Abstract
Fetal growth may be a precursory factor in observed association between birthweight and atopic dermatitis (AD), however, recent studies utilizing fetal ultrasound-based data have reported contradictory results. This study aims to clarify previous findings through comprehensive investigation of association between several trimester-specific ultrasound-based anthropometric measures with AD diagnosis by age 3 years. Measurements of 386 newborns in the Ulm SPATZ Health Study were converted into adjusted z-scores categorized as “low” (≤1 SD below mean), “normal,” or “high” (≥1 SD above mean). AD cases were defined using parent- or pediatrician-report of physician-diagnosis or clinical diagnosis. Adjusted risk ratios (RR) with 95% confidence intervals (95% CI) were calculated using modified Poisson regression. Compared to normal, both low and high 2nd trimester abdominal circumference [RR 1.51, (95% CI 1.01; 2.24) and 1.83 (1.21; 2.76)], high 2nd trimester head- abdominal circumference ratio [1.69 (1.16; 2.48)], and faltering 2nd to 3rd trimester [1.59 (1.04; 2.43)] head circumference were associated with greater AD risk. High 3rd trimester femur length [0.54 (0.31; 0.94)] was associated with lower risk. Using more inclusive exposure cut-points (0.8 SD), lower 1st trimester crown-rump length was also associated with greater AD risk. Our data suggest several different patterns of fetal growth may be differentially associated with AD.
Similar content being viewed by others
Introduction
Several studies have reported cross-sectional associations between birthweight, neonatal adiposity, or other anthropometric measures at birth and increased risk of atopic dermatitis (AD) beginning in infancy or early childhood1,2,3,4,5. Though these results suggest that overall fetal growth may be a risk factor for AD, studies investigating fetal growth throughout gestation may offer more detailed data for causative research on potential determinants of this, and potentially other, atopic diseases6,7,8,9.
Though few previous studies have directly investigated associations between fetal growth using ultrasound-based anthropometric measurements and AD or other atopic outcomes10,11,12,13,14, several potentially important but inconsistent results have been reported. Greater 1st to 2nd trimester ratio of crown-rump length to biparietal diameter and 2nd to 3rd trimester abdominal circumference have been separately reported to be associated with increased risk of parent-reported physician diagnosis of AD by age 10 years11 and by age 4 years12 respectively. In contrast to the latter finding, a smaller but more recent study reported accelerated 2nd to 3rd trimester abdominal circumference was associated with decreased risk of parent-reported physician diagnosis of AD by age 2 years14. Another study observed that greater 2nd trimester head circumference may be associated with decreased odds of parent-reported physician diagnosis of AD by age 5 years13. Finally, it should also be noted that several of the studies mentioned also reported null results which contrast with some of these statistically significant findings.
Inconsistencies between results may be attributable to several factors including differences in study populations, gestational age and anthropometric calculations, antenatal assessment periods and exposure definitions, AD case definition and inconsistencies related to parent reporting of AD diagnosis, data quality, statistical methodology, or a combination of these and other factors6,9,14. In particular, linear analysis of non-linear growth patterns typical during certain periods of gestation may have resulted in differential or null findings in some studies but not others.
The primary aim of this analysis was to determine whether fetal anthropometric measurements obtained via ultrasound during the 1st, 2nd, and 3rd trimester of pregnancy or 2nd to 3rd trimester growth trajectory were associated with physician diagnosis of AD by 3 years of age. To clarify and expand on results from previous studies and provide a more consistent basis for future research, we analyzed several anthropometric measurements cross-sectionally and longitudinally comparable to those reported piecemeal in previous literature. To further improve upon previous studies, we comprehensively investigated the effect of potentially important non-linear fetal growth. Finally, to reduce potential bias commonly encountered in observational studies of childhood AD, we implemented an inclusive case definition based on both parent-reported and pediatrician-reported physician diagnosed AD supplemented by clinical examination.
Methods
Study design and population
The Ulm SPATZ Health Study cohort consists of 1006 live newborns of 970 mothers (49% of all 1999 eligible families) recruited from the general population shortly after delivery in the University Medical Center Ulm, Southern Germany, from April 2012 until May 2013. Further details pertaining to study methodology and data collection are available elsewhere15,16. In brief, exclusion criteria were outpatient delivery, maternal age <18 years, transfer of the newborn or the mother to intensive care immediately after delivery, and/or insufficient knowledge of the German language. Ethical approval was obtained from and all study protocols were carried out in accordance with guidelines approved by the ethics board of Ulm University (no. 311/11). Participation was voluntary and written informed consent obtained in each case.
Fetal anthropometrics
Anthropometric measurements were obtained from data recorded during routine obstetric screenings regularly conducted in Germany at 9–12 weeks (1st trimester), 19–22 weeks (2nd trimester), and 29–32 weeks (3rd trimester) gestation. From these records, data were obtained for gestational age at measure, 1st trimester crown-rump length (CRL) and 2nd and 3rd trimester head circumference (HC), abdominal circumference (AC), and femur length (FL). In cases where AC or HC was not directly documented (22% for AC in 2nd and 3rd trimester each; 42% for HC in 2nd trimester and 43% in 3rd trimester), measures were computed using the Ramanujan formula for calculating the circumference of an ellipse and using documented abdominal anteroposterior and transverse diameters and biparietal and occipitofrontal diameters, respectively17. In addition to these computations, trimester-specific HC to AC ratio (HC:AC) and estimated fetal weight (EFW) were calculated using the Hadlock 3 formula [Log10EFW=1.326–0.00326*AC*FL + 0.0107*HC + 0.0438*AC + 0.158*FL]18,19. No statistically significant differences in z-scores were observed between documented and calculated HC or AC among those with full documentation of both circumferences and diameters (see Supplement Fig. 1).
As obstetricians frequently updated gestational age estimates after 1st trimester screening, more accurate later gestational age estimates were given priority over potentially inaccurate initial estimates in our analyses. To preserve comparability between measurements, ultrasound data reported >21 days from median gestational age calculated for all trimester-specific screenings were not used. Further accounting for gestational age at measure; CRL, HC, AC, FL, HC:AC, and EFW results were converted into z-scores obtained from linear regression models of the effect of gestational age on the respective measure and stratified by child’s gender. Models used to derive z-scores for HC and AC were further adjusted for whether they were obtained directly from written documentation or calculated. Z-scores were then used to categorize measures into “low” (≤1 SD below mean), “normal,” or “high” (≥1 SD above mean) fetal growth.
AD diagnosis
Children whose parent or pediatrician reported physician AD diagnosis within the 12 months preceding the 1, 2 or 3 year questionnaire-based follow-up period were considered AD cases. In addition, as part of the study protocol, children with potential AD symptoms were invited to undergo dermatological examinations at 6 months (n = 55 attended), 1 year (n = 99 attended), and/or 2 years (n = 60 attended) of age. Children with confirmed dermatological diagnosis during any exam period were considered cases regardless of parent or pediatrician reports. Non-cases were the remaining children with at least one negative parent and pediatrician report within the same or separate time periods. To assess the effect of potential misclassification with the AD case group, disease severity20 was assessed with Patient-Oriented Eczema Measure (POEM) scores during the 3 year follow-up period21.
Maternal and birth-related factors
Maternal demographic and lifestyle data including age at delivery, nationality, pre-pregnancy BMI, duration of school education (<12 years or ≥12 years), parity (first birth or greater), history of smoking (within the year prior to pregnancy), and reported physician-diagnosed childhood AD up to 18 years of age were collected using a self-administered standardized questionnaire during the hospital stay following delivery. Clinical data related to the child’s delivery including gender, birthweight, and delivery mode were obtained from electronic hospital records.
Statistical analyses
Primary analyses were restricted to children with complete 1st, 2nd, and 3rd trimester fetal growth data and AD outcome. Potential selection bias due to missing data and restrictions was assessed by comparing demographic characteristics (proportions or means) within the analysis subsample to 95% confidence intervals for each characteristic within the full cohort. Modified Poisson regression models22 were used to compute risk ratios (RR) for “low” and “high” trimester-specific anthropometric values with AD using the “normal” category as a reference group. Maternal and birth-related factors described above were tested for use as potential covariates in adjusted models. As none of these variables produced a 10% change in point estimate for bivariate association between any fetal growth measure and any AD outcome definition, adjusted models included only the a-priori selected potential confounders child’s gender and maternal education, parity, smoking, and AD history (diagnosis by 18 years of age). The same modelling method was used to calculate crude RR for 2nd to 3rd trimester change from “normal” to “low” (faltering growth) and from “normal” to “high” (accelerated growth) anthropometric category with AD. Here, due to restriction to 2nd trimester “normal”, sample sizes were too low to attempt meaningful adjustment for potential confounders. As in a larger previous study12, confidence intervals for model results were not adjusted for multiple comparisons due to the exploratory nature of the investigation and high correlation observed between exposure measures. Finally, sensitivity analyses including removal of subjects with fetal measurements viewed as potential outliers (+/−3 SD from their respective mean), inclusion of subjects with incomplete fetal growth data, evaluation of less stringent cutoff values (0.8 SD and 0.66 SD) used to categorize “low” and “high” anthropometric categories, and separate modelling for association of trimester-specific growth with parent- or pediatrician-reported physician diagnosed AD were conducted. All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Results
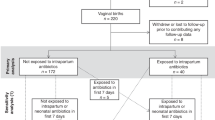
For the purposes of this analysis, the study population was restricted to mothers of singleton newborns (n = 934). Of these, ultrasound data could not be obtained for 69 children and a further 351 children were missing complete data (1st trimester CRL or 2nd or 3rd trimester HC or AC) at one or more trimesters. Among the 514 remaining children, 80 were lost to follow-up before the first dermatological assessment and 48 children were missing parent (n = 2), pediatrician (n = 25), or both (n = 21) reports at all time periods. In total, 386 children were included in our final analyses. Within this group, AD diagnosis was reported by either the parent or pediatrician for 61 children during the year 1 assessment, an additional 32 children in the year 2 assessment, and a further 17 children in the year 3 assessment.
Compared to the full cohort, mothers in this analysis sub-population were slightly older at delivery, more often higher educated, and had lower prevalence of smoking (Table 1). Children were of slightly higher birth weight and less likely to have been delivered via cesarean section. These differences were likely attributable to factors associated with higher participation-related factors among mothers and restriction to singleton deliveries. Gestational age at ultrasound measure and prevalence of AD diagnosis in the analysis sub-population were comparable to those observed in the full cohort.
Scatter plots with regression lines for all primary fetal anthropometric measurements by gestational age are available in Fig. 1. As expected, we observed linear increases for individual growth measures with increasing gestational age but non-linear increases for corresponding measures between the 2nd and 3rd trimester.
Among subjects for whom POEM scores were available, AD severity was highest for those with both parent and pediatrician report (see Fig. 2).
In crude trimester-specific models, both low and high 2nd trimester AC, high 2nd trimester HC:AC, and lower 3rd trimester HC were associated with higher AD risk (Supplement Table 1). Higher 3rd trimester FL was associated with lower risk of AD.
Following adjustment for potential covariates and confounders, point estimates were similar and remained statistically significant except for 3rd trimester HC which became only marginally significant (Table 2). Point estimates did not meaningfully change following removal of subjects (n = 19) with any measurement considered a suspected outliers (data not shown). In models including additional subjects with partial but incomplete fetal growth data (Supplement Table 2), we observed similar strength of association for 2nd trimester AC and HC:AC ratio while association for 3rd trimester FL attenuated toward the null.
Results from sensitivity analyses assessing fetal growth cut-point selection and restriction to subjects with complete ultrasound data yielded mostly predictable changes in point estimates. As cutoff values for “low” and “high” anthropometric categories were made less stringent by decreasing cut-points from 1.0 to 0.8 and 0.66 SD from the mean, some point estimates attenuated toward the null but in most cases remained significant (Supplement Tables 3 and 4). In models where 0.8 SD was used as a cutoff, lower 1st trimester CRL was associated with increased AD risk. This association also attenuated slightly toward the null in models using a 0.66 SD cutoff and was only marginally significant. Upon inclusion of all 602 subjects with 1st trimester CRL measures (e.g. excluded due to missing measurements in other trimesters) and using 0.8 and 0.6 SD cutoffs, point estimates were further attenuated but remained significant presumably due to increased power (data not shown). Point estimates were mostly similar regardless of case definition (parent- or pediatrician-reported physician diagnosed AD) but slightly weaker for lower 2nd trimester AC with parent-reported AD definition (data not shown).
Among children with “normal” 2nd trimester anthropometric measurements, faltering HC growth was marginally associated with higher AD risk in crude models (Table 3). When decreasing cutoff values for “low” and “high” anthropometric categories were applied, this result attenuated increasingly toward the null (Supplement Tables 5 and 6). Conversely, accelerated AC point estimates became increasingly protective reaching statistical significance in 0.66 SD cutoff models.
Discussion
Within this birth cohort study, we observed several associations for lower and higher fetal anthropometric measures throughout gestation with risk of AD diagnosis by 3 years of age. Associations observed for body length suggest that while early fetal growth impairment in the 1st trimester may be associated with increased risk, longer body length in 3rd trimester may be protective. However, low correlation between the two measures also suggests these associations are unlikely to be related. This is similar for observed associations of faltering 2nd to 3rd trimester HC and 2nd trimester HC:AC. Conversely, association for lower 2nd trimester HC:AC ratio is likely driven by AC. Associations observed separately for both lower and higher 2nd trimester AC may be indicative of a case where divergent growth patterns result in similar increased risk. These results suggest several patterns of fetal growth possibly beginning as early as the 1st trimester may influence AD risk or, more likely, may be indicative of other prenatal factors associated with AD risk.
Perhaps the most robust findings in our study are the previously unreported, and seemingly contradictory, associations for abnormal (lower and higher) 2nd trimester AC. Point estimates reported in previous studies were often derived from models investigating continuous linear association between trimester-specific fetal growth measures11,13,14 or fetal growth over time12,14 and AD. Though one study reported a protective effect for larger 2nd trimester HC with AD13, results from continuous linear analyses in other studies11,12,14 (none specifically including 2nd trimester HC) reported mostly null results. Significant point estimates for lower and higher trimester-specific measures in our study may suggest potentially meaningful non-linear associations may have been missed in previous reports.
We attempted to further account for potential non-linear growth patterns by restricting models investigating accelerated and faltering 2nd to 3rd trimester growth to subjects who fell within normal range (+/−1.0, 0.8, or 0.66 SD from expected) for 2nd trimester growth. Previous studies have separately reported contradictory findings in which accelerated 2nd to 3rd trimester AC growth was associated with both higher12 and lower14 AD risk. Point estimates for this association in our study were consistently protective but only became strong and significant when accelerated AC growth was categorized as greater than 0.66 SD higher than expected growth. Though inconsistent, our results may provide some further support to earlier findings of a protective effect of accelerated 2nd to 3rd trimester AC growth on AD risk. However further study with larger sample size is required to provide more clarity.
Several factors common in observational studies of this nature may have influenced our results and should be considered as potential limitations in the interpretation of our results. Most important among these are sample size, collection and interpretation of ultrasound data, and AD case definition.
To ensure comparability between point estimates across trimesters, the analysis sub-population was restricted to subjects with complete 1st, 2nd, and 3rd trimester fetal growth and AD outcome data up to 3 years. Following restriction, the proportion of remaining subjects (41% of those eligible) in the analysis was comparable to one larger study12 and higher than other smaller studies11,14 with similar aims. Nevertheless, sample size may have affected our power to detect statistical significance of some associations. In particular, faltering and accelerated fetal growth models included only a relatively small proportion of subjects due to previously mentioned restrictions. We performed several analyses to investigate the effects of sample size and participation on our results. In sensitivity analyses including all subjects with at least partial trimester-specific fetal growth measurements; our sample size increased to 602 (1st trimester), 445 (2nd trimester), and 461 (3rd trimester) subjects. Although we observed some shifts in point estimates toward the null, statistical significance was largely unaffected. These results may suggest that point estimates presented in our main results could be slightly overstated but were unlikely to have been greatly affected by selection bias. Though small sample size precluded us from adjusting faltering and accelerated growth models for potential confounders, we believe it is unlikely that adjustment for any of the factors we assessed in our analysis would have greatly affected point estimates as was the case in trimester-specific models.
Ultrasound data in our study was transcribed from paper documentation which mothers in Germany bring with them to each obstetric appointment which is then updated by their physician. Mothers were spread across 58 different obstetric practices resulting in some variation in measurement as well as documentation and potentially observer error. In some cases, documentation required calculation of HC and AC diameters. Amongst 5 practices which documented ultrasound results for 20 or more children, some individual fetal measurement results deviated from the expected mean but no practice showed consistent deviation for multiple results (data not shown). Though we could not account for potential variability within or between physician practice, one study from the UK reported inter-observer variability in interpretation of ultrasound imagery at roughly +/−4.9%, 8.8%, and 11.1% for measurement of HC, AC, and FL respectively23. Thus, it is possible that measurement error near cut-points defining “low” and “high” growth could have resulted in some misclassification of fetal growth. However, results from sensitivity analyses where categories were shifted from a z-score cut-point of 1.0 to 0.8 and 0.66 resulted in mostly predictable changes in estimates across this gradient. Further, individual measurements as reported in Fig. 1, were mostly within range of those reported by the Intergrowth-21 project for development of international guidelines on fetal growth24. Finally, model results were not noticeably different following exclusion of subjects with any single measurement considered a potential outlier. Therefore, it is unlikely our results were greatly affected by measurement error.
In contrast to previous studies which relied mostly on parent-only report of child of physician-diagnosed AD, AD cases in our study were defined using a combination of both parent and pediatrician report and, in a few cases, records from dermatological examinations. As we reported, AD severity at 3 years of age was highest for children with both a parent and pediatrician report of AD diagnosis while children with parent-only or pediatrician-only report included more mild cases yet obviously higher severity than among those with no report of AD. Thus, the AD case definition in our study may include a higher overall proportion of mildly symptomatic disease compared to other studies which may define AD using only parent report or health care data. However, when modeled separately, we observed similar point estimates for both parent-only and pediatrician-only reported outcomes. Nevertheless, as is the case in many studies of AD, some respondents or physicians may have confused AD with other forms of dermatitis. Therefore, we cannot completely rule out the possibility that some AD cases in our analysis may have been misclassified.
Several biological mechanisms have been proposed which may shed light on the potential role of fetal growth as a marker for risk of atopic disease. First trimester fetal growth restriction has been associated with several maternal demographic, lifestyle, and health-related factors25. Some of these factors such as maternal age and smoking are also associated with breastfeeding behavior16 which may thus be a proxy confounder for these maternal characteristics we accounted for when assessing associations of fetal growth with atopic outcomes. Another possibility is that fetal growth may be an indicator of other underlying factors related to fetal nutrition such as maternal diet, nutrient intake, or placental function7. These factors, or the resulting impact on fetal growth, may contribute to gene-environment interactions which could potentially influence skin development and/or function. Although we currently do not have data on genetic make-up of our study cohort, several gene loci have already been identified in other studies as potential study targets, the most well-known being Filaggrin26. In addition to genetic alteration, some evidence suggests factors associated with fetal environment may be associated with early-life programming of the inflammatory response27. Of note, we have recently reported association between gestational weight gain and levels of the inflammatory biomarker leptin in cord blood28. Finally, several studies have also suggested that postnatal growth which may be particularly associated with late-term fetal growth (e.g. “catch-up” growth) may also contribute to or explain associations with atopic dermatitis outcomes6,7.
Together with previous studies, our results may be interpreted as preliminary evidence suggesting that fetal growth may play a role in or be a marker for factors influencing the development of atopic disease. Most notably, our results may help clarify differential results in previous studies by demonstrating that several potential patterns of both accelerated and faltering fetal growth may be relevant. In addition, these patterns may vary depending upon developmental period of gestation. Further research may be warranted to investigate associations between non-linear patterns of fetal growth and disease particularly relatively unexplored 1st trimester growth acceleration. Therefore, larger studies incorporating more precise ultrasound measurements (particularly in the 1st and 2nd trimester), biomarkers for atopy such as IgE levels, and inclusion of additional factors which may affect fetal growth and possibly shed light on possible gene-environment interactions or immune-related fetal programming during gestation may be warranted. Finally, the generalizability of these results should be explored across geographically and culturally different study populations which may differ in both fetal growth and atopic outcome characteristics.
References
Lundholm, C., Ortqvist, A. K., Lichtenstein, P., Cnattingius, S. & Almqvist, C. Impaired fetal growth decreases the risk of childhood atopic eczema: a Swedish twin study. Clin. Exp. Allergy 40, 1044–1053 (2010).
Sevelsted, A. & Bisgaard, H. Neonatal size in term children is associated with asthma at age 7, but not with atopic dermatitis or allergic sensitization. Allergy 67, 670–675 (2012).
Lin, M.-H. et al. Fetal Growth, Obesity, and Atopic Disorders in Adolescence: a Retrospective Birth Cohort Study. Paediatr. Perinat. Epidemiol. 29, 472–479 (2015).
Egeberg, A., Andersen, Y. M. F., Gislason, G., Skov, L. & Thyssen, J. P. Neonatal risk factors of atopic dermatitis in Denmark - Results from a nationwide register-based study. Pediatr. Allergy Immunol. 27, 368–374 (2016).
O’Donovan, S. M. et al. Neonatal adiposity increases the risk of atopic dermatitis during the first year of life. J. Allergy Clin. Immunol. 137, 108–117 (2016).
Tedner, S. G., Örtqvist, A. K. & Almqvist, C. Fetal growth and risk of childhood asthma and allergic disease. Clin. Exp. Allergy 42, 1430–1447 (2012).
Turner, S. Perinatal programming of childhood asthma: early fetal size, growth trajectory during infancy, and childhood asthma outcomes. Clin. Dev. Immunol. 2012, 962923 (2012).
Jones, C. A., Holloway, J. A. & Warner, J. O. Does atopic disease start in foetal life? Allergy 55, 2–10 (2000).
Turner, S. W. & Devereux, G. Fetal ultrasound: shedding light or casting shadows on the fetal origins of airway disease. Am. J. Respir. Crit. Care Med. 185, 694–695 (2012).
Pike, K. C. et al. Patterns of fetal and infant growth are related to atopy and wheezing disorders at age 3 years. Thorax 65, 1099–1106 (2010).
Turner, S. et al. First- and second-trimester fetal size and asthma outcomes at age 10 years. Am. J. Respir. Crit. Care Med. 184, 407–413 (2011).
Sonnenschein-van der Voort, A. M. M. et al. Fetal and infant growth and asthma symptoms in preschool children: the Generation R Study. Am. J. Respir. Crit. Care Med. 185, 731–737 (2012).
Eviston, D. P., Minasyan, A., Mann, K. P., Campbell, D. E. & Nanan, R. K. In utero Head Circumference is Associated with Childhood Allergy. Front. Pediatr. 3, 73 (2015).
AlMakoshi, A., Ellahi, A., Sallout, B., Devereux, G. & Turner, S. Fetal growth trajectory and risk for eczema in a Saudi population. Pediatr. Allergy Immunol. 26, 811–816 (2015).
Braig, S. et al. Maternal prenatal stress and child atopic dermatitis up to age 2 years: The Ulm SPATZ health study. Pediatr. Allergy Immunol. 28, 144–151 (2017).
Logan, C. et al. Changing Societal and Lifestyle Factors and Breastfeeding Patterns Over Time. Pediatrics 137, e20154473 (2016).
Kurtz, A. B. & Kurtz, R. J. The ideal fetal head circumference calculation. J. Ultrasound Med. 8, 25–29 (1989).
Hadlock, F. P., Harrist, R. B., Carpenter, R. J., Deter, R. L. & Park, S. K. Sonographic estimation of fetal weight. The value of femur length in addition to head and abdomen measurements. Radiology 150, 535–540 (1984).
Kurmanavicius, J., Burkhardt, T., Wisser, J. & Huch, R. Ultrasonographic fetal weight estimation: accuracy of formulas and accuracy of examiners by birth weight from 500 to 5000 g. J. Perinat. Med. 32, 155–161 (2004).
Charman, C. R., Venn, A. J., Ravenscroft, J. C. & Williams, H. C. Translating Patient-Oriented Eczema Measure (POEM) scores into clinical practice by suggesting severity strata derived using anchor-based methods. Br. J. Dermatol. 169, 1326–1332 (2013).
Charman, C. R., Venn, A. J. & Williams, H. C. The patient-oriented eczema measure: development and initial validation of a new tool for measuring atopic eczema severity from the patients’ perspective. Arch. Dermatol. 140, 1513–1519 (2004).
Zou, G. A modified poisson regression approach to prospective studies with binary data. Am. J. Epidemiol. 159, 702–706 (2004).
Sarris, I. et al. Intra- and interobserver variability in fetal ultrasound measurements. Ultrasound Obstet. Gynecol. 39, 266–273 (2012).
Papageorghiou, A. T. et al. International standards for fetal growth based on serial ultrasound measurements: the Fetal Growth Longitudinal Study of the INTERGROWTH-21st Project. Lancet Lond. Engl. 384, 869–879 (2014).
Mook-Kanamori, D. O. et al. Risk factors and outcomes associated with first-trimester fetal growth restriction. JAMA 303, 527–534 (2010).
Liang, Y., Chang, C. & Lu, Q. The Genetics and Epigenetics of Atopic Dermatitis—Filaggrin and Other Polymorphisms. Clin. Rev. Allergy Immunol. 51, 315–328 (2016).
Briana, D. D. & Malamitsi-Puchner, A. Intrauterine growth restriction and adult disease: the role of adipocytokines. Eur. J. Endocrinol. 160, 337–347 (2009).
Logan, C. A. et al. Gestational Weight Gain and Fetal-Maternal Adiponectin, Leptin, and CRP: results of two birth cohorts studies. Sci. Rep. 7, 41847 (2017).
Acknowledgements
The Ulm SPATZ Health Study was funded through an unrestricted grant by the Medical Faculty of Ulm University. We thank the midwives, nurses and obstetricians of the Department of Gynecology and Obstetrics, University Medical Center Ulm for their study support and the caring pediatricians and the mothers and their families for participation. This funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. The contributing researchers are independent of the funder.
Author information
Authors and Affiliations
Contributions
C.A.L. contributed to study design and data collection, conducted the statistical analyses, interpreted the data, and wrote the manuscript. F.R. contributed to recruitment and data collection, critically reviewed the manuscript, and approved the final manuscript as submitted. J.M.W. contributed to the acquisition and interpretation of the data, critically reviewed the manuscript, and approved the final manuscript as submitted. D.R. conceived the SPATZ study, critically reviewed and revised the manuscript, and approved the final manuscript as submitted. J.G. conceived the SPATZ study and contributed to recruitment and data collection, interpretation of the data, writing the manuscript, and approval of the final manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Logan, C.A., Weiss, J.M., Reister, F. et al. Fetal growth and incidence of atopic dermatitis in early childhood: Results of the Ulm SPATZ Health Study. Sci Rep 8, 8041 (2018). https://doi.org/10.1038/s41598-018-26440-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26440-2
This article is cited by
-
Intrauterine growth restriction induces skin inflammation, increases TSLP and impairs epidermal barrier function
Journal of Molecular Medicine (2020)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.