Abstract
Genome editing is the introduction of directed modifications in the genome, a process boosted to therapeutic levels by designer nucleases. Building on the experience of ex vivo gene therapy for severe combined immunodeficiencies, it is likely that genome editing of haematopoietic stem/progenitor cells (HSPC) for correction of inherited blood diseases will be an early clinical application. We show molecular evidence of gene correction in a mouse model of primary immunodeficiency. In vitro experiments in DNA-dependent protein kinase catalytic subunit severe combined immunodeficiency (Prkdc scid) fibroblasts using designed zinc finger nucleases (ZFN) and a repair template demonstrated molecular and functional correction of the defect. Following transplantation of ex vivo gene-edited Prkdc scid HSPC, some of the recipient animals carried the expected genomic signature of ZFN-driven gene correction. In some primary and secondary transplant recipients we detected double-positive CD4/CD8 T-cells in thymus and single-positive T-cells in blood, but no other evidence of immune reconstitution. However, the leakiness of this model is a confounding factor for the interpretation of the possible T-cell reconstitution. Our results provide support for the feasibility of rescuing inherited blood disease by ex vivo genome editing followed by transplantation, and highlight some of the challenges.
Similar content being viewed by others
Introduction
Genome editing can repair defective genes, inactivate target genes and direct transgenes to safe harbours1. The targeted correction of mutations relies on homologous recombination (HR), a DNA repair pathway of insufficient efficiency for therapeutic approaches in primary cells2. Designer nucleases, which induce a double-strand break at the target locus and stimulate HR, can provide therapeutic-level genome editing3. Ex vivo genome editing of human haematopoietic stem/progenitor cells (HSPC) for correction of inherited blood diseases is feasible4,5,6,7, but clinical application should be preceded by demonstration of efficacy in animal models.
Primary immunodeficiencies (PIDs) are rare inherited disorders of the innate and acquired immune system. They result from more than 130 inherited mutations in genes required for production, differentiation or survival of specialised leukocytes like T or B lymphocytes, natural killer cells, neutrophils or antigen-presenting cells8,9. Patients regularly present with a higher vulnerability to opportunistic pathogens or infection with uncommon organisms and may also develop autoimmunity or autoinflammatory diseases and lymphoreticular malignancies10. Severe combined immunodeficiency (scid) disorders are fatal monogenic PIDs characterised by a severe decrease or lack of functional T-cells11. Typically, leukocytes originate from the multipotent HSPC in the bone marrow, so allogeneic haematopoietic stem cell transplantation (HSCT) is the preferred treatment. However; there are significant risks and some patients suffer from chronic complications, such as graft-versus-host disease12. Alternatively, promising gene therapy trials based on ex vivo retroviral-mediated gene addition prior to autologous HSCT have been performed in patients lacking a matched bone marrow donor8. The main obstacle facing this gene therapy approach is the possible risk of insertional mutagenesis leading to malignant outcomes13. Genome editing is a promising and more accurate approach, which may minimise the risk of unintended mutagenesis.
In this study the T- B- radiosensitive scid mouse14, a model of human DNA-dependent protein kinase catalytic subunit (DNA-PKcs; encoded by PRKDC) deficiency15,16, was used to provide proof-of-principle of phenotypic rescue by genome editing in the haematopoietic system. The scid mutation (T → A, Tyr-4046 → STOP) leads to an 83-amino acid C-terminal truncation which largely reduces protein stability of DNA-PKcs and kinase activity of DNA-PK17,18. This, in turn, causes failure of V(D)J recombination and hence T- B- immunodeficiency, and defective nonhomologous end-joining (NHEJ) resulting in radiation sensitivity19. This model was selected in the anticipation that Prkdc-corrected cells would have a selective survival advantage in vivo during the differentiation of T-cell progenitors. Mutant Prkdc has recently been corrected by genome editing in induced pluripotent stem cells, which have subsequently been differentiated to T-cells and characterised in vitro20.
We have used a ZFN and repair template to edit mutant Prkdc in mouse scid fibroblasts, and show evidence of correction at the molecular and functional levels. We then gene-edited Prkdc in scid HSPC and provide molecular evidence of correction. Upon transplantation to scid animals, gene-edited HSPC led to detectable levels of gene correction in several tissues of some of the recipients. In some cases we also observed double-positive CD4/CD8 T-cells in thymus, and single-positive T-cells in blood after primary and secondary transplants, but the evidence of cellular reconstitution is not entirely conclusive due to the leakiness of this model. We conclude that Prkdc gene-editing has been achieved in both scid fibroblasts and HSPC, and that the latter are able to mediate partial in vivo reconstitution, which can be detected at the molecular level and might lead to increased levels of T-cells in some of the recipients.
Results
Prkdc gene editing in scid fibroblasts
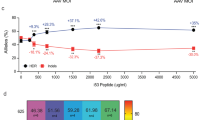
To edit Prkdc, we designed ZFNs to induce a DNA double stranded break (DSB) at this locus. We produced a ZFN heterodimer that recognizes a sequence in exon 85 of the murine Prkdc gene, 32 nucleotides downstream from the scid point mutation (Fig. 1a and Supplementary Figure 1). The ZFN monomers, carrying triple FLAG epitopes to allow facile detection by Western blotting, were cloned into lentiviral (LV) transfer plasmids (Supplementary Figure 2). Integration-Proficient Lentiviral Vectors (IPLVs) and Integration-Deficient Lentiviral Vectors (IDLVs) were produced and the expression of the ZFN monomers in Tert-immortalised scid fibroblasts was confirmed by Western blotting following LV transduction (Fig. 1b). Fibroblasts were immortalised for the purpose of obtaining clones for downstream analysis, as preliminary experiments had shown an inability of the primary cells to grow as clones. As expected from transductions of dividing cells21, this revealed strong expression from ZFN IPLVs and lower levels from ZFN IDLVs. To test the functionality of the ZFN at the target locus we used a mismatch-sensitive endonuclease assay, the Surveyor-nuclease (Cel-I) assay22. It should be noted that this assay underestimates the actual cutting frequency as most of the DSBs are repaired error-free23. With both IPLVs and IDLVs we observed dose-dependent increases in ZFN cutting activity, reaching 20% and 16%, respectively (Fig. 1c). Deep sequencing of the IPLV-treated sample at qPCR multiplicity of infection (MOI) 125 showed that 18% of the sequence reads carried small insertions and deletions at the site of ZFN cleavage (Table 1), corroborating the result of the Cel-I assay. As Prkdc is an autosomal gene, the percentages of indels or correction at the molecular level given throughout this paper would translate into approximately double the percentages of cells, if the vast majority of cells were gene modified at only a single allele. In practice, it has been shown previously that ~50% of the cells may be biallelically modified3.
Prkdc gene editing strategy and outcomes, ZFN design and gene expression, and cutting activity in scid fibroblasts. (a) Schematic of Prkdc, including location of scid and ZFN target sites, structure of ZFN and sequences of various Prkdc alleles around scid site. (b) Production of ZFN monomers from SFFV-driven IPLV and IDLV (qPCR MOI 100). Proteins were extracted 3 d after transduction. The blotting revealed FLAG-tagged ZFN monomers (42 and 38.5 kDa for ZFN1 & ZFN2, respectively), with α-tubulin used as a loading control (49 kDa). (c) Cel-I analysis of Prkdc cutting by ZFN. Scid fibroblasts were transduced with IPLV- and IDLV-ZFN at the indicated MOI and genomic DNA was extracted 3 d later. The Cel-I-digested PCR products were separated on a polyacrylamide gel and diagnostic bands (black arrows) were used to calculate the % of indels induced by the various ZFN treatments and indicated on the gel image.
A plasmid-based gene targeting experiment was performed to assess the frequency of ZFN-mediated homology-dependent repair (HDR) at the Prkdc locus. The Prkdc ZFN plasmids and a recombination template, carrying a 7.5 kb Prkdc genomic DNA including exons 82–86 and a neomycin phosphotransferase (neo) gene, were transfected separately or in combination into Tert-immortalised scid fibroblasts. Neo confers resistance to G418 and upon HDR will be targeted for insertion into intron 85 (Supplementary Figure 3). Random integration of the recombination template is also expected to produce a background of G418-resistant colonies. A significant ~30-fold increase in the number of G418-resistant colonies, to ~1.2% of total cells, was observed in the presence of both ZFN and template together compared to template alone (p < 0.0001; Fig. 2a). An inside-out PCR screening of 36 G418-resistant clones from the ZFN + template groups showed 20 of them to be Prkdc-targeted by HDR (Supplementary Figure 3), a percentage that is only achievable through either promotor-trap2,24,25 or designer nuclease-mediated strategies3. When SFFV or CMV-driven IPLV-ZFN and the plasmid template were used, the frequencies of G418-resistant colonies were approximately 50% of those obtained in the all-plasmid paradigm (not shown). This lower frequency could be related to sub-optimal experiments combining transduction/transfection, or increased cytotoxicity from IPLV-ZFN compared to plasmid-ZFN.
Phenotypic and molecular analyses of Prkdc gene editing in scid fibroblasts. (a) Gene editing in scid fibroblasts by plasmid transfection. Prkdc-neo template plasmid and ZFN monomer plasmids were used. All samples but the mock received repair template. G418-resistant CFUs were stained with crystal violet and counted. Statistical significance was evaluated using one-way ANOVA with Dunnett’s post hoc, comparing against template-only sample; ****p < 0.0001. (b) Prkdc gene editing in scid fibroblasts by transduction. Cells were transduced with IPLV-ZFN/IDLV-template or IDLV-ZFN/template at the indicated qPCR MOI and genomic DNA was extracted 10 d post-transduction. Scid locus was PCR-amplified with primers external to template, and ZFN-mediated gene correction was quantified from the diagnostic BsaWI band (arrow) and shown as %Prkdc correction. All samples shown were run in the same gel, from which irrelevant lanes have been cropped at places shown as thin white strips. The uncropped gel is shown in Supplementary Figure 6. (c) Restoration of DNA-PK activity after ZFN-mediated gene correction. Scid fibroblasts were transduced as above with IPLV or IDLV ZFN/template at the indicated MOI. The DNA-PK activity assay was performed using nuclear proteins extracted 10 d post transduction. Specific DNA-PK enzyme activity is plotted as a ratio between sample (gene edited cells) and mock (scid fibroblasts). Statistical significance was evaluated using a one-way ANOVA with Dunnett’s post hoc, comparing against mock sample; ***p < 0.0005. (d) Enhanced resistance of Prkdc gene-edited cells to DNA damage. Scid fibroblasts transduced with IDLV-ZFN/template at the indicated MOI were exposed to 10 μM melphalan for 1 h weekly for 3 weeks, then colonies were stained with crystal violet and CFU scored. Statistical significance was evaluated using a one-way ANOVA with Dunnett’s post hoc, comparing against mock sample; ****p < 0.00005. (e) Melphalan enrichment correlates with increase in molecular signature of Prkdc gene editing. Scid fibroblasts transduced with IDLV-ZFN/template at the indicated MOI were maintained in culture with or without weekly 10 μM melphalan treatment. Genomic DNA was extracted 3 weeks post-transduction and subjected to BsaWI digestion. The %Prkdc gene correction calculated from the intensity of the diagnostic band (arrow) is indicated on the gel.
We then analysed gene correction at Prkdc by combined delivery of the ZFNs (via IPLV or IDLV) with a 5-fold shorter (1.6 kb, Fig. 1a) repair template suitable for ex vivo IDLV delivery strategies. In this template, a silent diagnostic BsaWI restriction site has been introduced via site-directed mutagenesis two nucleotides upstream from the scid site (Fig. 1a and Supplementary Figure 1). Upon HDR-mediated gene editing, the BsaWI site is incorporated into the target locus alongside the correct nucleotide at the scid site (Supplementary Figure 4) and can be subsequently used as diagnostic for gene editing26. For experiments with IPLV-ZFN the template for gene correction (the “donor” construct) was supplied on a separate IDLV; for experiments with IDLV-ZFN, the donor construct was present in both IDLVs carrying the nuclease monomer genes. The ZFN-mediated frequency of targeted gene correction reached 7.7% with IPLV delivery of the ZFN, and 4.7% with IDLVs (Fig. 2b). Deep sequencing of the IDLV sample at qPCR MOI 1,000 confirmed the presence of 1.53% corrected sequences including the diagnostic BsaWI site and the wild-type nucleotide at the scid site, both of which result from ZFN-driven targeted correction.
Next, we used these pools of fibroblasts to determine whether ZFN-mediated gene correction of Prkdc led to restoration of DNA-PK activity, using a previously described assay27. Our results showed that DNA-PK activity in gene-corrected fibroblasts increased in a vector dose-dependent manner, reaching statistical significance for the IPLV at qPCR MOI 1,000 (Fig. 2c). Prkdc gene correction also led to increased resistance to melphalan-induced DNA interstrand cross-linking damage, the repair of which is dependent on an active DNA-PK complex28. We used 10 μM melphalan, which reduces the viability of scid fibroblasts by 80% and that of wild-type cells by 40% (Supplementary Figure 5). We observed a significant vector dose-dependent increase in the number of colony forming units (CFU) produced in the presence of melphalan by IDLV-gene edited scid fibroblasts (Fig. 2d). The increase in CFU was mirrored by an elevated level of the BsaWI gel band diagnostic for gene correction, enriched up to 3.7-fold (from 3.3% to 10.7% Prkdc modification; Fig. 2e). No CFU were obtained from IPLV-ZFN-treated fibroblasts, suggestive of cytotoxicity associated with constitutive expression of the Prkdc ZFNs in fibroblasts.
Prkdc gene editing in scid HSPC and transplantation
To attempt Prkdc gene editing in HSPC we used lin− scid bone marrow cells29. Using IPLV-delivered ZFN we detected up to 18% target gene cutting according to a Cel-I assay (Fig. 3a). Prkdc gene correction determined by deep sequencing ranged 4–17%. In contrast, when using IDLV, deep sequencing showed Prkdc indels with SFFV-ZFN peaking at ~0.4%, corroborating the known weakness of the SFFV promoter when used in the context of an IDLV vector to deliver transgenes21. Given the poor efficiency obtained with the SFFV promoter in the IDLV configuration, we performed a search for an optimised non-integrating delivery method including the use of a variety of promoters in IDLVs, and a number of adenoviral vectors. Optimal eGFP expression in HSPC (considering both transduction efficiency and mean fluorescence intensity) was obtained with IDLVs driven by a CMV promoter. In preliminary experiments, IDLV-mediated Prkdc gene correction in HSPC reached 2.1% of sequences bearing both the diagnostic BsaWI site and the wild-type nucleotide at the scid site as detected by deep sequencing (data not shown).
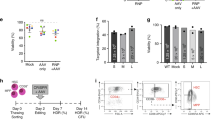
ZFN activity in HSPC and rescue of scid mouse T-cell deficiency. (a) Cel-I analysis of ZFN-mediated Prkdc cutting. Scid HSPC were transduced with IPLV-ZFN at the indicated MOI and genomic DNA was extracted 3 d later. Diagnostic Cel-I digestion products are indicated with black arrows and the % of indels induced by ZFN treatments shown on the gel image. (b–d) T-cell populations in PBMC from primary transplant recipients, 27 weeks post-transplantation. Primary transplantation of irradiated female scid mice was done with gene-edited male scid HSPC, while secondary transplantation of female scid mice was with whole bone marrow (BM) cells. Flow cytometry analyses of (b) CD3, (c) CD4 and (d) CD8 T-cell populations from wild-type balb/c and scid mice, and balb/c, scid-IPLV-eGFP, scid-IPLV-ZFN/IDLV-template and scid-IDLV-ZFN/template transplant recipients are shown. (e) Thymic T-cell precursors in primary transplant recipients, 27–32 weeks post-transplantation. 50% of gene-edited transplant recipients were responders with double-positive CD4 CD8 cells. (f) T-cell populations in PBMC of secondary scid-IPLV-ZFN/IDLV-template transplant recipient, 35 weeks post-transplantation. (g) Proliferative response of purified spleen CD3 T-cells at 35 weeks post-secondary transplantation. CFSE-stained T-cells were cultured in the presence (black line) or absence (grey overlay) of concanavalin A and IL-2 or anti-CD3/CD28 and IL-2. After 72 h, cell expansion of the T-cell population was assessed by CFSE dilution measured by flow cytometry.
Having determined that Prkdc gene-corrected HSPC could be obtained, we set up an experimental schedule (Supplementary Table 1) including ex vivo Prkdc gene editing in scid HSPC, primary transplantation into sub-lethally irradiated scid mice, and secondary transplantation of whole bone marrow into similarly irradiated scid mice, with periodic phenotypic analyses. HSPC were transduced with IPLV-SFFV-ZFN or IDLV-CMV-ZFN, albeit as shown above IDLV delivery of the ZFN monomers was clearly suboptimal in HSPC; the repair template was always provided in an IDLV as described above for fibroblasts. Control groups included animals receiving (i) a wild-type transplant of lin- HSPC, and (ii) lin- scid HSPC transduced with IPLV-eGFP. For molecular analyses, blood samples were collected at 10, 17 and 27 weeks post-transplantation from all primary transplant recipients, and at 9, 20 and 35 weeks from secondary transplant recipients. Peripheral blood mononuclear cells (PBMC) were analysed by flow cytometry to quantitate T-cells (CD3, CD4 and CD8), B-cells (B220) and myeloid (CD11b) cells. Table 2 summarises engraftment, vector copy number (VCN), phenotypical analyses and deep-sequencing data for Prkdc gene correction. As expected from animals receiving scid HSPC, the myeloid cells were considerably increased, as the recipients essentially did not develop B-cells and had varying levels of T-cells. VCNs were negligible in animals receiving transplants from IDLV-only treated cells, as these non-replicating vectors will dilute progressively with the cell population expansion.
One primary recipient from each ZFN + template HSPC group, IPLV and IDLV, showed increasing levels of CD3 T-cells over time, reaching 14.37 and 2.46% of PBMC respectively (Fig. 3b and Table 2). Similar results were obtained with CD4 and CD8 T-cell stainings (Fig. 3c,d and Table 2). When we analysed the thymus of the primary transplant recipients, fifty percent of animals from the ZFN + template groups (3/5 from the integrating group and 2/5 from IDLV group) showed double-positive CD4/CD8 T-cells (Fig. 3e and Table 2). Some of these CD4/CD8 patterns were somewhat abnormal, with very high levels of double-positive cells. Spleen and bone marrow were also analysed at culling, with unremarkable results.
Unfractionated bone marrow cells from the best primary responders (mice presenting with the highest levels of CD3 and CD4/8) in groups treated with ZFN + template combinations, as well as representative mice from control groups, were used for secondary transplantation. PBMC T-cells were detected in all responder BM recipients (Fig. 3f and Table 2). To assess spleen CD3 T-cell functionality we measured proliferation in response to non-specific stimulation with concanavalin A/IL-2 or anti-CD3/CD28/IL-2. Proliferative responses were observed for both study groups (Fig. 3g and Table 2). However, as scid animals are well known to develop thymic lymphomas that can lead to the generation of T-cells30, the mere detection of T-cells in primary and secondary transplant recipients is not definite confirmation for ex vivo genome editing.
Conclusive evidence of low-level reconstitution with Prkdc gene-corrected cells was obtained by deep-sequencing in a variety of tissue samples from both primary and secondary recipients (Table 2). Two primary recipients of IPLV-ZFN + template and three of IDLV-ZFN + template showed varying levels of correction. One of the primary recipients of the IPLV-ZFN + template group had the following percentages of corrected sequences: thymus, 0.4–13.7%; blood, 5.6%; spleen, 0.45%; and spleen CD8 T-cells, 26.9%. A secondary recipient from the same group showed 5.4% in blood, 8.8% in spleen, and 24.6% in spleen CD3 T-cells. Other recipients from each ZFN + template group showed lower but detectable reconstitution levels, suggesting some stability of the gene-corrected population across the transplantation experiment.
Off-target analyses
To study potential deleterious effects of the genome editing process we analysed off-target cutting, the presence of indels at the ZFN target site on the same haplotype as the corrected allele, and assessed the incidence of tumours. Off-target cutting was measured at 10 sites predicted by bioinformatic analysis based on data obtained by systematic evolution of ligands by exponential enrichment (SELEX; Supplementary Table 2). Samples used to evaluate off-target cutting included mock or ZFN-treated scid fibroblasts (with 0.05 and 18% indels at target site, respectively; Table 1), mock or ZFN-treated HSPC (0.08 and 0.60% indels at target site, respectively; Table 1), and ZFN + template treated mouse samples (Table 3), including spleen (1.23% indels, 0.07% gene correction), thymus (46.03% indels, 11.81% gene correction), and spleen CD8 T-cells (68.06% indels, 22.18% gene correction). As shown in Tables 1 and 3 for cultured cells and mice, respectively, very low levels of indels were detected at essentially all the potential off-target sites in control and ZFN-treated samples, with very small differentials between the two (differential average 0.03%, range −0.88 to 1.34%). This suggests that off-target indels detected in the ZFN-treated samples are essentially background levels due to the nature of the assays involved.
Deep sequencing analysis of genomic DNA from samples treated with ZFN showed that a significant proportion of indels were a recurrent 44-bp deletion including the ZFN target site (Fig. 1a). In samples treated with ZFN + template, indels (particularly the recurrent deletion) were also observed in association to gene correction (frequencies of gene correction quoted in Table 2 exclude events associated to an indel). The percentage of gene correction with associated indel was on average 11.25% of the total gene correction frequency (range 0–100%). The recurrent deletion results in the loss of the 3′ end of exon 85, and hence presumably aberrant splicing of the final exon 86. The likely result at the protein level is an aberrant C-terminal tail downstream of the scid site, with unclear functional outcome. No obvious sequence microhomology is present around the deletion breakpoints which could explain the high-frequency event. One possible explanation is that, although competent for indel generation in response to ZFN-induced DSBs (as we have shown by Cel-I assay and deep sequencing), the defect in Prkdc scid cells could create a bias towards a particular microdeletion around the ZFN target site.
Potential tumorigenicity was assessed by gross pathological examination of organs after sacrifice in all animals that could be examined. We observed occasional suspected tumours in transplanted animals, including two splenomegalies in primary transplanted animals (IPLV-ZFN, IDLV-ZFN + template), and a possible leukaemia (IPLV-eGFP; control) and a thymomegaly (IDLV-ZFN + template) in secondary transplanted animals (Table 2). The latter animal was a typical case of the reported leakiness of the Prkdc scid model, in which T-cells can be detected in animals that have developed thymic lymphomas30, as we observed a thymomegaly and T-cells but failed to detect gene editing at the molecular level. We analysed by deep sequencing genomic samples of the primary transplant spleens; bone marrow, thymus and spleen from the leukaemic control animal; and thymus, spleen and PBMCs from the thymomegalic animal. In all cases we observed Prkdc indel frequencies at the low end of the background range, (0.00–0.04%) and no gene correction events. As (i) a suspected tumour occurred in a control animal in this experiment, (ii) a tumour in a ZFN-treated animal was a very likely spontaneous thymic lymphoma typical of this model, and (iii) there is no evidence of significant editing of the target site in any of the tumour samples from ZFN-treated animals, we conclude that the potential tumours are unrelated to ZFN or lentiviral treatment of the cells.
Discussion
It has been recently shown that human HSPC corrected by genome editing can be transplanted into immunodeficient mice, where they regenerate an active, humanised haematopoietic system4,5,6,7. Demonstration of genetic modification and phenotypic rescue in animal models would be further support for such gene therapies in advance of clinical application. We have focused on a mouse model of primary immunodeficiency, with the aim of showing feasibility of genome editing as a therapeutic approach. In this Prkdc scid model, we have provided conclusive evidence of molecular and phenotypic correction using scid fibroblasts in vitro. We have demonstrated induction of targeted indels by the Prkdc ZFN through a Cel-I assay and deep sequencing, targeted correction in the presence of the ZFN and a corrective template by the introduction of a BsaWI diagnostic restriction site and deep sequencing, as well as phenotypic rescue of DNA-PK enzymatic activity and increased resistance to melphalan DNA damage. Likewise, using HSPC in culture we have shown the introduction of targeted indels and Prkdc gene correction.
We have transplanted the gene-edited HSPC into irradiated Prkdc scid recipients to analyse the efficiency of ex vivo rescue. Some primary recipients of the ZFN + template groups have shown increasing levels of CD3, CD4 and CD8 T-cells. At culling, some of the mice also showed increased levels of double-positive CD4/CD8 T-cells in the thymus. Some of the secondary transplant recipients have also shown enhanced levels of CD3, CD4 and CD8 cells. While these are important observations regarding possible rescue of T-cells, Prkdc scid animals are well known to develop thymic lymphomas that can lead to the generation of T-cells30 and hence the presence of T-cells per se is not conclusive proof of rescue. These immunodeficient animals also lack B-cells, but we have not observed recovery of B-cell levels. This was not entirely unexpected, as observations in immunodeficient mice indicate that significantly higher levels of gene addition in HSPC are required to rescue B-cells compared to T-cells31. A similarly favoured rescue of T- over B-cell deficiency has been observed in some clinical trials of gene addition therapy, albeit this may be at least in part related to absence of conditioning32.
Definite proof of the ability of gene-edited HSPC to mediate some level of reconstitution upon transplantation has been obtained by deep sequencing of animal tissues from some of the recipients. The expected signature for genome editing (correction of the scid mutation associated to the introduction of the BsaWI diagnostic site) has been observed in blood PBMC, thymus, spleen and purified spleen CD3 or CD8 T-cells. Levels are variable in the positive primary transplanted animals, higher with IPLV-ZFN and were also observed in secondary recipients. We consider the presence of the genome editing signature proof that reconstitution can be achieved, albeit inefficiently both in terms of the number of positive animals and the levels in different tissues.
Pharmacological inhibition of DNA-PKcs has been shown to increase the frequency of CRISPR/Cas9-mediated genome editing33; thus, it is possible that in Prkdc-deficient cells the defect in NHEJ favours HDR genome editing. It is also conceivable that Prkdc deficiency could contribute to the recurrence of the 44-bp deletion including the ZFN target site that we have observed in multiple samples from genome editing in vitro and ex vivo. Inhibition of DNA-PK during repair of CRISPR/Cas DSBs has been reported to inhibit canonical NHEJ and promote alternative (microhomology-mediated) NHEJ, resulting in a bias towards larger deletions34. These results highlight the importance of considering the possible effect of DNA damage repair defects (and targeted manipulation of the DSB repair systems34) on genome editing strategies. We have alleviated concerns over cytotoxicity and genotoxicity by showing background levels of off-target indels in scid fibroblasts and HSPC treated with the ZFN. We have also analysed the expected, occasional tumours observed in the irradiated, transplanted immunodeficient animals, showing they appear unrelated to ZFN treatment.
We have shown that some level of reconstitution can be achieved using ex vivo genome editing with ZFN and Prkdc template delivered with IDLV, an advantageous non-integrating system which will be diluted out as the gene-edited population expands. Reconstitution was also achieved with IPLV-delivered ZFN, somewhat surprisingly. Reconstitution has not been achieved in all primary recipients of gene-edited cells, so significant improvements in efficiency are required. In conclusion, a mouse model of primary immunodeficiency can be partially reconstituted by ex vivo HSPC genome editing and transplantation. The tremendous expansion in the genome editing field witnessed over the last few years35,36 attests to the power of the technology and forecasts fast advance of ground-breaking HSPC gene repair towards the clinic, where ZFN-mediated gene disruption in T-cells is already showing excellent results in the context of therapy for HIV37.
Methods
ZFN design and production
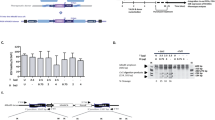
ZFNs targeting Prkdc exon 85 were designed by modular assembly using an archive of zinc finger proteins. Optimised zinc-finger domains were linked to obligatory heterodimeric FokI domains38. ZFN genes were cloned and initially tested on pVAX plasmid backbones, and then transferred onto self-inactivating lentiviral transfer plasmids pCCLsc_S_W (where transgene is driven by SFFV promoter and followed by Woodchuck hepatitis virus post-transcriptional regulatory element, WPRE) or pRRLsc_C_W (CMV promoter and WPRE).
Targeting constructs
Two Prkdc repair templates were made: (i) a classical, pBlueScript plasmid-based long construct including a neo gene within the homology region, and (ii) a short, lentiviral vector-based PCR-amplified repair template with a diagnostic restriction site. The long construct included the 7.5-kb HindIII fragment spanning Prkdc exons 82–86, subcloned from a wild-type yeast artificial chromosome (YAC) previously shown to complement the scid mutation in cell culture39. A neo cassette expressed from a PGK promoter was inserted in intron 85, at a PmlI site, allowing for positive selection after calcium phosphate-mediated plasmid transfection. The short Prkdc template was constructed by PCR amplification of a 1,626 bp sequence from wild-type Balb/c mouse fibroblast genomic DNA using forward and reverse primers 5′-AATGTTTAGTTTTTATGAGTGTTTTGC-3′ and 5′-CAAGCCATCTCTCTAGCCCTAC-3′, respectively. Two silent point mutations were introduced via site-directed mutagenesis 3- and 6-bp upstream of the scid site to create a diagnostic BsaWI restriction site (Site-Directed Mutagenesis Kit, Stratagene, USA). The short Prkdc template was cloned into the self-inactivating lentiviral vector plasmids, which then encoded either the Prkdc template alone or one ZFN monomer and the Prkdc template together. The template was cloned upstream of the central polypurine tract/central termination sequence (cPPT/cTS) and in reverse orientation.
Cells, transfection and transduction
HEK293T, HeLa, wild-type and scid mouse fibroblasts were maintained in DMEM with stable glutamine and 4.5 g/l glucose supplemented with 10% FBS and 1% penicillin/streptomycin at 37 °C and 5% CO2. Tert-immortalised scid fibroblasts, obtained by transduction with a retroviral vector encoding mouse Tert and puromycin-acetyltransferase (pac) were maintained likewise but in the presence of 3 μg/ml puromycin and used in all fibroblast experiments. Wild-type and scid HSPC were obtained from bone marrow, by flushing the femur and tibia of 6 to 8-week-old Balb/cOlaHsd or Balb/cJHan(tm)Hsd-Prkdc scid mice. Lin− HSPC were isolated using a MACS Lineage cell depletion kit (Miltenyi Biotec, Germany) as directed by the manufacturer. HSPC were cultured without splitting, in 24-well non-tissue culture-treated plates at 37 °C and 5% CO2, using serum-free StemSpan medium (Stemcell Technologies) containing 100 ng/ml murine stem cell factor (SCF), 100 ng/ml murine Fms-related tyrosine kinase 3 (Flt-3), 20 ng/ml human interleukin-6 (IL-6) and 1% penicillin/streptomycin. Fibroblast transfections for genome editing experiments were done using 106 cells/plate (n = 3), calcium phosphate co-precipitation and 10 μg (template plasmid in transfection/transduction experiments) or 30 μg of DNA (10 μg of each if co-transfecting ZFN monomer and template plasmids, using an irrelevant plasmid to normalise total amount of DNA in controls). Cells were transduced with IPLV or IDLV in the presence (HeLa, fibroblasts) or absence (HSPC) of 8 μg/ml polybrene, and then processed for analysis or injection as indicated. Fibroblast genome editing experiments involving ZFN vector transduction and template plasmid transfection were done with an optimum 48 h delay before the latter was carried out. 800 μg/ml G418 added 48 h post-transfection was used for selection, and colonies were either stained with crystal violet for counting 9 d post-transfection or picked and expanded for PCR analysis of Prkdc gene editing.
Lentiviral vector production
Third-generation lentiviral vectors were prepared as described40,41. Briefly, HEK293T cells were co-transfected with four plasmids by calcium phosphate precipitation, at a molar ratio of 1:1:1:2 (packaging:rev:envelope:transfer). For IPLVs the packaging plasmid was pMDLg/pRRE and for IDLVs it was pMDLg/pRRE-intD64V41. Rev was delivered on pRSV-rev and the vectors were pseudotyped with VSV-G protein using plasmid pMD2.VSV-G. The transfer plasmids encoded either the short Prkdc template alone, one of two ZFN monomers or the template and one of the monomers. Vector particles were concentrated by ultracentrifugation and titrated by real time-PCR using HeLa cell transductions41.
Western blot
Cells were homogenized in RIPA lysis buffer containing protease and phosphatase inhibitors. Protein concentrations were determined using the DC protein assay (Bio-Rad) and proteins ran on 5–12% acrylamide gradient gels. For FLAG epitope detection, the transferred membrane was incubated with mouse anti-FLAG M2 monoclonal antibody (Stratagene, UK) at 1:1000 dilution, using IRDye® 800CW goat anti-mouse (Li-cor GmbH, Germany) at 1:2000 dilution as secondary antibody. For loading controls, rabbit polyclonal anti α-tubulin (Abcam, UK) at 1:2000 dilution was used as primary antibody, and then Alexa Fluor® 680 Goat anti-rabbit (Invitrogen, USA) at 1:5000 dilution as secondary antibody. Blots were scanned at 700 and 800 nm channels for α-tubulin and anti-FLAG, respectively, using an Odyssey imager (Li-cor Bioscience, Germany).
Cel-I assay
ZFN-induced indels were detected using the SURVEYOR mutation detection kit (Transgenomic, USA) as described previously42. At day 3 post-transduction, genomic DNA from 1 × 106 mock- or ZFN-treated scid fibroblasts or HSPC was extracted using DNeasy kit (Qiagen, Germany) and PCR-amplified using forward and reverse primers 5′-GCAGACAATGCTGAGAAAAGG-3′ and 5′-GCACAAAACAGACAAGGGTGT-3′, respectively. Thermocycling conditions were as follows: 95 °C, 5 min; 95 °C, 30 s, 60 °C, 30 s, and 68 °C, 40 s for 35 cycles; and a final extension step of 68 °C for 2 min. The PCR product was denatured and allowed to reanneal, and processed as per instructions of Surveyor kit. The final products were analysed by polyacrylamide gel electrophoresis to assess ZFN-induced indels, using Image J/V2 software to quantify the intensity of the bands.
PCR screening for plasmid-based Prkdc gene editing
Following plasmid transfection or lentiviral transduction/plasmid transfection of ZFN monomer genes and the long Prkdc-neo targeting template, G418-resistant colonies were screened using an inside-out PCR strategy. Genomic DNA was extracted with the DNeasy® kit (Qiagen). The forward primer (5′-TCGCCTTCTTGACGAGTTCT-3′) hybridised within the neo cassette, whereas the reverse primer (5′-TTTTCCCCCTCATGTCACTC-3′) was directed to Prkdc genomic DNA downstream of the template right homology arm. The amplicon length was 1335 bp. For PCR amplification, 50 µl reactions were prepared containing 10 µl 5X GoTaq® (Promega, UK) reaction buffer, 1.5 µl of 10 mM dNTPs, 4 µl of 25 mM MgCl2, 1.5 µl of 10 µM of each forward and reverse primer, 200 ng DNA template, and 0.5 µl of 5 u/µl GoTaq DNA polymerase. Thermo-cycling conditions were: initial denaturation at 95 °C for 2 min, then 35 cycles of 95 °C for 45 s, 59 °C for 60 s, 72 °C for 30 s, followed by final extension at 72 °C for 5 min. PCR products were purified using a PCR purification kit (Qiagen) and digested with restriction enzymes predicted to cut the amplicons.
BsaWI assay
Prkdc gene editing was measured by gel assay as described previously26. Briefly, genomic DNA from mock- or ZFN/template-treated scid fibroblasts or HSPC was extracted using DNeasy kit (Qiagen, Germany), and 100 ng subjected to PCR using primers external to the Prkdc template (forward 5′-AACAATCCTCCTCCGAACCT-3′ and reverse 5′-TGGAGGTGGAAGAACCAAAC-3′). Thermocycling conditions were as follows: 95 °C, 30 s; 95 °C, 30 s, 59 °C, 60 s, and 65 °C, 75 s for 35 cycles; and a final extension step of 65 °C for 10 min. The PCR products were digested overnight with BsaWI (NEB, UK) and separated on a 0.7% agarose gel. The DNA was transferred overnight onto GeneScreen Plus Membrane (Perkin Elmer, USA), hybridised overnight using as probe [α-32P]-ATP labeled Prkdc template, washed and exposed to a phosphoimager screen. The screen was scanned using a Typhon-8600 (Amersham Pharmacia Biotech, UK) and the bands were quantified using Image J/V2 software26.
DNA-PKcs Assay
The SignaTECT DNA-dependent protein kinase assay system (Promega, USA) was used as previously described27. Nuclear proteins from mock- or ZFN/template-transduced scid fibroblasts were extracted using the CelLytic Nuclear Extraction kit (Sigma Aldrich, USA), and processed as per SignaTECT system instructions. Biotinylated peptide substrates were captured by spotting reactions onto individual squares of high binding capacity capture membrane. After several washes of the membrane, DNA-PK activity was quantified using the above phosphoimaging system.
MTT assay
Cellular viability was determined by the MTT assay as described previously43. Wild-type and scid fibroblasts were exposed to increasing concentrations of melphalan for 1 h, washed, and then cultured for a further 5 days. Cells were then exposed to the MTT tetrazolium salt for 4 h at 37 °C, and the formation of formazan was measured at 560 nm using a microplate reader (Promega, USA). The concentration giving the maximum difference between wild-type and scid fibroblast viability was chosen for selection. All values are averages of 3 independent experiments each done in duplicate.
Colony Forming Unit (CFU) assay
105 mock- or ZFN/template-transduced scid fibroblasts were cultured in the presence of 10 μM melphalan (optimal dose determined using MTT assay) for 1 h, and the treatment was repeated once a week for three weeks, until colonies formed. Colonies were either stained using crystal violet and counted or pooled for analysis using the gene editing BsaWI assay; n = 3.
Deep sequencing analysis
Deep sequencing was performed using the MiSeq system (Illumina, San Diego CA). Specific primer pairs (Supplementary Table 3) were designed, each amplifying 100–200 bp fragments spanning the target site or one of the predicted top 10 putative off-target sites (Supplementary Table 3), and each incorporating adaptor sequences for the MiSeq processing. Briefly, genomic DNA samples were initially amplified using primers listed in Supplementary Table 3, then a nested adaptor PCR was run using the primers listed in the footnote to Supplementary Table 3. Following amplifications, PCR products were barcoded by running a 14-cycle barcode PCR. The barcoded PCR products were purified using a PCR purification kit (Qiagen), pooled and sequenced using the Illumina MiSeq platform. A custom-written computer script was used to extract “high-quality sequence reads”, based on their alignment with the wild-type template sequence. Sequences that contained a deletion or insertion resulting in shorter or longer amplicons which involve any base in the ZFN cleavage site were classified as an NHEJ-mediated deletion or insertion, respectively; together, such mutations are classified as “indels”. Sequences with the expected changes (3 substitutions, 1 to wild-type and 2 for novel BsaWI site (Fig. 1a) were classified as “corrected”.
HSPC transplantation
All experiment procedures were approved by the Institutional Research Ethics Committee (Institute of Child Health, University College London, UK) and performed according to UK Home Office Animal Welfare Legislation. For primary transplantation, cells were collected 16 h post-transduction, washed twice in phosphate-buffered saline (PBS) and resuspended in PBS. Female BALB/cJHan(tm)Hsd-Prkdc scid mice were sub-lethally irradiated (2.5 Gy) and then transplanted with ~1 × 106 male cells in 200 µl PBS by tail vein injection (n = 5 per transplant group; Supplementary Table 1). For secondary transplantation, 32 weeks post-primary transplantation, whole bone marrow of control and responder mice was isolated and ~1 × 106 cells transplanted by tail vein injection onto female Balb/cJHan(tm)Hsd-Prkdc scid mice previously sub-lethally irradiated (2.5 Gy; n = 2 for wild-type transplant, n = 3 for IPLV-eGFP transplant, n = 4 for IPLV-ZFN and IDLV-ZFN groups). Blood samples were collected at different intervals by tail vein withdrawal. Table 2 includes animals for which molecular data are available.
Engraftment and vector copy number measurements
Engraftment of male donor-derived cells in the female recipient mice was calculated against standard curve with known percentage of male DNA isolated from C57 Balb/c male mice bone marrow. DNA was extracted using the Qiagen DNeasy® kit. A 102-bp sex-determining region (Sry) on the Y chromosome was amplified by qPCR using primers forward 5′-TCATCGGAGGGCTAAAGTGTCAC-3′ and reverse 5′-TGGCATGTGGGTTCCTGTCC-3′. Thermocycling conditions were: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 20 s, 72 °C for 20 s. A melting curve analysis was performed at the end with continuous reading between 50 and 100 °C. Titin was used as a reference gene to normalise the total number of cells. qPCR was carried using primers forward 5′-AAAACGAGCAGTGACGTGAGC-3′ and reverse 5′-TTCAGTCATGCTGCTAGCGC-3′. Cycling conditions were: 95 °C for 10 min, 40 cycles of 95 °C for 15 s, 60 °C for 60 s, 72 °C for 15 s, followed by a melting curve analysis.
To analyze the number of vector integration events (vector copy number), DNA was prepared using the DNeasy kit (Qiagen) according to the manufacturer’s instructions. The vector-specific WPRE was amplified with forward primer 5′-TGGATTCTGCGCGGGA-3′, and reverse 5′-GAAGGAAGGTCCGCTGGATT-3′ with the probe 5′-FAM-CTTCTGCTACGTCCCTTCGGCCCT-TAMRA-3′ (Eurofins). The WPRE copy number was normalized against the PCR product of mouse titin exon 5, which was amplified with the primers described above with the addition of the probe 5′-FAM-TGCACGGAAGCGTCTCGTCTCAGTC-TAMRA-3′ (Eurofins) for quantification. The real-time PCR was performed on samples and plasmid standards which contained both target genes, using 2 µl DNA, 1x Taqman Universal Mastermix (Applied Biosystems), 900 nM each of forward and reverse primers and 200 nM FAM-labeled probe in a 20 μl reaction (in triplicate) and analysed using the ABI Prism 7000 Real Time PCR System (Applied Biosystems, California, USA).
Flow cytometry analysis
Peripheral blood mononuclear cells were labelled with anti-mouse CD3-PECy7 (or Pacific blue) antibody, anti-mouse CD4-PE or anti-mouse CD8-APC. Flow cytometry data were acquired with a CyAn ADP (Beckman Coulter, USA) and analyzed using FlowJo software (TreeStar Inc, Ashland, OR, USA).
T-cell proliferation assay by CFSE dilution analysis
CD3 T-cell splenocytes were purified by magnetic capture and incubated in RPMI-1640 (Sigma) supplemented with 100 IU/ml penicillin, 100 μg/ml streptomycin, 2 mM L-glutamine, 0.01 M Hepes, 50 μM 2-β-mercaptoethanol (Gibco-Invitrogen), 10% heat-inactivated FCS (EuroClone, UK), supplemented with 1 μM CFSE (Celltrace, Invitrogen). The efficiency of CFSE staining was greater than 99%. CFSE-labelled cells were then incubated in the presence of 3 µl/ml ConA (Sigma) and 100 IU/ml IL-2 (R&D Systems) or 0.5 µl mouse CD3/CD28 T Expander (Dynal Biotech, Invitrogen) and 100 IU/ml IL-2. After 72 h, expanded cells were labelled with anti-CD3-PE (145–2C11 clone; BD Pharmingen). CFSE dilution in the T-cell population was measured by flow cytometry using an LSR Fortessa cell analyzer (Becton Dickinson) running FACSDiva software, and data processed using FlowJo software (TreeStar Inc, Ashland, OR, USA).
Data availability
The datasets generated during the current study are included in this published article (and its Supplementary Information files) or available from the corresponding author on reasonable request.
References
Urnov, F. D., Rebar, E. J., Holmes, M. C., Zhang, H. S. & Gregory, P. D. Genome editing with engineered zinc finger nucleases. Nat Rev Genet 11, 636–646, https://doi.org/10.1038/nrg2842 (2010).
Yáñez, R. J. & Porter, A. C. G. Therapeutic gene targeting. Gene Ther 5, 149–159 (1998).
Urnov, F. D. et al. Highly efficient endogenous human gene correction using designed zinc-finger nucleases. Nature 435, 646–651, https://doi.org/10.1038/nature03556 (2005).
Genovese, P. et al. Targeted genome editing in human repopulating haematopoietic stem cells. Nature 510, 235–240, https://doi.org/10.1038/nature13420 (2014).
Hoban, M. D. et al. Correction of the sickle cell disease mutation in human hematopoietic stem/progenitor cells. Blood 125, 2597–2604, https://doi.org/10.1182/blood-2014-12-615948 (2015).
Dever, D. P. et al. CRISPR/Cas9 beta-globin gene targeting in human haematopoietic stem cells. Nature 539, 384–389, https://doi.org/10.1038/nature20134 (2016).
DeWitt, M. A. et al. Selection-free genome editing of the sickle mutation in human adult hematopoietic stem/progenitor cells. Sci Transl Med 8, 360ra134, https://doi.org/10.1126/scitranslmed.aaf9336 (2016).
Aiuti, A. & Roncarolo, M. G. Ten years of gene therapy for primary immune deficiencies. Hematology Am Soc Hematol Educ Program 682–689, https://doi.org/10.1182/asheducation-2009.1.682 (2009).
Notarangelo, L. D. Primary immunodeficiencies. J Allergy Clin Immunol 125, S182–194, https://doi.org/10.1016/j.jaci.2009.07.053 (2010).
Rivat, C., Santilli, G., Gaspar, H. B. & Thrasher, A. J. Gene therapy for primary immunodeficiencies. Hum Gene Ther 23, 668–675, https://doi.org/10.1089/hum.2012.116 (2012).
Alexander, B. L. et al. Progress and prospects: gene therapy clinical trials. Gene Ther 14(part 1), 1439–1447, https://doi.org/10.1038/sj.gt.3303001 (2007).
Kohn, D. B. Update on gene therapy for immunodeficiencies. Clin Immunol 135, 247–254, https://doi.org/10.1016/j.clim.2009.12.003 (2010).
Hacein-Bey-Abina, S. et al. A serious adverse event after successful gene therapy for X-linked severe combined immunodeficiency. N Engl J Med 348, 255–256, https://doi.org/10.1056/NEJM200301163480314 (2003).
Bosma, G. C., Custer, R. P. & Bosma, M. J. A severe combined immunodeficiency mutation in the mouse. Nature 301, 527–530 (1983).
van der Burg, M. et al. A DNA-PKcs mutation in a radiosensitive T-B- SCID patient inhibits Artemis activation and nonhomologous end-joining. J Clin Invest 119, 91–98, https://doi.org/10.1172/JCI37141 (2009).
Woodbine, L. et al. PRKDC mutations in a SCID patient with profound neurological abnormalities. J Clin Invest 123, 2969–2980, https://doi.org/10.1172/JCI67349 (2013).
Beamish, H. J. et al. The C-terminal conserved domain of DNA-PKcs, missing in the SCID mouse, is required for kinase activity. Nucleic Acids Res 28, 1506–1513 (2000).
Blunt, T. et al. Identification of a nonsense mutation in the carboxyl-terminal region of DNA-dependent protein kinase catalytic subunit in the scid mouse. Proc Natl Acad Sci USA 93, 10285–10290 (1996).
Jackson, S. P. & Jeggo, P. A. DNA double-strand break repair and V(D)J recombination: involvement of DNA-PK. Trends Biochem Sci 20, 412–415 (1995).
Rahman, S. H. et al. Rescue of DNA-PK Signaling and T-Cell Differentiation by Targeted Genome Editing in a prkdc Deficient iPSC Disease Model. PLoS genetics 11, e1005239, https://doi.org/10.1371/journal.pgen.1005239 (2015).
Wanisch, K. & Yáñez-Muñoz, R. J. Integration-deficient lentiviral vectors: a slow coming of age. Mol Ther 17, 1316–1332, https://doi.org/10.1038/mt.2009.122 (2009).
Guschin, D. Y. et al. A rapid and general assay for monitoring endogenous gene modification. Methods in molecular biology 649, 247–256, https://doi.org/10.1007/978-1-60761-753-2_15 (2010).
Certo, M. T. et al. Coupling endonucleases with DNA end-processing enzymes to drive gene disruption. Nature methods 9, 973–975, https://doi.org/10.1038/nmeth.2177 (2012).
Yáñez, R. J. & Porter, A. C. G. Gene targeting is enhanced in human cells overexpressing hRAD51. Gene Ther 6, 1282–1290 (1999).
Yáñez, R. J. & Porter, A. C. G. A chromosomal position effect on gene targeting in human cells. Nucleic Acids Res 30, 4892–4901 (2002).
Rocca, C. J., Abdul-Razak, H. H., Holmes, M. C., Gregory, P. D. & Yáñez-Muñoz, R. J. A southern blot protocol to detect chimeric nuclease-mediated gene repair. Methods Mol Biol 1114, 325–338, https://doi.org/10.1007/978-1-62703-761-7_21 (2014).
Goueli, B. S., Hsiao, K., Tereba, A. & Goueli, S. A. A novel and simple method to assay the activity of individual protein kinases in a crude tissue extract. Analytical biochemistry 225, 10–17, https://doi.org/10.1006/abio.1995.1100 (1995).
Muller, C., Calsou, P. & Salles, B. The activity of the DNA-dependent protein kinase (DNA-PK) complex is determinant in the cellular response to nitrogen mustards. Biochimie 82, 25–28, S0300-9084(00)00341-2 [pii] (2000).
Mikkola, H. et al. Lentivirus gene transfer in murine hematopoietic progenitor cells is compromised by a delay in proviral integration and results in transduction mosaicism and heterogeneous gene expression in progeny cells. J Virol 74, 11911–11918 (2000).
Schuler, W. et al. Rearrangement of antigen receptor genes is defective in mice with severe combined immune deficiency. Cell 46, 963–972 (1986).
Huston, M. W. et al. Correction of murine SCID-X1 by lentiviral gene therapy using a codon-optimized IL2RG gene and minimal pretransplant conditioning. Mol Ther 19, 1867–1877, https://doi.org/10.1038/mt.2011.127 (2011).
Williams, D. A. & Thrasher, A. J. Concise review: lessons learned from clinical trials of gene therapy in monogenic immunodeficiency diseases. Stem cells translational medicine 3, 636–642, https://doi.org/10.5966/sctm.2013-0206 (2014).
Robert, F., Barbeau, M., Ethier, S., Dostie, J. & Pelletier, J. Pharmacological inhibition of DNA-PK stimulates Cas9-mediated genome editing. Genome medicine 7, 93, https://doi.org/10.1186/s13073-015-0215-6 (2015).
van Overbeek, M. et al. DNA Repair Profiling Reveals Nonrandom Outcomes at Cas9-Mediated Breaks. Mol Cell 63, 633–646, https://doi.org/10.1016/j.molcel.2016.06.037 (2016).
Doudna, J. A. & Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096, https://doi.org/10.1126/science.1258096 (2014).
Gupta, R. M. & Musunuru, K. Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J Clin Invest 124, 4154–4161, https://doi.org/10.1172/JCI72992 (2014).
Tebas, P. et al. Gene editing of CCR5 in autologous CD4 T cells of persons infected with HIV. The New England journal of medicine 370, 901–910, https://doi.org/10.1056/NEJMoa1300662 (2014).
Miller, J. C. et al. An improved zinc-finger nuclease architecture for highly specific genome editing. Nat Biotechnol 25, 778–785, https://doi.org/10.1038/nbt1319 (2007).
Blunt, T. et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 80, 813–823 (1995).
Follenzi, A. & Naldini, L. HIV-based vectors. Preparation and use. Methods in molecular medicine 69, 259–274 (2002).
Yáñez-Muñoz, R. J. et al. Effective gene therapy with nonintegrating lentiviral vectors. Nat Med 12, 348–353, https://doi.org/10.1038/nm1365 (2006).
Qiu, P. et al. Mutation detection using Surveyor nuclease. Biotechniques 36, 702–707 (2004).
van Meerloo, J., Kaspers, G. J. & Cloos, J. Cell sensitivity assays: the MTT assay. Methods Mol Biol 731, 237–245, https://doi.org/10.1007/978-1-61779-080-5_20 (2011).
Acknowledgements
The authors would like to thank John Thacker for balb/c and scid 3T3 mouse fibroblasts, Penny Jeggo for the mouse Prkdc YAC and general advice on DNA-PK, Lisa Woodbine for support on DNA-PK assays, Luigi Naldini for lentiviral plasmids, Chris Baum for retroviral plasmids and Ramón Alemany for adenoviral vectors. The authors acknowledge financial support from the 7th EU Framework Programme (PERSIST project, grant agreement No. 222878), the Primary Immunodeficiency Association (ref. 2002/13), Ministerio de Economía, Comercio y Competitividad y Fondo Europeo de Desarrollo Regional (FEDER, SAF2015–68073-R), MOHESR (Iraq) and Royal Holloway, University of London.
Author information
Authors and Affiliations
Contributions
R.J.Y.-M. conceived and directed the study, and designed most of the experiments. M.C.H. and P.D.G. designed and produced the ZFNs and developed the off-target analyses. H.H.A.-R. and C.J.R. carried out most of the molecular, in vitro and ex vivo work, produced the viral vectors and contributed to design of in vivo experiments. S.J.H., C.H.V.G., A.R., V.P., F.J.M.-E. and J.P. contributed to various aspects of the molecular and cell biology work. M.P.B. performed the injections for transplantation. H.H.A.-R., S.J.H., V.P. and M.P.B. processed samples from in vivo experiments. M.E.A.-F. performed the flow cytometry analyses of in vivo experiments. M.I.G. performed CFSE analyses. J.W., R.G. and C.C.B. carried out deep-sequencing analyses. G.G., C.K. C.v.K., M.S., J.A.B. and A.J.T. provided reagents, facilities and expertise, and participated in experimental design. All authors contributed to data analyses. R.J.Y.-M., C.J.R., H.H.A.-R. and M.C.H. wrote the paper, with contributions from all authors.
Corresponding author
Ethics declarations
Competing Interests
M.C.H. is an employee and P.D.G. and J.W. are former employees of Sangamo Therapeutics Inc, respectively. S.J.H. and M.E.A.-F. are employees of GSK. R.J.Y.-M. is a Trustee of the Genetic Alliance UK. The other authors declare no potential conflict of interest.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Abdul-Razak, H.H., Rocca, C.J., Howe, S.J. et al. Molecular Evidence of Genome Editing in a Mouse Model of Immunodeficiency. Sci Rep 8, 8214 (2018). https://doi.org/10.1038/s41598-018-26439-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26439-9
This article is cited by
-
A novel PRKDC mutation caused B lymphocytes V(D)J rearrangement disorder in the SLE-DAH like symptoms patient
Pediatric Rheumatology (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.