Abstract
The survival of HIV-infected patients has increased with the advent of antiretroviral therapy with the emergence of new comorbidities. Vertebral fracture is a manifestation of reduced bone strength and osteoporosis. This study aims to assess the frequency of spine fractures in HIV-positive men and women aged over 18 years. We performed a systematic review of randomized controlled trials, cohort studies, cross-sectional studies, and case-control studies. Studies that evaluated morphometric and/or clinical vertebral fracture were included. In total 488 studies were found, of which 53 had their full texts evaluated. A total of 85,411 HIV positive individuals were identified in 26 studies. The meta-analysis of the prevalence of vertebral fractures included 12 studies with 10,593 subjects. The prevalence was 11.1% [95% confidence interval (95% CI) 4.5%, 25.0%, I2 98.2% p < 0.00001]. When we evaluated independently studies of clinical vertebral fracture and morphometric vertebral fracture, the prevalence was 3.9% (95% CI 0.9, 15.8, I2 96.4% p < 0.00001) and 20.2% (95% CI 15.7%, 25.6%, I2 69.9% p = 0.003) respectively. HIV-infected individuals had an odds ratio of vertebral fractures of 2.3 (95% CI 1.37, 3.85, I2 98.2% p < 0.00001) when compared with HIV-uninfected patients (n = 9 studies). In conclusion, HIV-positive subjects had a higher risk of vertebral fractures when compared with HIV-negative subjects.
Similar content being viewed by others
Introduction
With the advent of antiretroviral therapy (ARV), the survival of HIV-positive individuals has increased1,2. Consequently, the spectrum of comorbidities they exhibit has increased3. Many of these comorbidities, common in the older population, arise at a younger age in individuals infected with HIV and one of the affected systems is the skeleton. Although studies showing the incidence and prevalence of fractures in HIV-infected patients have different designs concerning the sample size, age and gender, study population, type of fracture, and method of fracture assessment, the vast majority show a significant increase in the risk of fracture4. Reduction in bone mineral density (BMD) has been reported in HIV-infected patients regardless of sex and age, with an odds ratio of 6.4 for reduced BMD and 3.7 for osteoporosis when compared with uninfected controls5.
Vertebral fractures are one of the most common manifestations of osteoporosis. Radiographically confirmed vertebral fractures are a sign of impaired bone quality and strength and a strong predictor of future vertebral and non-vertebral fractures6. The presence of one vertebral fracture is associated with a five-fold increase in the risk of subsequent vertebral fractures and a three-fold increase in hip fracture risk7. Most vertebral fractures are asymptomatic, and only about one-fourth of incident radiographic vertebral deformities are clinically diagnosed8. The prevalence of vertebral fractures in the general population increases with age, occurring in approximately 25% of women over 50 years9.
Recently, studies have demonstrated an increased prevalence of vertebral fracture in HIV patients10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31. Although the studies that evaluate the risk of vertebral fractures in people living with HIV appear to be consistent, the studies that evaluate the prevalence and incidence of spine fractures have shown different results. Moreover, these studies have different designs, sample sizes, and fracture assessment, which make the findings of each study difficult to interpret and generalize. Thus, we have performed a systematic review and meta-analysis to quantify the prevalence, incidence, and risk of vertebral fractures in HIV-individuals. To the best of your knowledge, this is the first metanalysis evaluating spine fractures in people living with HIV.
Materials and Methods
The meta-analysis was carried out according to the PRISMA Guidelines32. The study was registered at PROSPERO, an international database of prospectively registered systematic reviews, with protocol number CRD42016048702 9 https://www.crd.york.ac.uk/PROSPERO/display_record.asp?ID=CRD42016048702). It was approved by the Research Committee of Health Sciences Centre of the Federal University of Santa Maria (045911).
Studies were included in the meta-analysis if they met the following criteria: (1) Randomized controlled trials, cohort studies, cross-sectional, and case-control; (2) men and women aged over 18 years, HIV-positive with or without antiretroviral therapy; (3) have evaluated both morphometric or clinical vertebral fracture; and (4) the primary outcome of interest was prevalence or incidence of vertebral fractures, and/or the secondary outcome was hazard ratio of fracture. Animal studies, studies that evaluated specific cohorts of patients with HIV (for example, studies that assess a particular variable only in patients e.g. HIV with hepatitis-C, or just HIV with lipodystrophy), or those that did not meet the inclusion criteria were excluded from the initial review. The last search was on September 27th, 2017.
The search for studies was performed in EMBASE (Elsevier), PubMed, the Regional Library of Medicine (BIREME), and the Cochrane Library (Cochrane Database of Systematic Reviews - CDSR). Also, studies based on the reference lists of the included articles were analyzed. Studies written in any language and with no publication date limits were considered. The terms used for the search are described in the supplementary material.
Whenever different articles from the same database were obtained, all the articles necessaries to complete the extraction were included in the metanalysis. The authors were contacted by email when more data were required.
Selection process
The selection of the studies was performed by two protocol members independently. Firstly, the studies were screened based on their titles and abstracts. The studies that could not be ruled out in this procedure had their full texts evaluated. Additionally, for all selected items, the full texts were sought, and their eligibility was double-checked. If there was dis- agreement between the two reviewers regarding the identification, eligibility, and inclusion of items, they were checked again by a third reviewer and, if necessary by a fourth reviewer.
Data collection process
The data of each study were extracted, independently, by two protocol members (TSI and RMC). The agreement between the two extractors should be 100%. In the cases where there was disagreement, a third and, if necessary, a fourth party adjudicated. The following data were extracted from each article: the name of the first author, year of publication, study design, site, age, gender, ART use, number of vertebral fractures, method of vertebral fracture evaluation (clinical, morphometric, ICD, self-reported), and outcome (vertebral fracture prevalence, incidence, odds/hazard ratio). When any of these data could not be extracted from the full text of the study, the authors were contacted.
Risk of bias (quality) assessment
Assessment of the risk of bias of the included studies was performed independently by two authors and was ranked as high, low and uncertain. Possible discrepancies were adjudicated by the other protocol members. All included studies were cohort, randomized clinical trials, case–control, or cross-sectional studies. For RCTs and cohort or case-control studies, bias risk assessment was conducted by the Cochrane Collaboration tool33 and Newcastle-Ottawa scale34 respectively. The Newcastle-Ottawa scale assessed the selection, comparability and exposure of a case-control study and selection, comparability, and outcome of a cohort study. In it, 9 stars represent maximum score for a study, and the study with over 6 stars would be regarded as relatively high quality. For cross-sectional study assessment quality Crombie´s Scale33 was used. Crombie´s Scale is composed of 7 items, and each item is graded as “Yes” (1 point), “Unclear” (0.5 points), or “No” (0 points).
Vertebral fracture definition
There are differing criteria for the definition of vertebral fracture and thus rates may vary within the same population depending on the approach selected. In addition, some studies use just ICD coding to detect the incidence or prevalence of vertebral fracture, which restricts data to inclusion only of fractures that present clinically. Because of the heterogeneity of methods used for vertebral fracture assessment among the studies, for the purposes of this analysis we separated the studies into two groups according to the approach utilised. Studies that used imaging methods (X-ray, DXA or CT), independent of protocol, were allocated to the morphometric vertebral fracture group. Studies that used ICD, self-report, or did not describe the method of vertebral fracture assessment were allocated to the clinical vertebral fracture group.
Data synthesis and statistical analysis
Data on the prevalence, incidence, and the odds ratio of vertebral fracture were summarized separately. We pooled proportions (data for prevalence or incidence) applying LOGIT transformation, using random effects model, with DerSimonian and Laird as variance estimator35. Results were presented as a pooled proportion (prevalence or incidence), with 95% confidence intervals. The pooled odds ratio was estimated, also using random effects model, with DerSimonian and Laird as variance estimator35. The statistical heterogeneity among studies was assessed using Cochran’s Q test and the inconsistency I2 test36. We performed an additional analysis with a fixed-effects model to qualitatively evaluate differences in point estimates provided by models with random and fixed effects. For sensitivity analysis, to explain heterogeneity and potential effects modifiers, we performed subgroup analysis for the type of fracture assessment and type of study, and meta-regression for age, gender, study location, sample size, study year, and study quality using a mixed-effects model (with the explaining variable as fixed)37,38. The publication bias was evaluated thru a qualitative inspection of funnel plot39 and the Begg test as a statistical parameter for testing funnel plot asymmetry40. All the analyses were made using the software R [R version 3.2.4, 2016, The R Foundation for Statistical Computing, Platform: x86_64-apple-darwin13.4.0 (64-bit)] and RStudio [RStudio Team (2015). RStudio: Integrated Development for R. RStudio, Inc., Boston, MA http://www.rstudio.com/].
Approval, Registration and Availability
Approval for this study was obtained from the Research Committee of Health Sciences Centre of the Federal University of Santa Maria (045911). The study protocol is registered with the International Prospective Register of Systematic Reviews (PROSPERO) under the number CRD42016048702. All research data will be available upon publication at https://www.dcmufsm.com.
Results
Study selection
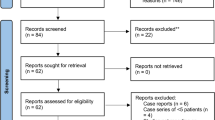
In total 488 studies were found in the electronic database search, of which 90 were duplicates. After screening the title and abstracts, 53 relevant studies remained and underwent detailed full-text review. Of these, 29 were excluded due to lack of a suitable outcome, inappropriate study design, inclusion of selected cohorts of patients with HIV, or redundant publication. A total of 24 articles met the eligibility criteria and were included in the meta-analysis. The process of relevant studies selection and the number of articles excluded at each stage are outlined in the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (Fig. 1). In two different studies from the same author, carried out with the same cohort, the results were overlapping25,26. To achieve full information from this population, data were extracted from both articles and the values were pooled and analyzed together.
PRISMA 2009 Flow Diagram of the studies included in the review. The meta-analysis was carried out according to the PRISMA Guidelines32.
Study characteristics
Table 1 describes the study characteristics of the 24 studies included in our meta-analysis. A total of 84,628 individuals HIV positive were identified in 24 studies. We found 10 cross-sectional studies with a total of 1,221 subjects; 10 cohort studies with 74,116; 3 case-control studies with 8,689 and 1 randomized clinical trial with 602. For vertebral fracture ascertainment, 9 studies used imaging methods, 7 used ICDs or databases, 4 used self-reported questionnaires, 1 used clinical charts, and 2 did not describe the method used. Seventeen studies recruited male and female participants, 5 studies recruited only males, and 2 studies recruited only females. Most of the subjects in the studies were taking antiretroviral therapy. Apart from the study conducted by Gallant et al.17, which was a multicenter study enrolling participants from several countries (South America, Europe, USA), the studies took place in individual countries: 8 from Italy, 7 from USA, 2 from Denmark and one each from Taiwan, France, Australia, UK and Japan.
Study quality
The Newcastle-Ottawa Scale was applied to assess the selection, comparability, and exposure of the case-control and cross-sectional study, and the selection, comparability, and outcome of the cohort study. These scores are displayed in Table 4 (Supplementary material). The Crombie’s items assessment for cross-sectional studies is described in Table 5 (Supplementary material).
HIV and vertebral fracture
Twelve studies were used in the assessment of the prevalence of vertebral fracture in HIV-positive subjects (Fig. 2). The mean age of the included subjects varied from 40 to 53 years. Two subgroups were evaluated independently: clinical vertebral fracture and morphometric vertebral fracture, with 5 and 7 studies, respectively. The overall prevalence was 11.1% (95% confidence interval 4.5% to 25.0%; prediction interval 0.3% to 85.6%). The prevalence of morphometric vertebral fracture was 20.2% (95% confidence interval 15.7 to 25.6; prediction interval 8.8% to 39.6%), and of clinical vertebral fracture 3.9% (95% confidence interval 0.9 to 15.8; prediction interval 0.02% to 91.6%). The p-value for the publication bias evaluated by the Begg test was 0.131. The funnel plot is displayed in Figure 4, supplementary material. There were no major differences in the point estimates provided by models with random and fixed effects (data not shown).
Forest plot of the prevalence of vertebral fractures in HIV-positive subjects. The proportions were pooled applying LOGIT transformation, using random effects model, with DerSimonian and Laird as variance estimator. Results were presented as a pooled prevalence, 95% confidence intervals. The p-value for subgroup analysis on the differences of the prevalence by type of fracture assessment is 0.021.
A sensitivity analysis was carried out to investigate the effect of each study on results of the meta-analysis of the prevalence of vertebral fracture. We excluded the included articles one by one; however, the pooled results were not affected by this procedure and no single article could explain the source of the heterogeneity.
The meta-analysis of the incidence of vertebral fractures included 10 studies with 422,799 PY HIV- infected individuals. The pooled estimate of the incidence was 0.8 (95% confidence interval 0.4 to 1.9) per 1000 person-years. The assessment for heterogeneity was significant for vertebral fractures (Q = 1.459, p = 0.0001, I² = 95.9%). However, when we excluded one outlier study (Young et al. 2011), the incidence estimate decreased to 0.6 (95% confidence interval 0.4 to 0.8) per 1000 person-years and the heterogeneity remained significant, but lower (Q = 0.194, p = 0.0001, I² = 74.9%). The incidence rate of each study is described in Table 2.
In a total of 9 studies, 56,117 HIV-infected patients were compared with 517,1132 HIV-uninfected controls. HIV patients had a 2.3-fold increase in the odds ratio of vertebral fracture (95% confidence interval 1.37 to 3.85) when compared with HIV-uninfected patients (Fig. 3). There was no interaction between the assessment method [morphometric or clinical vertebral, P = 0.211] and the study type (case-control and cross-sectional, P = 0.918]; (data not shown). The p-value for the publication bias evaluated by the Begg test was 0.421. The funnel plot is displayed in Figure 4, supplementary material. There were no major differences in the point estimates provided by models with random and fixed effects (data not shown).
Forest plot of the odds ratio of vertebral fractures in HIV-positive subjects. The pooled odds ratio was estimated using random effects model, with DerSimonian and Laird as variance estimator. Results were presented as a pooled odds ratio, 95% confidence intervals. The p-value for subgroup analysis on the differences of the prevalence by type of fracture assessment is 0.211.
Meta-regression
The results for the meta-regression of the prevalence analysis are shown in Table 3. There was no interaction between age, gender, and study year and the prevalence of vertebral fractures. Nonetheless, the heterogeneity was explained, at least in part, by the study location, sample size, and study quality.
Discussion
This systematic review included 24 studies, with 84,628 HIV-infected subjects. The pooled results indicated that HIV infection was associated with an increase in the risk of vertebral fracture. When compared with HIV-negative subjects, HIV patients had a 2.3-fold increased risk of vertebral fracture, independent of age and gender.
Previous studies have shown that HIV infection has adverse effects on bone health. In 2006, a meta-analysis of cross-sectional studies reported an odds ratio of 6.4 for reduced BMD and 3.7 for osteoporosis, defined as a BMDT-score ≤−2.5 in HIV-infected subjects compared with uninfected controls5. In another meta-analysis, Shiau et al.41 found a modest increase in the incidence of fracture in HIV-infected individuals, with an incidence rate ratio of 1.35 (95% CI 1.10–1.65) for fragility fracture and 1.58 (95% CI 1.25–2.00] for any fracture.
The exact mechanisms underlying bone disease in the HIV-infected population remain unclear. Risk factors for fracture include many of those seen in the general population, for example smoking, alcohol abuse, and glucocorticoid therapy, as well as HIV-specific risk factors such as anti-retroviral therapy, co-infection with hepatitis C, and hypogonadism. In addition, co-morbidities associated with HIV infection such as renal disease and diabetes also increase fracture risk42.
In our study, the meta-regression did not show an association between age or gender and vertebral fracture. Although individual studies have included older subjects, the age range in the analyzed studies in our meta-regression was from 40 to 53 years, which may have contributed to this finding. The positive association between age and vertebral fracture prevalence in the general population has been well documented in several large population-based studies43. The narrow age range in our study could have blunted this association.
The influence of gender on vertebral fractures in the general population appears to vary with age. Studies that include men and woman have reported that the incidence is higher in men than women under the age of 50–55 years43. For men and woman aged 50–79 years, data from European Vertebral Osteoporosis Study (EVOS)44 have shown that the prevalence of vertebral fracture in the age-standardized population in Europe was 12.2% and 12% respectively. Other studies show that the risk rises in women after the age of 60 years and substantially after 70 years of age45. There was no interaction between gender and vertebral fracture in our study.
Although the vast majority of the subjects in the studies evaluated in this systematic review and meta-analysis were on ART, the type and the duration of therapy were not described in several studies; therefore, we could not study its influence on vertebral fractures. The effects of ART on bone mineral density in HIV infected individuals are well documented, with losses of between 2% and 6% during the first year of therapy and a tendency to stabilise thereafter, this effect varying with the drugs used. Bolland et al.46 investigated temporal changes in BMD of adults with HIV in longitudinal studies. The authors found that BMD was stable in HIV cohorts established on antiretroviral therapy, whereas cohorts initiating therapy had short-term accelerated BMD loss followed by a longer period of stability. To examine whether ART initiation is associated with increased fracture rate, Yin28 evaluated the incidence of fracture in HIV-positive subjects from 26 randomized ART studies (n = 4640) followed in the AIDS Clinical Trials Group (ACTG) Longitudinal-Linked Randomized Trial study for 5 years. The authors found that in ART-naive subjects, fracture rates were higher in the first 2 years after ART initiation [(0.53/100 person-years) when compared to the subsequent years (0.30/100 person-years)]. Nonetheless, the data on vertebral fracture incidence after initiation ART are sparse. In addition, most studies did not include morphometric evaluation of vertebral fractures.
The disparity between the prevalence of morphometric (20.2%) and clinical vertebral fractures (3.9%) in our study is in agreement with findings in the general population. Clinical vertebral fracture rates are considerably lower than radiographic vertebral fracture rates, reflecting the minority of these fractures that come to clinical attention47. In the general population, the prevalence of morphometric vertebral fractures in people age 50 years and over varies between 9.5 and 37%43,47, the value increasing with age and also varying according to the definition of vertebral fracture. In the EVOS study, the prevalence varied from 12.2% for men and 12% for women using the McCloskey method to 20.2% and 20.2%, respectively, when the Eastell method was used. In our analysis, studies using semiquantitative and quantitative methods were pooled together. This approach might have generated a conservative bias, underestimating the frequency of fractures. Studies that used the semiquantitative method could have missed mild fractures, classified as grade 1 by Genant48 because of the difficulty of detecting this type of fracture in younger individuals.
In our study, the data for vertebral fracture incidence were obtained from 10 studies. Nine of them used ICD data or self-reporting. Since only around one third of vertebral fractures are symptomatic, use of self-reporting data will underestimate the incidence. Furthermore different coding is used in ICD9 and ICD10, which may have contributed to the variation in reported incidence. The most recent version of ICD 10 defines several subtypes of vertebral fractures, and depending on the coding used by each study, different types and sites of vertebral fracture may be included. Hence, high impact fractures or fractures in cervical vertebrae may be included. Furthermore, the incidence ratio found in our study should be interpreted with caution as there were insufficient data to provide a robust estimate.
Our study has some limitations. It is a systematic review and meta-analysis of observational studies that aimed to evaluate the frequency and risk of vertebral fractures, therefore it is to be expected that our pooled analysis showed heterogeneity. Differences in the design of the studies, the study location, the sample size, the populations studied, the method used for vertebral fracture evaluation, and the quality of the studies may all have contributed to the observed heterogeneity. Another limitation is the fact that we included cohort, case-control, and cross-sectional studies in the estimated odds ratio, thus causality may not be concluded. Nevertheless, there was no interaction between the study type or method used for fracture and the pooled odds ratio of spine fractures. Finally, some studies included in our systematic review were not specifically designed to evaluate vertebral fracture and/or vertebral fracture was not the primary endpoint. Because many vertebral fractures are asymptomatic, they might be underreported in those studies. Hence the prevalence of clinical vertebral fractures and the incidence of vertebral fractures may be underestimated in our metanalysis.
Our study demonstrates that the risk of vertebral fracture in HIV-positive subjects is approximately double that of HIV-negative subjects, a finding that has important clinical implications. Although the risk factors for vertebral fractures have not been clearly established in the ‘HIV infected population, identification and correction of risk factors recognized in other populations, such as smoking, alcohol abuse, sedentary lifestyle, vitamin D deficiency, and the use of glucocorticoids should be recommended. Moreover, screening for spine fractures with X-ray should be considered in high risk individuals. Further studies are required to establish vertebral fracture incidence in HIV-positive individuals, to identify the risk factors involved, and to develop effective strategies for their prevention
References
Fang, C. T. et al. Life expectancy of patients with newly-diagnosed HIV infection in the era of highly active antiretroviral therapy. QJM 100, 97–105, https://doi.org/10.1093/qjmed/hcl141 (2007).
Palella, F. J. et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med 338, 853–860, https://doi.org/10.1056/NEJM199803263381301 (1998).
Deeks, S. G. & Phillips, A. N. HIV infection, antiretroviral treatment, ageing, and non-AIDS related morbidity. BMJ 338, a3172, https://doi.org/10.1136/bmj.a3172 (2009).
Compston, J. Osteoporosis and fracture risk associated with HIV infection and treatment. Endocrinol Metab Clin North Am 43, 769–780, https://doi.org/10.1016/j.ecl.2014.05.001 (2014).
Brown, T. T. & Qaqish, R. B. Antiretroviral therapy and the prevalence of osteopenia and osteoporosis: a meta-analytic review. AIDS 20, 2165–2174, https://doi.org/10.1097/QAD.0b013e32801022eb (2006).
Cosman, F. et al. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Osteoporos Int 25, 2359–2381, https://doi.org/10.1007/s00198-014-2794-2 (2014).
Ross, P. D., Davis, J. W., Epstein, R. S. & Wasnich, R. D. Pre-existing fractures and bone mass predict vertebral fracture incidence in women. Ann Intern Med 114, 919–923 (1991).
Fink, H. A. et al. What proportion of incident radiographic vertebral deformities is clinically diagnosed and vice versa? J Bone Miner Res 20, 1216–1222, https://doi.org/10.1359/JBMR.050314 (2005).
Melton, L. J. III et al. Prevalence and incidence of vertebral deformities. Osteoporos Int 3, 113–119 (1993).
Gazzola, L. et al. Assessment of radiological vertebral fractures in HIV-infected patients: clinical implications and predictive factors. HIV Med 16, 563–571, https://doi.org/10.1111/hiv.12267 (2015).
Porcelli, T. et al. Role of bone mineral density in predicting morphometric vertebral fractures in patients with HIV infection. Osteoporos Int 25, 2263–2269, https://doi.org/10.1007/s00198-014-2760-z (2014).
Bedimo, R. et al. Mechanisms of bone disease in HIV and hepatitis C virus: impact of bone turnover, tenofovir exposure, sex steroids and severity of liver disease. AIDS 30, 601–608, https://doi.org/10.1097/qad.0000000000000952 (2016).
Borderi, M. et al. Prevalence of sub-clinical vertebral fractures in HIV-infected patients. New Microbiol 37, 25–32 (2014).
Ciullini, L. et al. Trabecular bone score (TBS) is associated with sub-clinical vertebral fractures in HIV-infected patients. J Bone Miner Metab 36, 111–118, https://doi.org/10.1007/s00774-017-0819-6 (2018).
Clo, A. et al. Calcaneal quantitative ultrasound (QUS) and dual X-ray absorptiometry (DXA) bone analysis in adult HIV-positive patients. New Microbiol 38, 345–356 (2015).
Collin, F. et al. Ten-year incidence and risk factors of bone fractures in a cohort of treated HIV1-infected adults. AIDS 23, 1021–1024 (2009).
Gallant, J. E. et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: A 3-year randomized trial. Journal of the American Medical Association 292, 191–201 (2004).
Hansen, A. B. E. et al. Incidence of low and high-energy fractures in persons with and without HIV infection: A Danish population-based cohort study. AIDS 26, 285–293 (2012).
Kurita, T., Kitaichi, T., Nagao, T., Miura, T. & Kitazono, Y. Safety analysis of Epzicom® (lamivudine/abacavir sulfate) in post-marketing surveillance in Japan. Pharmacoepidemiology and Drug Safety 23, 372–381 (2014).
Mazzotta, E. et al. Prevalence and predictors of low bone mineral density and fragility fractures among HIV-infected patients at one Italian center after universal DXA screening: Sensitivity and specificity of current guidelines on bone mineral density management. AIDS Patient Care and STDs 29, 169–180 (2015).
Prieto-Alhambra, D. et al. HIV infection and its association with an excess risk of clinical fractures: A nationwide case-control study. Journal of Acquired Immune Deficiency Syndromes 66, 90–95 (2014).
Sharma, A. et al. Increased fracture incidence in middle-aged HIV-infected and HIV-uninfected women: Updated results from the women’s interagency HIV study. Journal of Acquired Immune Deficiency Syndromes 70, 54–61 (2015).
Torti, C. et al. High prevalence of radiological vertebral fractures in HIV-infected males. Endocrine 41, 512–517, https://doi.org/10.1007/s12020-011-9586-7 (2012).
Triant, V. A., Brown, T. T., Lee, H. & Grinspoon, S. K. Fracture prevalence among human immunodeficiency virus (HIV)-infected versus non-HIV-infected patients in a large U.S. healthcare system. J Clin Endocrinol Metab 93, 3499–3504, https://doi.org/10.1210/jc.2008-0828 (2008).
Womack, J. A. et al. Increased risk of fragility fractures among HIV infected compared to uninfected male veterans. PloS One 6, e17217, https://doi.org/10.1371/journal.pone.0017217 (2011).
Womack, J. A. et al. Physiologic frailty and fragility fracture in HIV-infected male veterans. Clin Infect Dis 56, 1498–1504, https://doi.org/10.1093/cid/cit056 (2013).
Yang, N. P. et al. Treatment incidence of orthopedic injuries among hiv-infected subjects in Taiwan: A dynamic cohort survey, 2005-2008. HealthMED 6, 2700–2708 (2012).
Yin, M. T. et al. Fractures after antiretroviral initiation. AIDS 26, 2175–2184 (2012).
Yin, M. T. et al. Low bone mass and high bone turnover in postmenopausal human immunodeficiency virus-infected women. J Clin Endocrinol Metab 95, 620–629 (2010).
Yong, M. K., Elliott, J. H., Woolley, I. J. & Hoy, J. F. Low CD4 count is associated with an increased risk of fragility fracture in HIV-infected patients. Journal of Acquired Immune Deficiency Syndromes 57, 205–210 (2011).
Young, B., Dao, C. N., Buchacz, K., Baker, R. & Brooks, J. T. Increased rates of bone fracture among HIV-infected persons in the HIV outpatient study (HOPS) compared with the US general population, 2000–2006. Clin Infec Dis 52, 1061–1068 (2011).
Moher, D. et al. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Plos Med 6, e1000097, https://doi.org/10.1371/journal.pmed1000097 (2009).
Zeng, X. et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 8, 2–10, https://doi.org/10.1111/jebm.12141 (2015).
Stang, A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 25, 603–605, https://doi.org/10.1007/s10654-010-9491-z (2010).
Veroniki, A. A. et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 7, 55–79, https://doi.org/10.1002/jrsm.1164 (2016).
Higgins, J. P. & Thompson, S. G. Quantifying heterogeneity in a meta-analysis. Stat Med 21, 1539–1558, https://doi.org/10.1002/sim.1186 (2002).
Berkey, C. S., Hoaglin, D. C., Mosteller, F. & Colditz, G. A. A random-effects regression model for meta-analysis. Stat Med 14, 395–411 (1995).
van Houwelingen, H. C., Arends, L. R. & Stijnen, T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 21, 589–624 (2002).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997).
Begg, C. B. & Mazumdar, M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994).
Shiau, S., Broun, E. C., Arpadi, S. M. & Yin, M. T. Incident fractures in HIV-infected individuals: a systematic review and meta-analysis. AIDS 27, 1949–1957, https://doi.org/10.1097/QAD.0b013e328361d241 (2013).
Casado, J. L. et al. Prevalence of causes of secondary osteoporosis and contribution to lower bone mineral density in HIV-infected patients. Osteoporos Int 25, 1071–1079 (2014).
Ballane, G., Cauley, J. A., Luckey, M. M. & El-Hajj Fuleihan, G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int 28, 1531–1542, https://doi.org/10.1007/s00198-017-3909-3 (2017).
O’Neill, T. W. et al. The prevalence of vertebral deformity in european men and women: the European Vertebral Osteoporosis Study. J Bone Miner Res 11, 1010–1018, https://doi.org/10.1002/jbmr.5650110719 (1996).
Schousboe, J. T. Epidemiology of Vertebral Fractures. J Clin Densitom 19, 8–22, https://doi.org/10.1016/j.jocd.2015.08.004S1094-6950(15)00168-7 (2016).
Bolland, M. J., Wang, T. K. M., Grey, A., Gamble, G. D. & Reid, I. R. Stable bone density in HAART-treated individuals with HIV: A meta-analysis. J Clin Endocrinol Metab 96, 2721–2723 (2011).
Cauley, J. A., Chalhoub, D., Kassem, A. M. & Fuleihan Gel, H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol 10, 338–351, https://doi.org/10.1038/nrendo.2014.51nrendo.2014.51 (2014).
Genant, H. K., Wu, C. Y., van Kuijk, C. & Nevitt, M. C. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8, 1137–1148, https://doi.org/10.1002/jbmr.5650080915 (1993).
Pepe, J. et al. The combination of FRAX and Ageing Male Symptoms scale better identifies treated HIV males at risk for major fracture. Clinical Endocrinol 77, 672–678, https://doi.org/10.1111/j.1365-2265.2012.04452.x (2012).
Short, C. E. S., Shaw, S. G., Fisher, M. J., Walker-Bone, K. & Gilleece, Y. C. Prevalence of and risk factors for osteoporosis and fracture among a male HIV-infected population in the UK. International Journal of STD and AIDS 25, 113–121 (2014).
Acknowledgements
This study was sponsored by the Federal University of Santa Maria. The UFSM had no role in the design, analysis or writing up this protocol. All authors state that they have no conflict of interest regarding this manuscript. The authors would like to thank Maicon Falavigna for his support with the statistical analysis. The authors received no specific funding for this work. The Federal University of Santa Maria sponsored this study. The sponsor had no role in the design, conduct, or analysis of our study or in the decision to submit this manuscript for publication.
Author information
Authors and Affiliations
Contributions
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drª Premaor had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Authors’ roles: Study design: T.I., F.C., J.C. and M.P. Study conduct: T.I., R.C. Data collection: T.I., R.C. Data analysis: T.I., M.P. Data interpretation: T.I., F.C., J.C. and M.P. Drafting manuscript: T.I., J.C. and M.P. Revising manuscript content: T.I., R.C., F.C., J.C. and M.P. Approving final version of manuscript: T.I., R.C., F.C., J.C. and M.P. M.P. takes responsibility for the integrity of the data analysis.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ilha, T.A.S.H., Comim, F.V., Copes, R.M. et al. HIV and Vertebral Fractures: a Systematic Review and Metanalysis. Sci Rep 8, 7838 (2018). https://doi.org/10.1038/s41598-018-26312-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26312-9
This article is cited by
-
Evaluation of bone mineral density, microarchitecture, and detection of fractures on young patients living with human immunodeficiency virus: when and how to screen?
Endocrine (2023)
-
Prevalence of and risk factors for vertebral fracture and low bone mineral density among Peruvian women aging with HIV
Archives of Osteoporosis (2023)
-
Impact of hypogonadism on bone mineral density and vertebral fractures in HIV-infected men
Journal of Endocrinological Investigation (2022)
-
Osteoporosis and HIV Infection
Calcified Tissue International (2022)
-
Aging with HIV in Latin America and the Caribbean: a Systematic Review
Current HIV/AIDS Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.