Abstract
Multiple retinal nerve fiber layer (RNFL) defects develop uncommonly, even though glaucomatous RNFL loss is typically observed as one RNFL defect in each quadrant. We investigated the risk factors associated with multiple RNFL defects to increase our understanding of the nature and pathogenesis of various RNFL defect patterns. Data from subjects with multiple RNFL defects (28 patients) and glaucoma patients without multiple RNFL defects (194 patients) were analyzed. The term “multiple RNFL defects” refers to three or more isolated defects separated by a comparatively normal area. Patients with multiple RNFL defects showed a higher prevalence of hypertension, end-stage renal disease, and cerebrovascular disease than those without multiple RNFL defects, both before and after propensity score matching for age and mean deviation (all P < 0.05). The number of patients with parafoveal visual field points depressed <5% on pattern deviation plots was higher in subjects with multiple RNFL defects than in those without multiple RNFL defects (P = 0.048). In conclusion, the presence of multiple RNFL defects had clinical relevance for systemic vascular risk factors and a higher risk of parafoveal scotoma. Clinicians should be aware of the possibility of concomitant systemic vascular disease when evaluating patients with multiple RNFL defects.
Similar content being viewed by others
Introduction
Focal retinal nerve fiber layer (RNFL) defects are rarely detected in normal eyes and are highly specific for optic nerve damage1. Localized RNFL defects are useful as a clinical sign of early glaucoma1,2. They may also indicate a substantial loss of retinal ganglion cells, as has been found in glaucoma patients with localized RNFL loss apparent on stereophotographs3.
In glaucomatous optic neuropathy, RNFL loss is typically observed as one RNFL defect in each sector. Uncommonly, multiple RNFL defects develop in one quadrant. They are divided by a normal RNFL within a single sector4,5. We speculated that multiple RNFL defects were related to systemic vascular risk factors for the following reasons. At first, multiple RNFL defects are not limited to the superior or inferior sectors of the optic disc, and many are also located at the temporal region of the optic disc5. In glaucoma patients, localized RNFL defects are generally detected in the inferotemporal region, followed by the superotemporal region3. RNFL defects adjacent to the center of the macula or paracentral visual field defects are associated with systemic vascular risk factors, rather than high intraocular pressure (IOP) in glaucoma6,7,8. Secondly, Xu et al. found that localized RNFL defects were associated with arterial hypertension9. In that study, the authors presented one case with arterial hypertension and multiple localized RNFL defects located at 10, 11, and 12 o’clock9. Given those findings, it is possible that multiple RNFL defects are related to systemic vascular factors. Another previous study reported that ocular factors, such as myopia and a small optic disc, were associated with multiple RNFL defects in glaucoma5. However, there has been no study directly analyzing the influence of systemic vascular disease on the development of multiple RNFL defects.
In this study, we evaluated whether systemic vascular risk factors are related to multiple RNFL defects. The characteristics of visual field defects in patients with multiple RNFL defects were also assessed. This investigation will increase our understanding of the nature and pathogenesis of various RNFL loss patterns.
Methods
The Institutional Review Board of the Catholic University of Korea, Seoul, Korea, approved this cross-sectional study and waived the need for written informed consent because of its retrospective design. This study followed the tenets of the Declaration of Helsinki. Inclusion criteria were a best-corrected visual acuity of 20/40 or better, spherical equivalent >−7 diopters (D), and a normal open angle. Patients with trauma or uveitis or proliferative diabetic retinopathy or rim pallor or diseases that might affect the peripapillary area were excluded. Patients with abnormal color vision were excluded. All patients underwent complete ophthalmic examination, including slit-lamp biomicroscopy, Goldmann applanation tonometry, gonioscopy, central corneal thickness measurement, and dilated fundus biomicroscopy. All subjects underwent stereoscopic optic disc imaging. Color vision testing was performed using the 24-plate edition of the Ishihara test book published in 1997 (Kanehara & Co., Ltd, Tokyo, Japan). The spherical equivalent was calculated as the spherical error +1/2 cylinders. Myopia was defined as a spherical equivalent ≤−1 diopter. Systemic risk factors, including diabetes, hypertension, end-stage renal disease, heart disease, cerebrovascular disease, and hematologic disease, were analyzed. The systemic diseases were divided into common and uncommon diseases. The cut-off value for the prevalence of common diseases was 10.0% in total subjects.
Multiple RNFL defects were defined as 3 or more RNFL defects separated by relatively normal RNFL regions5. Multiple RNFL defects were composed of only focal RNFL defects or those mixed with diffuse defects. Focal RNFL defects were defined as a dark gray slit or wedge without sliver striations. The width of the focal defect was wider than a major retinal vessel at a one-disc diameter length from the margin of the optic disc. Patients with multiple RNFL defects who met the inclusion criteria were consecutively included from all patients examined for glaucoma at the glaucoma clinic of Seoul St. Mary’s Hospital between May 2012 and May 2016. When both eyes met the inclusion criteria, one eye per individual was randomly selected for the study.
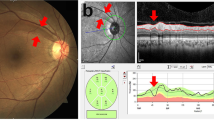
Data for preperimetric or perimetric glaucoma patients were collected from all patients examined for glaucoma at the glaucoma clinic of Seoul St. Mary’s Hospital between September and December 2015. This study included eyes with the presence of a glaucomatous optic disc, such as those with diffuse or focal rim thinning, notching, or retinal nerve fiber layer defects with or without corresponding glaucomatous visual field (VF) defects. Subjects with and without multiple RNFL defects were compared based on demographics including systemic risk factors, RNFL and optic disc parameters, posterior pole profiles, and the characteristics of visual field defects. The location of split RNFL defects was indicated as a quadrant (superior, temporal, inferior, nasal, each 90 degrees) around the optic disc on red-free photography (Fig. 1).
Demonstration of quadrant locations of split retinal nerve fiber layer (RNFL) defects and posterior pole profiles. (A) The location of split RNFL defects was determined by quadrant (superior, temporal, inferior, nasal, equal to 90 degrees) around the optic disc on red-free imaging. (B) Tilt ratio was identified as the ratio between the longest diameter (LD) and the shortest diameter (SD) of the optic disc. The β-zone parapapillary atrophy (PPA) area was calculated using the Image J program. (C) Torsion degree was calculated as the angle between the LD from the vertical meridian, which was at right angles to the reference line. (A) I, inferior; N, nasal; S, superior; T, temporal (B) LD, longest diameter; SD; shortest diameter.
Optical Coherence Tomography
Spectral-domain optical coherence tomography (SD-OCT) imaging was performed using Cirrus HD-OCT version 6.0 (Carl Zeiss Meditec, Inc.). RNFL thickness was measured using optic Disc Cube 200 × 200 scan mode. The Cirrus SD-OCT automatically determines the center of the disc and then draws a circumpapillary circle (radius: 1.73 mm) from the cube data set for RNFL thickness analysis. Poor quality images with signal strength less than 6 were discarded. Mean average RNFL thickness, or the mean thickness in each of 4 quadrants, was provided by the software and included in the comparison between the two groups according to multiple RNFL defects. Optic disc parameters such as disc area, rim area, average cup-to-disc ratio (CDR), vertical CDR, and cup volume were analyzed.
Posterior Pole Profiles
The red-free RNFL images were measured to evaluate the tilt ratio, optic disc torsion, and ratio of βPPA area to disc area. Two examiners (K.I.J., K.S.J.) blinded to clinical information measured the images using the Image J 1.48 program (https://imagej.nih.gov/ij/download.html, National Institutes of Health, Bethesda, MD). The means of the 2 measurements of the 2 examiners were included in this study (Fig. 1).
Tilt ratio (shortest diameter/shortest diameter) was employed to express optic disc tilt10,11. Optic disc torsion was determined as the deviation of the long optic disc axis from the vertical meridian, which was at right angles to a reference line coupling the fovea and disc center. The absolute value of optic disc torsion was used in the analysis12,13. β-zone parapapillary atrophy (PPA) was indicated as an inner crescent of chorioretinal atrophy with observable sclera and choroidal vessels. The disc and βPPA border were outlined using a measurement tool in Image J software, which assessed the areas of the disc and βPPA and displayed them in a separate window. The ratio of βPPA area to disc area was used.
Visual Field Testing
All subjects underwent standard automated perimetry using 24-2 Swedish interactive threshold algorithm (SITA) standard programs with a Humphrey field analyzer II 750i (Carl Zeiss Meditec, Dublin, CA). Mean deviation (MD) and PSD were used in the analysis. A glaucomatous VF defect was defined as a cluster of 3 or more points with a P value <5%, one of which had a P value of <1% on the pattern deviation plot. Reliable tests were defined as < 15% fixation losses, false positives, or false negatives.
On four central 10° VF points of pattern deviation plot, the presence or percentages of significantly depressed points at P < 0.05 were evaluated. On 12 central VF points of pattern deviation plot, each pattern deviation value was compared between subjects with and without multiple RNFL defects.
Statistical Analysis
Differences between the different groups were evaluated by Student’s t test or chi-squared test. Multiple logistic regression analysis was performed to determine which systemic vascular disease was associated with multiple RNFL defects. To decrease the chance of selection bias, propensity score analysis matching was performed. This analysis can perform a quasi-randomized comparison from a retrospective study14. To determine the propensity score, multiple logistic regression analysis was performed using age and MD with SPSS software (ver. 22.0; SPSS Inc., Chicago, IL)15. The propensity score was defined for each participant as the probability (from 0 to 1) of assignment to the case group, given covariates such as age and MD. A logistic regression model is estimated in which an indicator variable denoting the group status (Z, where Z equals 1 if the subject is in the case group or 0 if the subject is in the control group) is regressed according to covariates (independent variables, xi: age, MD in this study). A 1:3 matching method was chosen with the nearest neighbor approach without replacement, based on a greedy matching method. A 1:3 matching means that 3 control units with similar propensity scores were matched to a single unit in the case group. Eighty-four patients, three times the number of subjects with multiple RNFL defects (n = 28), were selected from the group of 194 glaucoma patients without multiple RNFL defects. One-to-many nearest neighbor matching can provide higher precision with little cost in bias compared with simple 1:1 matching16. The technical details are described as follows. We first installed the R package (R 2.15.0), the program to connect R and SPSS (essentials for R), and the PS matching program. The PS matching program was written as a custom dialog in SPSS. This program operates in R through the SPSS-R-plugin. P < 0.05 indicated statistical significance.
Results
Data from 28 patients with multiple RNFL defects and 194 patients with glaucoma were analyzed. Twenty-six (92.9%) of 28 patients with multiple RNFL defects exhibited abnormalities in the thickness map obtained with SD-OCT. After 1:3 propensity score matching, 84 patients with glaucoma were selected from among 194 patients with glaucoma. Patients without multiple RNFL defects (average age 57.0 ± 13.3 years) were older than those with multiple RNFL defects (average age 52.7 ± 11.4 years), but the difference was not statistically significant (P = 0.103; Table 1). MD in the visual field showed more severe glaucomatous damage in subjects without multiple RNFL defects (−6.7 ± 7.3 dB) than in those with multiple RNFL defects (−2.8 ± 3.2 dB; P < 0.001). After propensity score matching according to age and MD, the two groups demonstrated similar glaucomatous stage by MD (P = 0.995). There were no significant differences in best corrected visual acuity, spherical equivalent, untreated IOP, or occurrence of disc hemorrhage between the two groups before and after propensity score matching. In terms of systemic factors, the proportion of patients with hypertension was higher in patients with multiple RNFL defects than those without them among common vascular diseases, both before and after propensity score matching (all P < 0.01) Among uncommon diseases, the proportion of patients with end-stage renal disease, and cerebrovascular disease was higher in patients with multiple RNFL defects than in those without them, both before and after propensity score matching (all P < 0.01).
Table 2 shows optic disc characteristics and RNFL thickness parameters according to the presence of multiple RNFL defects. Average or quadrant RNFL thickness did not show a difference between patients with multiple RNFL defects and those without after propensity score matching. Disc area did not differ depending on the presence of multiple RNFL defects both before (P = 0.162) and after (P = 0.142) propensity score matching. Rim area, average CDR, vertical CDR, and cup volume showed more glaucomatous damage in subjects without multiple RNFL defects compared to those with multiple RNFL defects. Patients with multiple RNFL defects (+) presented with a smaller degree of disc torsion and smaller PPA area to disc area ratio than the multiple RNFL defect (−) group both before (Both P < 0.001) and after (P = 0.012, 0.018, respectively) propensity score matching.
Many of the split RNFL defects were in the superior quadrant of the optic disc (n = 18), followed by the temporal (n = 11) and inferior (n = 3) regions of the optic disc (Fig. 2).
Table 3 presents the involvement of the paracentral visual field defect within the 4 central points. The proportion of patients with paracentral visual field points depressed <5% on pattern deviation plot was higher in the multiple RNFL defect (+) group than in the multiple RNFL defect (−) group (P = 0.048). Among these patients, 10 (58.8%) had an isolated parafoveal scotoma, and 7 (41.2%) had combined parafoveal scotomas. Other visual field defect patterns were found as follows: Three patients had nasal scotoma. Five patients had visual field defects in the Bjerrum area. Three patients had no visual field defect.
On a pattern deviation plot, 12 paracentral visual field points were compared between the two groups. In subjects with multiple RNFL defects, the pattern deviation value (−2.3 dB) of the lower paracentral visual field points was lower than in those without them (−1.1 dB, P = 0.015; Supplementary Fig. S1). Pattern deviation (−6.6 dB) of the upper paracentral VF points displayed a lower value in patients without multiple RNFL defects than in patients with multiple RNFL defects (−3.2 dB, P = 0.004).
Among common vascular diseases, hypertension was associated with multiple RNFL defects by multiple logistic regression analysis (P < 0.001; Table 4). Among uncommon diseases, end-stage renal disease and cerebrovascular disease were both related to multiple RNFL defects by multiple logistic regression analysis (both P < 0.001; Table 4)
A representative case with multiple RNFL defects is shown in Fig. 3. A 47-year-old male with a history of systemic hypertension showed multiple RNFL defects in his left eye. The best-corrected visual acuity in their left eyes was 20/20, and no abnormal color vision was identified.
Among subjects with multiple RNFL defects, only two cases were associated with retinal vascular disease. A 43-year-old female with moderate diabetic retinopathy, hypertensive retinopathy, and history of cerebral infarction showed multiple cotton-wool spots in both eyes (Fig. 4). Six months later, she was referred to the glaucoma clinic because of elevated IOP (22–23 mmHg). Her fundi showed multiple RNFL defects in both eyes. Second, a 42-year-old female with systemic hypertension developed scotoma suddenly in her left eye and visited our hospital. Her fundus imaging demonstrated branched retinal vein obstruction at the superotemporal arcade. After loss to follow-up for one year, she was referred to our glaucoma clinic from a local clinic because of atypical retinal nerve fiber layer defects. She showed multiple RNFL defects in the superotemporal sector of the optic disc corresponding to the location where branched retinal vein obstruction had occurred.
Two cases with multiple retinal nerve fiber layer (RNFL) defects related to retinal vascular disease. (A) A 43-year-old female with moderate non-proliferative diabetic retinopathy, hypertensive retinopathy, and a history of cerebral infarction presented with multiple cotton-wool spots in both eyes. Six months later, multiple RNFL defects occurred in both eyes. (B) A 42-year-old female with systemic hypertension showed multiple RNFL defects at the sector of the optic disc corresponding to the location where a branched retinal vein obstruction had occurred.
Discussion
We demonstrated that multiple RNFL defects were associated with systemic hypertension among common systemic vascular diseases, and end-stage renal disease and cerebrovascular disease among uncommon diseases. Patients with multiple RNFL defects had higher odds for parafoveal field defects than glaucoma patients without multiple RNFL defects.
In 1992, a previous study reported that myopia and a small optic disc were related to multiple RNFL defects in glaucoma5. However, that study did not investigate systemic risk factors. Optic disc size was measured only via red-free fundus imaging. They did not exclude eyes with high myopia. In our study, highly myopic eyes (spherical equivalent >−7 D) were excluded because atrophic changes of the neural retina and choroid could obscure a clear pattern of RNFL defects. Posterior pole profiles, such as a tilted optic disc, greater degree of disc torsion, and β-zone PPA, were not associated with multiple RNFL defects. Posterior pole profiles are influenced by myopia12. At least within non-highly myopic eyes, myopia and its associated characteristics did not seem to play a critical role in the development of multiple RNFL defects.
Seventeen patients (60.7%) with multiple RNFL defects had a history of systemic hypertension. Systemic hypertension increased the risk of developing glaucoma in meta-analyses17,18. CDR or cup volume were smaller in patients with multiple RNFL defects than glaucoma patients without multiple RNFL defects and with similar VF defects. The pathogenesis of multiple RNFL defects in patients with systemic hypertension does not seem to be identical to that of glaucomatous optic neuropathy. One report found that localized RNFL defects were related to arterial hypertension, especially in the severe stage of hypertension9. The odds ratio for the connection between systemic hypertension and localized RNFL defects was higher than for the connection between systemic hypertension and retinal microvascular abnormalities9. In moderate hypertensive retinopathy, cotton-wool spots, which are accumulations of axoplasmic material of RNFLs, can be detected as a result of focal infarcts of the RNFL. In several case reports, a giant cotton-wool spot left a RNFL defect as a sequela to the cotton-wool spot19,20. In our study, only one case showed a previous history of cotton-wool spots, although we could not exclude the possibility that cotton-wool spots developed and disappeared completely in other cases. We cannot conclusively determine whether multiple RNFL defects in subjects with a history of systemic hypertension were caused by glaucoma, cotton-wool spots or a combination of both. Systemic hypertension is the major factor contributing to target organ damage involving the brain, kidney, and heart. Among uncommon vascular diseases, end-stage renal disease and cerebrovascular disease were related to systemic hypertension in five cases (71.4%) and four cases (80.0%), respectively.
In patients with multiple RNFL defects, the number of RNFL defects was greater than in those with single glaucomatous RNFL defects in one quadrant. However, multiple RNFL defects were separated by relatively normal RNFL regions. Total area of RNFL defects may be similar between the multiple RNFL defect (+) and (−) groups. Therefore, we speculate that there was no difference in average RNFL thickness between the two groups because the total areas of the RNFL defects were similar between the two groups.
Split RNFL defects appeared more often in the superior quadrant (18 cases) of the optic disc than in the inferior quadrant (3 cases), even though OCT showed thinner RNFL thicknesses in the inferior quadrant (88.9 ± 21.3 µm) than in the superior quadrant (99.3 ± 20.3 µm) in cases with multiple RNFL defects. The VF pattern deviation plot showed more significant glaucomatous defects on the inferior paracentral field in subjects with multiple RNFL defects compared to those without them (P = 0.015). In glaucoma, localized RNFL defects are usually observed in the inferotemporal region, followed by the superotemporal region3. Larger lamina pore size with less supporting connective tissue at the inferotemporal optic nerve head may make retinal ganglion cells in this region vulnerable to glaucomatous damage and cause initial inferotemporal RNFL defects in many cases of glaucoma21,22. The strong association with systemic vascular disease seemed to play a role in the predominance of split RNFL defects in the superior sector of the optic disc. Vascular insufficiency or disturbances to the optic nerve head may lead to more split RNFL defects at the superior side of the optic disc due to gravitational effects23. Several studies, including a previous study from our group, have found that RNFL loss in type 2 diabetic patients showed a predominance in the superior region of the optic nerve23,24,25. Multiple RNFL defects have different characteristics in the susceptible region of the optic disc compared to typical glaucomatous RNFL defects.
Damage to the papillomacular bundle or central fibers can induce central or paracentral visual field defects. Early glaucomatous damage in the macula is clinically critical because central visual disturbance puts patients at greater risk of losing visual function and interferes with everyday activities26,27,28. Accumulating clinical evidence suggests that paracentral scotoma may occur with non-pressure factors either in addition to or rather than IOP-dependent factors6,7,8. Parafoveal scotoma developed more frequently in patients with normal tension glaucoma than in patients with high tension glaucoma in several studies29,30, while other studies did not show consistent findings31,32. Our group found that glaucoma patients with autonomic dysfunction and abnormal peripheral microcirculation more often presented with paracentral visual field defects than patients without these dysfunctions7. Therefore, we speculated that the development of multiple RNFL defects related to systemic vascular disease might affect frequent involvement of paracentral visual field defects. However, multiple RNFL defects did not result in a distinct loss of visual acuity because they did not involve the fovea. Mean best corrected visual acuity of patients with multiple RNFL defects was 0.03 ± 0.05 (logMAR), similar to that of glaucoma patients without multiple RNFL defects.
In two cases, split RNFL defects were associated with the previous occurrence of retinal vascular disease, such as hypertensive retinopathy or branched retinal vein occlusion. Several studies have found RNFL defects or thinning after resolution of retinal vein occlusion33,34. However, a pattern of defects, such as multiple RNFL defects, has not been reported.
One of the limitations of this study is its retrospective nature. We sought to overcome the limitations of our study by using propensity-score matching according to age and MD in the comparison of the characteristics of eyes with multiple RNFL defects to those with typical glaucomatous damage. This study also has a possibility of selection bias due to its hospital-based nature with a small sample size. A small group of patients with multiple RNFL defects may not be representative of the larger population with them. Several subjects with multiple RNFL loss and rare diseases, such as trauma and pancreatitis, might not be included. Our research group attempted to overcome the limitations by reviewing charts over a long period of time—approximately 4 years. Hospital-based studies can provide important clues as to whether particular attributes are related to disease. Systemic hypotension has been suggested to be a risk factor for glaucoma. However, blood pressure was not measured directly in this retrospective study. We could not obtain exact information with regard to systemic hypotension due to the nature of this retrospective study. That is one of limitations of this study. Lack of detailed information on systemic hypertension such as its duration or stage, is also one of this study’s limitations. Overall, the clinical findings supporting the diagnosis of glaucoma in patients with multiple RNFL defects are more prominent. Patients with multiple RNFL defects showed definitely the presence of the RNFL defect. RNFL thickness was thinner in the inferior quadrant (88.9 ± 21.3 µm) than in the superior quadrant (99.3 ± 20.3 µm) in cases with multiple RNFL defects. There was no significant difference in the maximal IOP between the group with multiple RNFL defects and those without multiple RNFL defects (P = 0.144). We excluded patients with trauma, rim pallor, abnormal color vision and a best-corrected visual acuity of less than 20/40 to decrease the possibility of including other optic nerve diseases. In fact, no one with multiple RNFL defects was excluded because of abnormal color vision or decreased best-corrected visual acuity. Vascular insufficiency or instability has been suggested as a vascular pathogenic mechanism underlying development or progression of glaucoma. In this study, multiple RNFL defects were associated with systemic vascular risk factors. We speculated that vascular insufficiency might play a prominent role relatively than the compression of lamina cribrosa in patients with multiple RNFL defects, even though the confirmatory study is needed. Therefore, patients with multiple RNFL defects seemed to have multiple RNFL defects and less optic disc change than glaucoma patients without multiple RNFL defects. When glaucomatous optic neuropathy occurs predominantly with vascular insufficiency, it seemed that there might be a high possibility of developing multiple RNFL defects. Alterations in the optic disc or RNFL due to glaucomatous structural changes might induce hemodynamic changes at that location. Blood flow stasis found by angiography in glaucoma patients can result in endothelial damage or thrombosis formation35. Therefore, we assumed that glaucomatous damage also could contribute to formation of multiple RNFL defects because multiple RNFL defects were related to systemic vascular risk factors. However, it is not clear whether all cases with multiple RNFL defects independently develop from the pathogenesis of glaucoma or in combination with glaucoma. It was one of the limitations of our study. Larger and more carefully controlled population-based studies are needed to confirm the results of this study.
In conclusion, multiple RNFL defects were associated with systemic vascular risk factors and frequent involvement of parafoveal scotoma. Further studies are needed to determine whether nerve damage with multiple RNFL defects progresses or not, and if this occurs rapidly or slowly. Clinicians must consider the possibility of concomitant systemic vascular disease when evaluating patients with multiple RNFL defects.
References
Jonas, J. B. & Schiro, D. Localised wedge shaped defects of the retinal nerve fibre layer in glaucoma. Br J Ophthalmol 78, 285–290 (1994).
Sommer, A. et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol 109, 77–83 (1991).
Tatham, A. J. et al. Estimated retinal ganglion cell counts in glaucomatous eyes with localized retinal nerve fiber layer defects. Am J Ophthalmol 156, 578–587 e571 (2013).
Iwata, K., Nanba, K. & Abe, H. Typical slit-like retinal nerve fiber layer defect and corresponding scotoma (author’s transl). Nippon Ganka Gakkai Zasshi 85, 1791–1803 (1981).
Chihara, E. & Honda, Y. Multiple defects in the retinal nerve fiber layer in glaucoma. Graefes Arch Clin Exp Ophthalmol 230, 201–205 (1992).
Park, S. C. et al. Initial parafoveal versus peripheral scotomas in glaucoma: risk factors and visual field characteristics. Ophthalmology 118, 1782–1789 (2011).
Park, H. Y., Jung, K. I., Na, K. S., Park, S. H. & Park, C. K. Visual field characteristics in normal-tension glaucoma patients with autonomic dysfunction and abnormal peripheral microcirculation. Am J Ophthalmol 154, 466–475 e461 (2012).
Kim, D. M., Seo, J. H., Kim, S. H. & Hwang, S. S. Comparison of localized retinal nerve fiber layer defects between a low-teen intraocular pressure group and a high-teen intraocular pressure group in normal-tension glaucoma patients. J Glaucoma 16, 293–296 (2007).
Xu, L. et al. Localized retinal nerve fiber layer defects and arterial hypertension. Am J Hypertens 26, 511–517 (2013).
Vongphanit, J., Mitchell, P. & Wang, J. J. Population prevalence of tilted optic disks and the relationship of this sign to refractive error. Am J Ophthalmol 133, 679–685 (2002).
Felius, J., Locke, K. G., Hussein, M. A., Stager, D. R. Jr. & Stager, D. R. Sr. Photographic assessment of changes in torsional strabismus. J AAPOS 13, 593–595 (2009).
Park, H. Y., Lee, K. & Park, C. K. Optic disc torsion direction predicts the location of glaucomatous damage in normal-tension glaucoma patients with myopia. Ophthalmology 119, 1844–1851 (2012).
Choi, J. A., Park, H. Y. & Park, C. K. Difference in the posterior pole profiles associated with the initial location of visual field defect in early-stage normal tension glaucoma. Acta Ophthalmol 93, e94–99 (2015).
Griswold, M. E., Localio, A. R. & Mulrow, C. Propensity score adjustment with multilevel data: setting your sites on decreasing selection bias. Ann Intern Med 152, 393–395 (2010).
D’Agostino, R. B. Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 17, 2265–2281 (1998).
Rassen, J. A. et al. One-to-many propensity score matching in cohort studies. Pharmacoepidemiol Drug Saf 21(Suppl 2), 69–80 (2012).
Bae, H. W. et al. Systemic hypertension as a risk factor for open-angle glaucoma: a meta-analysis of population-based studies. PLoS One 9, e108226 (2014).
Zhao, D., Cho, J., Kim, M. H. & Guallar, E. The association of blood pressure and primary open-angle glaucoma: a meta-analysis. Am J Ophthalmol 158, 615–627 e619 (2014).
Chaum, E., Drewry, R. D., Ware, G. T. & Charles, S. Nerve fiber bundle visual field defect resulting from a giant peripapillary cotton-wool spot. J Neuroophthalmol 21, 276–277 (2001).
Zhang, L. et al. Cotton-wool spot and optical coherence tomography of a retinal nerve fiber layer defect. Arch Ophthalmol 130, 913 (2012).
Jonas, J. B., Fernandez, M. C. & Sturmer, J. Pattern of glaucomatous neuroretinal rim loss. Ophthalmology 100, 63–68 (1993).
Hood, D. C. et al. The Nature of Macular Damage in Glaucoma as Revealed by Averaging Optical Coherence Tomography Data. Transl Vis Sci Technol 1, 3 (2012).
Choi, J. A. et al. Retinal nerve fiber layer loss is associated with urinary albumin excretion in patients with type 2 diabetes. Ophthalmology 122, 976–981 (2015).
Lopes de Faria, J. M., Russ, H. & Costa, V. P. Retinal nerve fibre layer loss in patients with type 1 diabetes mellitus without retinopathy. Br J Ophthalmol 86, 725–728 (2002).
Sugimoto, M. et al. Detection of early diabetic change with optical coherence tomography in type 2 diabetes mellitus patients without retinopathy. Ophthalmologica 219, 379–385 (2005).
Kolker, A. E. Visual prognosis in advanced glaucoma: a comparison of medical and surgical therapy for retention of vision in 101 eyes with advanced glaucoma. Trans Am Ophthalmol Soc 75, 539–555 (1977).
Coeckelbergh, T. R., Brouwer, W. H., Cornelissen, F. W., Van Wolffelaar, P. & Kooijman, A. C. The effect of visual field defects on driving performance: a driving simulator study. Arch Ophthalmol 120, 1509–1516 (2002).
Fujita, K., Yasuda, N., Oda, K. & Yuzawa, M. Reading performance in patients with central visual field disturbance due to glaucoma. Nippon Ganka Gakkai Zasshi 110, 914–918 (2006).
Hitchings, R. A. & Anderton, S. A. A comparative study of visual field defects seen in patients with low-tension glaucoma and chronic simple glaucoma. Br J Ophthalmol 67, 818–821 (1983).
Caprioli, J. & Spaeth, G. L. Comparison of visual field defects in the low-tension glaucomas with those in the high-tension glaucomas. Am J Ophthalmol 97, 730–737 (1984).
Motolko, M., Drance, S. M. & Douglas, G. R. Visual field defects in low-tension glaucoma. Comparison of defects in low-tension glaucoma and chronic open angle glaucoma. Arch Ophthalmol 100, 1074–1077 (1982).
King, D., Drance, S. M., Douglas, G., Schulzer, M. & Wijsman, K. Comparison of visual field defects in normal-tension glaucoma and high-tension glaucoma. Am J Ophthalmol 101, 204–207 (1986).
Bartz-Schmidt, K. U. & Schmitz-Valckenberg, P. Retinal nerve fiber layer photography in retinal vein occlusion. Fortschr Ophthalmol 88, 466–472 (1991).
Kim, C. S., Shin, K. S., Lee, H. J., Jo, Y. J. & Kim, J. Y. Sectoral retinal nerve fiber layer thinning in branch retinal vein occlusion. Retina 34, 525–530 (2014).
Park, H. Y., Jeong, H. J., Kim, Y. H. & Park, C. K. Optic disc hemorrhage is related to various hemodynamic findings by disc angiography. PLoS One 10, e0120000 (2015).
Acknowledgements
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C1940). The authors declare that no competing interests exist with the funder. The funders had no role in study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Involved in Design of study (C.K.P., K.I.J.); conduct of study (K.I.J.); data collection (S.J.K., K.I.J.); analysis and interpretation of data (K.I.J., C.K.P.); statistical expertise (C.K.P., K.I.J.); writing the article (K.I.J.); and critical revision of the article (C.K.P.).
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jung, K.I., Kim, S.J. & Park, C.K. Systemic Vascular Risk Factors for Multiple Retinal Nerve Fiber Layer Defects. Sci Rep 8, 7797 (2018). https://doi.org/10.1038/s41598-018-26160-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-26160-7
This article is cited by
-
Association between localised retinal nerve fibre layer defects and cardiovascular risk factors
Scientific Reports (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.