of diabetes-related complications (Brownlee 2001). Additionally, AGEs might also contribute to glycemic impairment, by activating oxidative, endoplasmic reticulum (ER) and inflammatory stress in tissues related to insulin-regulated plasma glucose disposal1. Regarding that, some studies have suggested that AGEs can impair adipocyte glucose disposal in vitro2,3. However, in the major site of insulin-regulated glucose disposal, the skeletal muscle4, the effects of AGEs upon the glucose uptake markers, both in vivo and in vitro, are not known.
In muscles, insulin-stimulated glucose uptake is performed through the solute carrier family 2, facilitated glucose transporter member 4 (GLUT4), which is rapidly translocated to the plasma membrane in response to the hormone5. Besides, muscle contraction can also stimulate GLUT4 translocation, which operates in addition to insulin6. Although reduced GLUT4 translocation impairs skeletal muscle glucose uptake characterizing insulin resistance7, long-term established insulin resistance has currently been related to a defective glucose transporter gene and/or protein expression8,9.
AGEs interact with AGER (advanced glycosylation end product-specific receptor, former RAGE), activating the NFKB (nuclear factor NF-kappa-B) pathway and the expression of inflammatory genes1,10. By inducing inflammation, AGEs trigger ER-stress, which once activated, elicits the unfolded protein response (UPR) in order to restore cellular homeostasis1,10. In this process, a rapid activation of several chaperone networks is an early event, which can be monitored by the expression of GRP78 (78 kDa glucose-regulated protein) and GRP94 (endoplasmin/94 kDA glucose-regulated protein) proteins11,12. AGE/AGER interaction, directly or indirectly (via UPR), activates the inflammatory pathway, modulating distinct steps such as phosphorylation of IKKA and IKKB (inhibitor of nuclear factor kappa-B kinase, subunits alpha and beta, respectively), degradation of IKBA and IKBB (nuclear factor kappa-B inhibitor alpha and beta, respectively), and nuclear translocation of NFKB11,12. Recently, it was definitely demonstrated that, in nucleus, both NFKB1 (nuclear factor NF-kappa-B p105 subunit) and RELA (nuclear factor NF-kappa-B p65 subunit) bind into the promoter region of Slc2a4 gene, repressing its transcription, and hence decreasing GLUT4 expression13. Thus, in a converging way, AGEs-stimulated ER and inflammatory stress might reduce skeletal muscle glucose disposal, contributing to glucose homeostasis impairment.
On the other hand, the interaction of AGEs with DDOST (dolichyl-diphosphooligosaccharide-protein glycosyltransferase 48 kDa subunit, former AGER1) can counterbalance the AGER-mediated deleterious pathways14 by inducing antioxidant mechanisms.
In diabetes (DM), AGE formation increases as a consequence of hyperglycemia and oxidative stress; however, their own effects on glucose homeostasis have not yet been investigated. Besides, AGEs can also be obtained from exogenous sources, such as high fat/sugar heat-processed foods, abundant in modern diets15, reinforcing the importance of knowing its effects on glucose homeostasis. We hypothesized that AGEs, independent of the occurrence of hyperglycemia, can modulate skeletal muscle glucose disposal by altering GLUT4 expression, thus impairing glucose homeostasis. Considering that, the present study aimed to investigate: (1) in vivo, the chronic effects of AGEs on glucose homeostasis, and on the expression of Slc2a4/GLUT4 and molecular markers of both UPR and inflammation in soleus muscle; and (2) in vitro, the direct effects of AGEs on expression of Slc2a4/GLUT4 and molecular markers of both UPR and inflammation in soleus muscle.
Results
Treatment of rats with AGE-albumin induced insulin resistance
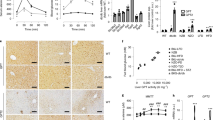
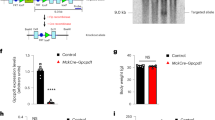
As previously described16, twelve-week treatment with AGE-albumin reduced the glucose decay constant rate (kITT) during the insulin tolerance test (Fig. 1D); however, the treatment did not alter rat body mass gain, blood glucose or plasma insulin concentrations (Fig. 1A–C). Our hypothesis that this insulin resistance involves a direct effect of AGE-albumin in soleus muscle was supported by the detection of reduced expression of Ddost gene (Fig. 1E). Besides, participation of reduced skeletal muscle glucose disposal in the whole-body insulin resistance was evinced by the repression of Slc2a4 mRNA and GLUT4 protein expression (Fig. 1F).
Treatment of rats with advanced glycated albumin induces insulin resistance and represses Slc2a4/GLUT4 expression. Body weight gain (A), blood glucose (B), plasma insulin (C), blood glucose decay and constant rate (kITT) during insulin tolerance test (D), skeletal muscle expression of advanced glycosylation end product-specific receptor (Ager), and of dolichyl-diphosphooligosaccharide-protein glycosyltransferase non-catalytic subunit (Ddost) mRNAs (E), solute carrier family 2 member 4 (Slc2a4) mRNA, and solute carrier family 2 facilitated glucose transporter member 4 (GLUT4) protein (F) were measured in rats chronically treated with advanced glycated- (AGE; black bars; closed circles) or control- (C; white bars; open circles) rat albumin. Representative autoradiograms and Ponceau S staining of respective lanes, used as protein loading control, are shown in (F). Data are mean ± SEM of 4 (panel D) or 7 (panels A–C,E and F) animals. Means were compared by unpaired two-tailed t test. *P < 0.05 and **P < 0.01 vs control-albumin.
Treatment of rats with AGE-albumin induced markers of reticulum endoplasmic and inflammatory stress in skeletal muscle
In vivo treatment with AGE-albumin increased the expression of GRP78 chaperone (Fig. 2A), revealing ER stress activation. Phosphorylation of IKKA and IKKB (Fig. 2B) and IKBA and IKBB content (Fig. 2C) were unchanged; however nuclear content of NFKB1 (Fig. 3) was increased, indicating the activation of a fundamental inflammatory pathway.
Treatment of rats with advanced glycated albumin activates markers of endoplasmic reticulum and inflammatory stress in skeletal muscle. Markers of endoplasmic reticulum stress: 78 kDa glucose-regulated protein (GRP78) and 94 kDa glucose-regulated protein (GRP94) (A), and markers of inflammatory stress: inhibitor of nuclear factor kappa-B kinase subunits alpha (IKKA) and beta (IKKB) (B), and nuclear factor kappa-B inhibitors alpha (IKBA) and beta (IKBB) (C) were measured in skeletal muscles of rats chronically treated with advanced glycated- (AGE; black bars) or control- (C; white bars) rat albumin. In each panel, representative autoradiograms and Ponceau S staining of respective lanes, used as protein loading control, are shown. Data are mean ± SEM of 7 animals. Means were compared by unpaired two-tailed t test. **P < 0.01 vs control-albumin.
Treatment of rats with advanced glycated albumin activates nuclear factor NF-kappa-B in skeletal muscle. Nuclear factor NF-kappa-B p50 protein (NFKB1) (A,B) and nuclear factor NF-kappa-B p65 protein (RELA) (A,C) contents were measured in cytosolic and nuclear subcellular fractions from skeletal muscles of rats chronically treated with advanced glycated albumin- (AGE; black bars) or control- (C; white bars) rat albumin. In (A), representative autoradiograms and Ponceau S staining of respective lanes, used as protein loading control, are shown. Data are mean ± SEM of 7 animals. Means were compared by unpaired two-tailed t test. *P < 0.05 vs control-albumin.
In vitro treatment of rat skeletal muscle with AGE-albumin repressed GLUT4 expression
To confirm a direct effect of AGE-albumin in skeletal muscle, soleus muscles from untreated healthy rats were incubated with AGE- or C-albumin. After 2.5-hour incubation, AGE-albumin decreased (50%) the Slc2a4 mRNA expression (Fig. 4A); but this period was not enough to decrease the total amount of cellular GLUT4 protein content. Nevertheless, extension of the incubation period to 5 and 7.5 hours (Fig. 4B and C) induced a progressive decrease in GLUT4 expression, which reached a 25% reduction (P < 0.05) after 7.5-hour challenge with AGE-albumin.
In vitro treatment of rat skeletal muscle with advanced glycated albumin represses Slc2a4/GLUT4 expression. Solute carrier family 2 member 4 (Slc2a4) mRNA (A) and solute carrier family 2 facilitated glucose transporter member 4 (GLUT4) protein (B,C) were measured in skeletal muscles of untreated rats incubated with advanced glycated- (left muscle; AGE; black bars) or control- (right muscle; C; white bars) bovine albumin during 2.5 hours. For GLUT4 analysis, incubation periods were additionally extended to 5 and 7.5 hours, and experiments of each period were performed separately (B,C). In (B), representative autoradiograms and Ponceau S staining of respective lanes, used as protein loading control, are shown. Data are mean ± SEM of 5 (GLUT4 protein) or 8 (Slc2a4 mRNA) animals. Means were compared by paired two-tailed t test. *P < 0.05 and ***P < 0.001 vs control-albumin.
Considering that AGE-albumin treatment might also impair GLUT4 vesicles translocation, we tested the glucose uptake in muscles incubated for 2.5 hours, when GLUT4 content was still unaltered. The results revealed that neither the basal (2.017 ± 0.185 vs 2.15 ± 0.21 μmol/g tissue, control- vs AGE-albumin, respectively; n = 6; P = 0.979) nor the insulin-stimulated (4.80 ± 0.326 vs 4.62 ± 0.213 μmol/g tissue, control- vs AGE-albumin, respectively; n = 6; P = 0.948) glucose uptake was altered by the presence of AGE-albumin, although the positive effect of insulin (P < 0.001) was observed in both conditions.
In vitro treatment of rat skeletal muscle with AGE-albumin induced endoplasmic reticulum and inflammatory stress markers
In order to specify the role of AGE-albumin, the expression of AGE receptors was investigated. The results show that Ager mRNA expression increased in muscles incubated (2.5 hours) with AGE-albumin (Fig. 5A).
In vitro treatment of rat skeletal muscle with advanced glycated albumin activates endoplasmic reticulum stress and inflammatory pathways. Advanced glycosylation end product-specific receptor (Ager) and dolichyl-diphosphooligosaccharide-protein glycosyltransferase non catalytic subunit (Ddost) mRNAs (A); 78 kDa glucose-regulated protein (GRP78) and 94 kDa glucose-regulated protein (GRP94) (B); phosphorylated inhibitor of nuclear factor kappa-B kinase subunits alpha (IKKA) and beta (IKKB) (C), and nuclear factor kappa-B inhibitors alpha (IKBA) and beta (IBB) (D) were measured in muscles of untreated rats incubated with advanced glycated- (left muscle; AGE; black bars) or control- (right muscle; C; white bars) bovine albumin for 2.5 hours. On the left side of panels B, C and D, representative autoradiograms and Ponceau S staining of respective lanes, used as protein loading control, are shown. Data are mean ± SEM of 6 (p-IKKA, p-IKKB and IKKA); 7 (Ager, Ddost and IKKB); or 8 (GRP78 and GRP94) animals. Means were compared by paired two-tailed t test. *P < 0.05, **P < 0.01 and ***P < 0.001vs control-albumin.
Two-and-a-half-hour incubation of muscles with AGE-albumin increased the expression of chaperones GRP78 and GRP94 proteins (Fig. 5B), revealing the activation of UPR. Besides, the results show that phosphorylation of IKKA and IKKB (Fig. 5C) increased, whereas the cellular content of IKBA and IKBB decreased (Fig. 5D), revealing the rapid activation of inflammatory pathway.
In vitro treatment of rat skeletal muscle with AGE-albumin increased nuclear protein binding into NFKB-binding site of Slc2a4 gene promoter
Firstly, we confirmed that the NFKB pathway activation culminates in increased nuclear content of both NFKB1 and RELA subunits of NFKB (Fig. 6A–C).
In vitro treatment of rat skeletal muscle with advanced glycated albumin increases nuclear content of NFKB1 and RELA proteins, and the nuclear proteins binding into Slc2a4 promoter NFKB-binding site. Nuclear factor NF-kappa-B p50 protein (NFKB1) (A,B) and nuclear factor NF-kappa-B p65 protein (RELA) (A,C) proteins were measured in cytosolic and nuclear subcellular fractions. Nuclear protein binding into NFKB-binding site of Slc2a4 gene promoter was analyzed by electrophoretic mobility shift assay and revealed two protein/DNA complexes a and b (D), which were separately quantified (E). Muscles were incubated for 2.5 hours with advanced glycated- (AGE, black bars) or control- (C, white bars) bovine albumin. In (A), representative autoradiograms and Ponceau S staining of respective lanes, used as protein loading control, are shown; and were cropped from different membranes. Data are mean ± SEM of 7 animals. Means were compared by paired two-tailed t test. *P < 0.05 vs control-albumin.
Once established that 2.5-hour incubation of muscle with AGE-albumin activated the NFKB inflammatory pathway, culminating with nuclear translocation of NFKB1 and RELA proteins, we investigated the nuclear protein binding into the NFKB-binding site of Slc2a4 gene promoter region (Fig. 6D,E). As previously described13, two protein/DNA complexes were detected, and both complex “a” and complex “b” increased by 100% and 50%, respectively, in response to AGE-albumin.
Discussion
AGEs are prevalent in DM and represent one of the basis for the development of long-term DM complications17. Albumin, the most abundant plasmatic protein, when modified by glycation displays an important role in cellular and tissue damage18. In the present study, we investigated whether glycated-albumin, regardless of a high glucose milieu, could modulate GLUT4 expression in skeletal muscle, which represents the latest downstream step for insulin-induced glucose disposal. In healthy subjects, skeletal muscle accounts for up to ~80% of glucose disposal under insulin-stimulated conditions, playing a fundamental role in glycemic homeostasis19. Regarding that, reduced GLUT4 expression in skeletal muscle was extensively reported in experimental models of type 2 DM (T2DM) in mice20,21,22,23,24. In human T2DM, although some pioneering studies have failed to detect reduced SLC2A4/GLUT4 expression in skeletal muscle25,26,27, this was firstly reported by Dohm and colleagues28, and further definitely confirmed by studies employing more sensitive analyses of GLUT4 quantification8,29,30. Furthermore, studies that have been investigated epigenetic regulation of Slc2a4 gene in muscles from T2DM patients have now given attention for gene repression in this condition30,31.
According to the hypothesis that AGEs contribute to glycemic control impairment, reduced insulin-induced glucose uptake by skeletal muscle of animals fed a high AGE content diet was already reported32,33. Besides, by intraperitoneal injection of AGE-albumin, we have recently reported that AGEs impair whole-body insulin sensitivity16, which now can be ascribed to a reduction in skeletal muscle Slc2a4/GLUT4 expression.
In order to clarify the mechanisms potentially involved in this GLUT4 regulation, we firstly measured the expression of AGEs receptors Ager and Ddost. Treatment with AGE-albumin reduced the expression of Ddost, a regulation already described in mice subjected to chronic ingestion of oral AGEs32. Cellular effects of AGEs include a convergent activation of oxidative, ER and inflammatory stress12. Accordingly, AGE-albumin treated rats showed increased cellular GRP78 and nuclear NFKB1 content, respective markers of ER and inflammation stress, which can be responsible for impaired Slc2a4/GLUT4 expression.
In in vitro incubations, a direct effect of AGE-albumin in soleus muscle was demonstrated by the Slc2a4 mRNA reduction after 2.5-hour AGE-albumin incubation, which reflected on decreased GLUT4 protein 2.5 hours later. Increased expression of Slc2a4 mRNA and GLUT4 protein has been observed as soon as 30 min to 120 min, respectively, after an enhancer stimulus33,34,35. However, decreasing effects have been described to occur later on; especially for GLUT4 protein, since the repressor effect depends not only on the transcriptional/translational inhibition, but also on the mRNA/protein half-life.
Decreased GLUT4 content was reported in muscle after 3-hour incubation with tumor necrosis factor alpha36, and in L6 muscle cells after 16-hour culture with linoleic or oleic fatty acids37. Here, GLUT4 protein reduction was detected 5 hours after muscle incubation with AGE-albumin, and that became more evident after 7.5 hours. These results can explain the AGE-induced reduction of glucose uptake described in L6 and in C2C12 cells after 8-hour AGEs treatment3,38.
Although the powerful effect of GLUT4 reduced expression in the AGE-induced impairment in muscle glucose disposal, we cannot discard the participation of impaired GLUT4 storage vesicles translocation to the plasma membrane. Reduced activity of some steps of insulin signaling pathway has been reported to occur in response to AGEs overload both in vivo32,33 and in vitro36. Considering that, we measured the 2-deoxy-D-glucose (2DG) uptake in muscles incubated in vitro with control- and AGE-albumin for 2.5 hours, a time point in which the total cellular GLUT4 content was not altered. No differences were observed in both basal and insulin-stimulated conditions, although the expected positive effect of insulin was clearly observed. This result indicates that, at least for 2.5 hours, the insulin-mediated traffic of GLUT4 storage vesicles was unaffected by AGEs, reinforcing the important role of Slc2a4/GLUT4 repressed expression.
In vitro incubation of soleus muscle confirmed that AGE-albumin can directly and rapidly activate ER and inflammatory stress, an effect that is probably related to the increased expression of the AGE receptor gene Ager. Muscle incubation with AGE-albumin for 2.5 hours increased GRP78 and GRP94 chaperones, evincing the initial activation of the UPR. Increased protein content of ER chaperone GRP78 has been observed in several tissues of 4-week high-AGEs fed mice39, but there is no report of this regulation in skeletal muscle. Curiously, the in vivo treatment with AGE-albumin did not alter GRP94; however, in lead- and polycystic ovary syndrome-induced UPR, the GRP78 increase was reported to be more significant than the GRP94 increase40,41.
As a direct effect and/or as an UPR-related effect, AGEs can induce inflammatory stress. In vitro, AGE-albumin induced a clear activation of the canonical NFKB pathway in muscle; an effect that culminated with increased nuclear content of NFKB1 and RELA proteins. Besides, electrophoretic mobility assay revealed an increased nuclear protein binding activity into a Slc2a4 promoter NFKB-binding site. NFKB-mediated repression of Slc24 expression was proposed to be an inflammatory effect of tumor necrosis factor alpha in adipocytes several years ago42. Only recently the NFKB repressor effect on Slc2a4 transcription was finally confirmed13, in both adipose and muscle tissues. Furthermore, the repressor effect involves both NFKB1 (p50) and RELA (p65) proteins acting as a heterodimer13. Thus, the present data clearly show that AGEs, in vitro, repress Slc2a4/GLUT4 expression by a NFKB-mediated pathway. Although NFKB is a powerful repressor of Slc2a4 transcription13, proposed to mediate AGEs effect in the present study, we cannot discard the possibility that AGEs also reduce the transcriptional activity of some Slc2a4 enhancer.
The present data reveal that chronically administered AGE-albumin, regardless of a hyperglycemic condition, is able to impair glycemic homeostasis, by activating ER and inflammatory stress in skeletal muscle, which culminates with repression of Slc2a4/GLUT4 expression. This effect was also observed in vitro, in muscles from normal rats incubated with AGE-albumin for a few hours, in which the ER and inflammatory stress lead to increased NFKB1 and RELA binding activity into the Slc2a4 promoter, thus explaining its gene transcription repression. These data reveal that AGEs may worsen glycemic control in diabetic subjects and impair glycemic homeostasis in non-diabetic states of increased AGEs concentration; and that involves an ER- and inflammatory-mediated repression of Slc2a4/GLUT4 expression.
Considering the high amount of AGEs in processed foods and high-fat diets, our results shed light on an important role of AGEs as inducers of insulin resistance, a key mechanism in the pathogenesis of T2DM. In this regard, strategies for AGEs intermediates detoxification and /or blocking AGEs signaling may be useful to prevent derangements in glucose homeostasis.
Material and Methods
Advanced glycation of albumin
The advanced glycation of rat (A6414; Sigma-Aldrich, Saint Louis, Missouri, USA) and bovine (A6003; Sigma-Aldrich, Saint Louis, Missouri, USA) albumin was performed in vitro by incubating albumin with freshly prepared 10 mM glycolaldehyde (Sigma Chemical Co., St. Louis, MO, USA) solution in phosphate buffer (PBS) at 37 °C, in a shaking water bath under N2 atmosfere, in the dark. Control albumin was incubated with PBS alone. Samples were extensively dialyzed against PBS and kept frozen at −80 °C until experiments. The amount of endotoxins was <50 pg endotoxin/mL as determined by the chromogenic Limulus amebocyte assay (Falmouth, MA, USA) (data not shown). Carboxymethyllysine determined by ELISA was 12.6 times greater in rat AGE-albumin as compared to C-albumin. In addition, carboxymethyllysine and pyrraline amounts (mmol/mmol of lysine) were determined by liquid chromatography-mass spectrometry being highly superior in glycated samples as compared to C, as previously described43.
Animals
The in vivo effect of AGEs was investigated in four-week old male Wistar rats obtained from de Central Animal Facility of the University of São Paulo Medical School, and housed in controlled environment (12-h light/dark cycle), with chow diet and water ad libitum. Animals were randomized into two groups receiving daily intraperitoneal (i.p.) injections of 20 mg/kg/day of rat control- (C) or AGE-albumin44 for 12 weeks. At the end of week 12, animals were anesthetized via i.p. injection with sodium thiopental (60 mg/kg, Cristália, São Paulo, Brazil), and subjected to an insulin tolerance test or to blood (inferior vena cava) and soleus muscle (left and right) collection. Blood was processed for glucose and insulin concentration measurement45. The muscles were immediately frozen and stored at −80 °C for further analyses. This experimental protocol was approved by the Institutional Care and Research Advisory Committee (CAPPesq HC-FMUSP #002/14).
The in vitro effect of AGEs was investigated in soleus muscle harvested from untreated healthy control 65- to 75-day-old male Wistar rats (180 to 200 g body weight), obtained from the Animal Center of the Institute of Biomedical Sciences, University of São Paulo. Animals were housed under controlled conditions as described above. After i.p. anesthesia with thiopental sodium (60 mg/kg, Cristália, São Paulo, Brazil), left and right soleus muscles were harvested for the in vitro study. The experimental protocol was approved by the Ethical Committee for Animal Research of the Institute of Biomedical Sciences of the University of São Paulo (protocol #124/134/2).
All procedures performed on animals were in accordance with the relevant guidelines and regulations.
Insulin tolerance test (ITT)
ITT was performed as previously described45. Tail blood samples were collected at 0, 4, 8, 12, 16 and 20 min after intravenous (penis vein) injection of regular insulin (0.75 U/kg, Humulin® R, Eli Lilly and Company, Indianapolis, IN, USA). Blood glucose values from 4 to 20 min were transformed to Napierian logarithm and subjected to a linear regression. The slope of the regression was multiplied by −100, to express the result as %/min. The tests were performed from 9:00 to 11:00 hours, in 4-hour food deprived rats.
In vitro muscle incubation
Both right and left soleus muscles were gently dissected to preserve the integrity of tendons. One tendon was fixed into a horizontal metallic support, whereas the other was connected to an isometric transducer by a pulley (TBM-4F, World Precision Instruments INC., Sarasota, FL, USA). Muscle length was adjusted to produce maximal twitch tension (~3 g). The muscles were immersed in 100 mL Krebs-Heinseleit buffer, pH 7.4, containing 8 mM D-glucose and 1 mg/mL of control- (right muscle) or AGE- (left muscle) bovine albumin. Muscles were incubated at 37 °C, continuously oxygenated with 95% O2: 5% CO2, for 2.5 hours, with buffer replacement every 1.25 hours. For GLUT4 protein analysis, incubation time was extended to 5 and to 7.5 hours, with buffer replacement every 2.5 hours. At the end of the incubation periods, muscles were immediately frozen and stored at −80 °C for further analyses.
Muscle glucose uptake in vitro
In vitro muscle glucose uptake was evaluated using deoxy-D-glucose-2-[1,2-3 H(N)] (2DG), as previously described24. Soleus muscle strips were previously incubated for 1.25 hours with control- or AGE-albumin, as described above. After that, the strips were removed to flasks containing the same buffers (control- or AGE-albumin), added by 0.2 mCi/mL 2DG (deoxy-D-glucose-2-[1,2-3 H(N)], PerkinElmer, Boston, MA, USA), and with or without 400 mM insulin (Humulin® R, Eli Lilly, Indianapolis, IN, USA), and thus incubated for more 1.5 hours. This protocol totalizes 2.5 hours of exposition to the different albumins. Results were expressed as μmol/g tissue.
Quantitative PCR analysis
Slc2a4, Ddost and Ager mRNAs, which codify GLUT4, DDOST and AGER proteins; respectively, were evaluated by reversed transcribed quantitative PCR (RT-qPCR). Total RNA was extracted by Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) from muscles obtained after the in vivo and in vitro treatments. cDNA was obtained by reverse transcription, and then amplified using the StepOnePlus System (Life Technologies), and the following TaqMan Gene Expression Assays (Life Technologies): Ddost (Rn01518759_m1), Ager (Rn01525753_g1) and B2m (Rn00560865_m1). For rat Slc2a4, the non-inventoried primers 5′-GGCTGTGCCATCTTGATGAC-3′ (fw) and 5′-CACGATGGACACATAACTCATGGAT-3′ (rv); and the probe 5′-FAM-AACCCGCTCCAGCAGC-MGB3′ (Taqman, Life Technologies) were used (Poletto et al., 2015). Data were normalized by the expression of the housekeeping gene B2m. Relative levels of mRNA expression were calculated using the comparative cycle threshold (Ct) (2−ΔΔCt) method.
Western blotting analyses
Total cellular membrane protein fraction for GLUT4 quantification was performed as previously described35,37. IKKA, IKKB, IKBA, IKBB, GRP78 and GRP94 were analyzed in a total cellular protein fraction: muscle samples were homogenized in buffer20, centrifuged at 12,000 g (at 4 °C, for 20 min), and the supernatants were subjected to electrophoresis. For NFKB1 and RELA protein analyses, cytosolic and nuclear protein fractions were obtained as previously described45. Protein concentration in the samples was determined by the Bradford method. Equal amounts of total protein (according to the target protein) were subjected to sodium dodecyl sulfated polyacrylamide gel electrophoresis, using 12% T and 2.7% C gel for GLUT4, NFKB1, RELA, IKBA and IKBB; and 8% T and 2,7% C gel for p-IKKA and p-IKKB. Proteins were then transferred to nitrocellulose membrane, and incubated with primary antibody against GLUT4 (rabbit anti-GLUT4, #07-1404, Millipore) phosphorylated-IKKA and -IKKB (rabbit anti-pIKKα/β Ser180/Ser181, sc-23470-R, Santa Cruz Biotechnology), IKBA (rabbit anti-IKBα, sc-371, Santa Cruz Biotechnology), IKBB (rabbit anti-IKBβ, sc-945, Santa Cruz Biotechnology), NFKB1 (goat anti-p50, sc-1190, Santa Cruz Biotechnology), RELA (rabbit anti-p65, ab-7970, Abcam), and GRP78/94 (mouse anti-KDEL, ADI-SPA-827-F, Enzo Lifesciences). The membrane was then incubated with horseradish peroxidase-linked secondary antibody, and signal was detected by chemiluminescence. Blots were quantified by optical densitometry (ImageScanner III, GE Healthcare, Uppsala, Sweden). Protein-loaded normalization was undertaken by analyzing the Ponceau-stained membrane46, and results were further normalized considering mean of control values as 100.
Electrophoretic mobility shift assay (EMSA)
Nuclear proteins for EMSA were extracted from muscles incubated in vitro, and EMSA was performed as previously described13. The oligonucleotide used as probe corresponds to the -134/-113 sequence of the mouse Slc2a4 gene, which was previously confirmed to bind NFKB1 and RELA using nuclear proteins from rat L6 muscle cells13. EMSA performed with this probe revealed two protein/DNA complexes (A and B) in rat muscle cells, and competition assays confirmed the presence of both NFKB1 and RELA in these complexes13.
Statistical Analyses
Comparison of results from rats treated or not with rat AGE-albumin in vivo was performed by unpaired two-tailed t test, after confirmation that the variances were not significantly different. Comparison of results from muscles incubated (left) or not (right) with bovine AGE-albumin was performed by paired two-tailed t test. Glucose uptake was analyzed by one-way ANOVA. Differences were considered significant when P < 0.05, and the number of samples is informed in the legends.
Data availability
There is no restriction on the availability of materials and data.
References
Vlassara, H. & Uribarri, J. Advanced Glycation End Products (AGE) and Diabetes: Cause, Effect, or Both? Current Diabetes Reports. 14, 453–462 (2014).
Unoki, H. et al. Advanced glycation end products attenuate cellular insulin sensitivity by increasing the generation of intracellular reactive oxygen species in adipocytes. Diabetes Res. Clin. Pract. 76, 236–244 (2007).
Wu, C. H. et al. AGE-Induced interference of glucose uptake and transport as a possible cause of insulin resistance in adipocytes. J. Agric. Food Chem. 59, 7978–7984 (2011).
Zierath, J. R., Krook, A. & Wallberg-Henriksson, H. Insulin action and insulin resistance in human skeletal muscle. Diabetologia. 43, 821–835 (2000).
Klip, A. The many ways to regulate glucose transporter 4. Appl. Physiol. Nutr. Metab. 34, 481–487 (2009).
Jessen, N. & Goodyear, L. J. Contraction signaling to glucose transport in skeletal muscle. J. Appl. Physiol. (1985). 99, 330–337 (2005).
Herman, M. A. & Kahn, B. B. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 116, 1767–1775 (2006).
Kampmann, U. et al. GLUT4 and UBC9 protein expression is reduced in muscle from type 2 diabetic patients with severe insulin resistance. PLoS One. 6, e27854 (2011).
Corrêa-Giannella, M. L. & Machado, U. F. SLC2A4 gene: a promising target for pharmacogenomics of insulin resistance. Pharmacogenomics. 14, 847–850 (2013).
Ramasamy, R., Yan, S. F. & Schmidt, A. M. Arguing for the motion: Yes, RAGE is a receptor for advanced glycation end products. Mol. Nutr. Food Res. 51, 1111–1115 (2007).
Anelli, T. & Sitia, R. Protein quality control in the early secretory pathway. EMBO J. 27, 315–327 (2008).
Piperi, C., Adamopoulos, C., Dalagiorgou, G., Diamanti-Kandarakis, E. & Papavassiliou, A. G. Crosstalk between advanced glycation and endoplasmic reticulum stress: Emerging therapeutic targeting for metabolic diseases. J. Clin. Endocrinol. Metab. 97, 2231–2242 (2012).
Furuya, D. T. et al. Identification of nuclear factor-κB sites in the Slc2a4 gene promoter. Mol. Cell Endocrinol. 370, 87–95 (2013).
Cai, W. J., He, J. C., Zhu, L., Lu, C. Y. & Vlassara, H. Advanced glycation end product (AGE) receptor 1 suppresses cell oxidant stress and activation signaling via EGF receptor. PNAS. 103, 13801–13806 (2006).
Uribarri, J. et al. Advanced glycation end products in foods and a practical guide to their reduction in the diet. J. Am. Diet. Assoc. 110, 911–916 (2010).
Fabre, N. T. et al. Hormetic modulation of hepatic insulin sensitivity by advanced glycation end products. Mol. Cell Endocrinol. 447, 116–124 (2017).
Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature. 414, 813–820 (2001).
Dozio, E., Di Gaetano, N., Findeisen, P. & Corsi Romanelli, M. M. Glycated albumin: from biochemistry and laboratory medicine to clinical practice. Endocrine. 55, 682–690 (2017).
DeFronzo, R. A. Pathogenesis of type 2 diabetes mellitus. Med. Clin. North Am. 88, 787–835 (2004).
Camps, M. et al. Effect of diabetes and fasting on GLUT-4 (muscle/fat) glucose-transporter expression in insulin-sensitive tissues. Heterogeneous response in heart, red and white muscle. Biochem J. 282, 765–772 (1992).
Hardin, D. S., Dominguez, J. H. & Garvey, W. T. Muscle Group-Specific Regulation of GLUT 4 Glucose Transporters in Control, Diabetic, and Insulin-Treated Diabetes Rats. Metabolism. 42, 1310–1315 (1993).
Machado, U. F., Shimizu, Y. & Saito, M. Decreased Glucose Transporter (GLUT4) Content in Insulin-Sensitive Tissues of Obese Aurothioglucose- and Monosodium Glutamate-Treated Mice. Horm. Metab. Res. 25, 462–465 (1993).
Klip, A., Tsakiridis, T., Marette, A. & Ortiz, P. A. Regulation of expression of glucose transporters by glucose: a review of studies in vivo and in cell cultures. FASEB J. 8, 43–53 (1994).
Mori, R. C., Hirabara, S. M., Hirata, A. E., Okamoto, M. M. & Machado, U. F. Glimepiride as insulin sensitizer: increased liver and muscle responses to insulin. Diabetes Obes. Metab. 10, 596–600 (2008).
Pedersen, O. et al. Evidence against altered expression of GLUT1 or GLUT4 in skeletal muscle of patients with obesity or NIDDM. Diabetes. 39, 865–870 (1990).
Garvey, W. T., Maianu, L., Hancock, J. A., Golichowski, A. M. & Baron, A. Gene expression of GLUT4 in skeletal muscle from insulin-resistant patients with obesity, IGT, GDM, and NIDDM. Diabetes. 41, 465–475 (1992).
Dela, F. et al. Physical training increases muscle GLUT4 protein and mRNA in patients with NIDDM. Diabetes. 43, 862–865 (1994).
Dohm, G. L. et al. Decreased expression of glucose transporter in muscle from insulin-resistant patients. Am. J. Physiol. 260, E459–463 (1991).
Gaster, M., Staehr, P., Beck-Nielsen, H., Schrøder, H. D. & Handberg, A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 50, 1324–1329 (2001).
Stentz, F. B. & Kitabchi, A. E. Transcriptome and proteome expression involved in insulin resistance in muscle and activated T-lymphocytes of patients with type 2diabetes. Geno. Prot. Bioinfo. 5, 216–235 (2007).
Massart, J. et al. Altered miR-29 expression in type 2 diabetes influences glucose and lipid metabolism in skeletal muscle. Diabetes 66, 1807–1818 (2017).
Cai, W. et al. Oral advanced glycation endproducts (AGEs) promote insulin resistance and diabetes by depleting the antioxidant defenses AGE receptor-1 and sirtuin 1. PNAS. 109, 15888–15893 (2012).
Cassese, A. et al. In skeletal muscle advanced glycation end products (AGEs) inhibit insulin action and induce the formation of multimolecular complexes including the receptor for AGEs. J. Biol. Chem. 283, 36088–36099 (2008).
Silva, J. L. et al. NF-kappaB, MEF2A, MEF2D and HIF1-a involvement on insulin- and contraction-induced regulation of GLUT4 gene expression in soleus muscle. Mol. Cell Endocrinol. 240, 82–93 (2005).
Lima, G. A. et al. Contractile activity per se induces transcriptional activation of SLC2A4 gene in soleus muscle: involvement of MEF2D, HIF-1a, and TRalpha transcriptional factors. Am. J. Physiol. Endocrinol. Metab. 296, E132–138 (2009).
Moraes, P. A. et al. Insulin acutely triggers transcription of Slc2a4 gene: participation of the AT-rich, E-box and NFKB-binding sites. Life Sci. 114, 36–44 (2014).
Poletto, A. C. et al. Oleic and linoleic fatty acids downregulate Slc2a4/GLUT4 expression via NFKB and SREBP1 in skeletal muscle cells. Mol. Cell Endocrinol. 401, 65–72 (2015).
Miele, C. et al. Human glycated albumin affects glucose metabolism in L6 skeletal muscle cells by impairing insulin-induced insulin receptor substrate (IRS) signaling through a protein kinase C alpha-mediated mechanism. J. Biol. Chem. 278, 47376–47387 (2003).
Adamopoulos, C. et al. Systemic effects of AGEs in ER stress induction in vivo. Glycoconjugate J. 33, 537–44 (2016).
Shinkai, Y., Yamamoto, C. & Kaji, T. Lead induces the expression of endoplasmic reticulum chaperones GRP78 and GRP94 in vascular endothelial cells via the JNK-AP-1 pathway. Toxicol. Sci. 114, 378–386 (2010).
Bañuls, C. et al. Metabolic syndrome enhances endoplasmic reticulum, oxidative stress and leucyte-entorhelium interactions in PCOS. Metabolism 71, 153–162 (2017).
Ruan, H., Hacohen, N., Golub, T. R., Parijs, L. V. & Lodish, H. F. Tumor necrosis factor-α supresses adipocyte-specific genes and activates expression of preadipocyte genes in 3T3-L1 adipocytes. Nuclear factor-κB activation by TNF-α is obligatory. Diabetes. 51, 1319–1336 (2002).
da Silva, K. S. et al. N-acetylcysteine counteracts adipose tissue macrophage infiltration and insulin resistance elicited by advanced glycated albumin in healthy rats. Front Physiol. 8, 723 (2017).
Coughlan, M. T. et al. Advanced glycation end products are direct modulators of beta-cell function. Diabetes. 60, 2523–2532 (2011).
Okamoto, M. M. et al. Intensive insulin treatment induces insulin resistance in diabetic rats by impairing glucose metabolism-related mechanisms in muscle and liver. J. Endocrinol. 211, 55–64 (2011).
Thacker, J. S., Yeung, D. H., Staines, W. R. & Mielke, J. G. Total protein or high-abundance protein: Which offers the best loading control for Western blotting. Anal. Biochem. 469, 76–78 (2016).
Acknowledgements
The authors thank Dr. Adauri Brezolin for English revision of the manuscript. This project was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) to Danilo C. Pinto-Junior (2012/20432-0), Caio Y. Yonamine (2016/25155-5), João V. Esteves (2012/20432-0), Karolline S. Silva (2012/18724-2), Nelly T. Fabre (2013/00713-7), Karina Thieme (2014/17251-9), and Maria Lúcia Corrêa-Giannella, Marisa Passarelli and Ubiratan Fabres Machado (2016/15603-0) Maria Lúcia Corrêa-Giannella, Marisa Passarelli and Ubiratan Fabres Machado are recipients of a fellowship from Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Contributions
D.C.P., C.Y.Y., M.M.O., M.L.M. and J.V.E. researched in vitro data; D.C.P., K.S.S., N.T.F., K.T. and S.C. researched in vivo data; P.M.S., M.L.C., M.P. and U.F.M. planned the experimental design, researched/analyzed data and wrote/edited/revised the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pinto-Junior, D.C., Silva, K.S., Michalani, M.L. et al. Advanced glycation end products-induced insulin resistance involves repression of skeletal muscle GLUT4 expression. Sci Rep 8, 8109 (2018). https://doi.org/10.1038/s41598-018-26482-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26482-6
This article is cited by
-
The combination of endurance exercise and SGTC (Salvia–Ginseng–Trigonella–Cinnamon) ameliorate mitochondrial markers’ overexpression with sufficient ATP production in the skeletal muscle of mice fed AGEs-rich high-fat diet
Nutrition & Metabolism (2022)
-
Circulating TRB3 and GRP78 levels in type 2 diabetes patients: crosstalk between glucose homeostasis and endoplasmic reticulum stress
Journal of Endocrinological Investigation (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.