Abstract
Parkinson’s disease (PD) is a degenerative movement disorder causing progressive disability that severely affects patients’ quality of life. While early treatment can produce significant benefits for patients, the mildness of many early signs combined with the lack of accessible high-frequency monitoring tools may delay clinical diagnosis. To meet this need, user interaction data from consumer technologies have recently been exploited towards unsupervised screening for PD symptoms in daily life. Similarly, this work proposes a method for detecting fine motor skills decline in early PD patients via analysis of patterns emerging from finger interaction with touchscreen smartphones during natural typing. Our approach relies on low-/higher-order statistical features of keystrokes timing and pressure variables, computed from short typing sessions. Features are fed into a two-stage multi-model classification pipeline that reaches a decision on the subject’s status (PD patient/control) by gradually fusing prediction probabilities obtained for individual typing sessions and keystroke variables. This method achieved an AUC = 0.92 and 0.82/0.81 sensitivity/specificity (matched groups of 18 early PD patients/15 controls) with discriminant features plausibly correlating with clinical scores of relevant PD motor symptoms. These findings suggest an improvement over similar approaches, thereby constituting a further step towards unobtrusive early PD detection from routine activities.
Similar content being viewed by others
Introduction
Parkinson’s disease (PD) is a common progressive neurodegenerative disorder1, characterised primarily by motor symptoms that contribute to significant disability2,3. The pathological hallmark of the disease is the loss of dopaminergic neurons in the substantia nigra, a basal ganglia structure of the human brain, and the presence of Lewy body-containing alpha-synuclein3, a protein widely distributed in the brain. The clinical spectrum of the disease is more extensive covering also a wide range of non-motor symptoms4 due to the degeneration of other dopaminergic and non-dopaminergic regions of the brain, spinal cord and peripheral nervous system5,6,7. The resultant decreased availability of dopamine in the basal ganglia leads to the motor symptomatology3,8. Due to the mildness of many early signs, including motor symptoms2, patients may not undergo clinical examinations for PD during early stages and therefore, the disease may be undiagnosed for many years9.
When PD is screened non-instrumentally - that is the majority of cases - the procedure traditionally involves the evaluation of subject’s overall condition according to standardised scales and questionnaires, such as the commonly used Unified Parkinson’s Disease Rating Scale (UPDRS)10. Motor status in particular, is often assessed by an expert based on the individual scoring and aggregated score of UPDRS Part III items10, which cover a broad range of PD motor symptoms, including among others, tremor (resting and action), rigidity, and bradykinesia. The latter examination, as is the case with other scales/questionnaires, requires a movement disorders specialist and the presence of the subject at the clinic. These factors limit the frequency of evaluation and monitoring of PD symptoms, while results are often of subjective nature as they rely on the expert’s experience or subject’s self-reports11. On the other hand, the development of more accessible tools that provide objective information with higher sampling rate can lead to timely diagnosis and consequent improvement of the prospective patient’s quality of life via early therapeutic interventions12.
To this end, data captured from electronic sensors have been used by works targeting objective PD symptoms monitoring, such as microphone-captured speech signals for voice impairment recognition13 and inertial measurement unit (IMU) data for hand tremor assessment14 or freezing of gait detection15, among others16, with real-life transferability potential. Moreover, the booming of mobile technology and user-mobile interaction17 has led to efforts of transferring PD screening and monitoring in the daily life, through mining of interaction data. The mPower study18 is the most prominent example of large-scale data collection for PD symptoms research, exploiting the deep penetration of smartphones in the general population. During the study, over 9,000 participants (PD patients and healthy users) remotely contributed multi-modal data by performing digitised tests bundled with a smartphone application. However, the drop-out rate concerning certain of these tests was over 90%. The latter highlights a common drawback of such approaches, i.e., data collection requires the active participation of the user and it is therefore, subject to adherence and impacted to a certain extent by the Hawthorne effect19.
In the modern era of computers and smartphones, typing on keyboards is a common, daily user-device interaction that involves dense (in terms of time), coordinated and successive finger/hand movements. Quantitative information arising from this type of interaction has been the focus of various applications, as it can be captured unobtrusively in the background during routine typing, reflecting in this way, the natural behaviour of the user. In particular, timing information associated with keystrokes, namely keystroke dynamics, has been exploited towards biometric authentication20, as well as Alzheimer’s disease21 and psycho-motor impairment detection, such as sleep inertia22. The fact that PD patients lack on rhythm stability of their finger movements23,24, while rigidity and bradykinesia affect their coordination25, renders keystroke dynamics, being the product of fine motor skills, an attractive source of information also for PD research. In fact, recent efforts have taken advantage of traditional keystroke dynamics timing variables, such as the hold time (HT) (the time a key is held down) and flight time (FT) (the time interval between releasing a key and pressing the next one), to classify subjects as having PD or not. Giancardo et al.26 used statistics of HTs, emerging from typing on a hardware keyboard, and Support Vector Machines (SVM) ensemble regression to produce a numerical index for distinguishing early PD patients and healthy controls with promising results [0.81 area under the ROC curve (AUC)]. To the same end, Arroyo-Gallego et al.27, based on data captured during typing on a touchscreen smartphone, evaluated the univariate and multivariate classification performance of various FT features and achieved a 0.91 AUC with a single feature. Both studies included tasks of continuous typing for more than five minutes.
Motivated by the aforementioned, the present work proposes a machine learning-based approach for discriminating early PD patients from healthy subjects based on enriched keystroke dynamics information, acquired during natural typing on a touchscreen smartphone. The dataset under scrutiny was recorded from 33 subjects (18 early PD patients/15 healthy controls) through a more ecologically-valid experiment compared to previous studies26,27 (see Dataset acquisition in Methods), which included fragmentary typing of short text excerpts, rather than continuous typing of longer duration. A systematic process is adopted to reach an optimised classification configuration by leveraging traditional variables of keystroke dynamics already exploited by relevant works26,27, such as HT and FT, which are further enriched for the first time, with information of normalised pressure (NP) applied on each keystroke/tap.
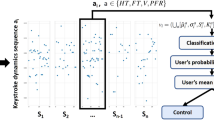
Figure 1 illustrates the feature extraction process and the proposed classification pipeline. Regarding the former (Fig. 1(a)), features are extracted on a typing session level, by aggregating statistical characteristics of time-windowed keystroke dynamics variables to construct feature vectors representing the session on the HT, conditionally-filtered/normalised FT (NFT) and NP dimensions, individually. A leave-one-subject-out (LOSO) scheme with nested cross-validations is employed for feature selection, classifiers optimisation, training and testing (see Classification methodology in Methods) of the proposed approach. The latter is a two-stage multi-model pipeline (Fig. 1(b)), in the process of which three models are employed to classify each typing session of a given subject based on HT, NFT and NP feature vectors, independently (First stage), followed by a classifier that uses the individual outcomes to produce a fused probability on whether the session belongs to a PD patient or not (Second stage). In the end, prediction probabilities assigned to individual typing sessions are subjected to mean-voting to reach a final verdict on the subject’s status against PD. The performance of our method is evaluated using the receiver operating characteristics (ROC) analysis (see Classification performance evaluation in Methods) and compared against the most recent FT-based univariate and multivariate classification approaches27. The relationship of UPDRS Part III items of interest with the most discriminant features that emerge from the analysis is further examined to interpret the results.
Illustration of (a) feature vector extraction from a given keystroke dynamics variable of a typing session and (b) classification pipeline of each subject based on hold time (HT), normalised flight time (NFT) and pressure (NP) information. (a) Given a keystroke dynamics variable sequence a n , a ∈ {HT, NFT, NP}: (1) The sequence is split in subsequences \({a}_{n}^{i}\) using 15-seconds non-overlapping time windows; (2) For each subsequence, the first- up to fourth-order statistical moments (mean μ i , standard deviation σ i , kurtosis K i , and skewness S i ) of the elements are computed; (3) The probability density function (PDF) f i(x) of each subsequence is estimated through kernel density estimation (KDE) and the matrix of sample covariance C(i, j) between the PDFs of all subsequences is calculated. Feature vectors v a representing each typing session are formed by the mean \(\mathop{\bullet }\limits^{\mbox{--}}\) and standard deviation (std) σ⋅ of the moments extracted in (2), across time windows (subsequences), and the mean, std and sum of absolute values of the upper triangle C U (i, j) of the covariance matrix calculated in (3). (b) The proposed two-stage multi-model pipeline for classifying subjects as PD patients or healthy controls: (1st Stage) Feature vector sets {v a } of a given subject, with each vector representing a typing session, serve as input to three trained models M a , each one dedicated to a keystroke dynamics variable, a ∈ {NFT, HT, NP}. Models M a yield three prediction probabilities P a which are then grouped in new feature vectors v P ; (2nd Stage) Feature vector set {v P } serves as input to a Logistic Regression classifier C LR that outputs the final classification probabilities {P f } denoting whether each typing session belongs to a PD patient or a healthy control. Finally, the mean of prediction probabilities P f is used to categorise the subject as PD patient or healthy control.
To exemplify the motivation behind the aforementioned classification approach, Fig. 2 depicts the HT, NFT, and NP sequences derived from 10 typing sessions of two healthy controls and two early PD patients that participated in our experiment. It can be observed that across sessions, all subjects exhibit a constant behaviour in terms of all keystroke dynamics variables. Nevertheless, while healthy subjects further exhibit similar behaviour across variables, PD patients present with differentiations when compared to each other and controls. In the light of this observation and after its generalisation, the two-stage multi-model approach was conceptualised. It, initially, granulates decisions to account for (non-)existing between-group differences in terms of each variable independently and afterwards, produces a final outcome on the basis of these individual decisions.
Indicative examples of keystroke dynamics variable sequences of healthy controls and PD patients. Sequences of normalised flight time (NFT) (blue), hold time (HT) (green), and normalised pressure (NP) (red) derived from 10 typing sessions (S1–S10), typed by two controls (Control 1 and Control 2) and two PD patients (PD Patient 1 and PD Patient 2), are presented along with overlayed probability density functions of each sequence, estimated for each typing session. Between two consecutive sessions, there was an one-minute interval. It is observed that controls exhibit similar behaviour across all variables. On the other hand, there are differentiations in the behaviour of PD patients when compared to each other, as well as healthy subjects. PD Patient 1 exhibits similar values to controls in terms of NFT and NP, but clearly higher HT values. In contrast, PD Patient 2 produced more wide-spread values for all keystroke dynamics variables in comparison to controls.
Results
ROC-based performance comparisons between the best configuration of our classification pipeline and recent multivariate and univariate benchmark methods27 are presented in Fig. 3(a). The best configuration consisted of Ridge28 feature selection (during LOSO training) - Random Forest29 classifier combination for the three first-stage models and mean-voting as the final step for reaching a decision on the left-out subject (PD patient or control). With this configuration, an average AUC of 0.92 (0.82–0.98; 95% Confidence Interval (CI)) was achieved over 33 iterations of LOSO validation and 1,000 bootstraps. On the other hand, FT-based multivariate and univariate methods proposed by Arroyo-Gallego et al.27 had a lower performance, i.e., average AUC of 0.82 (0.73–0.94; 95%CI) and 0.70 (0.53–0.85; 95%CI), respectively, when applied on our dataset and subjected to the same LOSO validation process.
Comparison of Receiver Operating Characteristics (ROC) curves and evolution of area under the ROC curve (AUC) with respect to the number of typing sessions used as input. (a) ROC curves demonstrating the classification performance of the proposed approach and of existing ones reported in literature and applied on our dataset (18 early PD patients/15 controls). The performance of the proposed model (green curve) is compared to the best multivariate method based on flight time (FT) features proposed by Arroyo-Gallego et al.27 (blue curve), as well as their best performing univariate method (red curve). Solid lines represent the mean ROC curve, while shadowed areas delimit the 95% confidence intervals, computed over 1,000 bootstraps. The legend shows the AUC and the 95% confidence intervals. The two-stage multi-model approach described in this paper achieves an AUC of 0.92 [0.82–0.98], outperforming the univariate 0.70 [0.53–0.85] and multivariate 0.82 [0.69–0.94] methods27. (b) Evolution of the AUC for the best performing configuration of the proposed classification pipeline with respect to the number of typing sessions used as input. The blue and green line correspond to the AUC obtained by using the mean and median of final classification probabilities {P f }, respectively, as the voting scheme to reach a decision on whether the subject has PD or not based on her/his individually classified typing sessions (see also Fig. 1(b)). As intuitively expected, as the number of typing sessions increases, a clear improvement in the classification performance is noticed, while the mean of probabilities produces better results compared to the median value.
The diagnostic performance [AUC, diagnostic accuracy] of the proposed method that is based on fused probabilities {P f } was also compared against the individual performance of prediction probabilities estimated based on each keystroke dynamics variable, i.e., {P HT }, {P NFT }, {P NP } (see Fig. 1(b) and Supplementary Material Table S.3 for additional performance metrics). In this context, for each of the latter sets of probabilities, mean-voting was applied to reach a decision on the subject’s condition (PD patient or control). Results obtained for individual predictions ({P HT }: [82.1%, 78.8%]; {P NFT }: [77%, 66.7%]; {P NP }: [67%, 66.7%]), extracted by the best first-stage classification configuration, denote that by combining information of keystroke dynamics (fusion via the second stage), diagnostic performance is increased ({P f }: [92%, 82%]).
To mitigate the risk of over-fitting, the classification pipeline was trained in a subject-agnostic fashion, which allowed the use of a larger observation space (275 typing sessions) compared to 33 input observations if features were a priori aggregated on a subject level. The impact of the number of the left-out (test) subject’s typing sessions - feature representations of which are used as input - on classification performance is illustrated in Fig. 3(b). As intuitively expected, performance improves as information from a larger number of typing sessions becomes available. Figure 3(b) also provides a comparison of two voting-schemes, i.e., median and mean, used to reach the final decision on the subject’s condition, with the latter yielding higher AUC values compared to the former as the sample of final prediction probabilities becomes larger.
Table 1 presents the results of group-level (early PD patients vs controls) statistical comparisons (two-sided Mann-Whitney U test) with respect to each individual feature, as well as the frequency of selection of each feature over all LOSO iterations of the best classification configuration. From Table 1, it is evident that certain features differ significantly between groups and are consistently selected during the LOSO training step, thereby their discriminative power is highlighted. Such consistency in not observed when using benchmark27 FT feature extraction and multivariate LOSO analysis on our dataset (see Supplementary Material Table S.4).
Distributions of the most discriminant features per subject group, computed over all typing sessions, are illustrated in Fig. 4. In general, as compared to healthy controls, PD patients exhibit longer and more variant HTs, lower pressure values, and a shift towards longer FTs, as indicated by lower skewness values of the zero-mean NFT distributions. These relationships still hold even when the group of early PD patients is clustered and examined with respect to PD medication (Supplementary Material Fig. S.1). De-novo patients’ (recently diagnosed with PD and never taken PD medication) data form an intermediate distribution between healthy controls and early PD patients under medication. Both early PD patients’ subgroups differ significantly from controls in terms of feature distributions, but not when compared to each other. These findings are similar to those that Giancardo et al.26 reported regarding their proposed HT-based discriminant index.
Group-wise comparison of distributions of the most frequently selected features as derived by the proposed approach. Box plots represent the distribution of HT \(\overline{\mu i}\), HT \(\overline{\sigma i}\), NFT \(\overline{{S}_{i}}\), NP \(\overline{\mu i}\) (see also Table 1) of PD patients and healthy controls, computed over 144 and 131 typing sessions, respectively. Each box plot visualises the interquartile range (height of rectangle), spanning the first (bottom) to the third quartile (top), the median value (horizontal line inside the rectangle), the minimum and maximum values (ends of “whiskers” below and above the box, respectively) still within the interquartile range, and outlier values (individual points below and above “whiskers”). The groups exhibit significant differences (two-sided Mann-Whitney U test) across all features of interest. **Statistically significant difference (p < 0.001).
To foster an interpretation of our outcomes with respect to PD motor symptomatology, results of Spearman correlation analysis between the most frequently selected features (see also Table 1) and scores of UPDRS Part III items of interest, as well as the compound score, are tabulated in Table 2. High to moderate correlation coefficients (\(|{r}_{s}| > 0.60\), p < 0.001) were obtained for scores of rigidity and finger taps (right hand), as well as body bradykinesia/hypokinesia and the general motor status (total UPDRS Part III score). These results point to an effect of the severity of the associated motor symptoms on keystroke dynamics and consequently, typing kinetics. On the other hand, scores associated with the upper-left extremity, as well as bilateral action tremor scores, yielded low and in certain cases, insignificant correlations (\(p > 0.05\)). The fact that all subjects of the study cohort were right-handed may explain the stronger unilateral (right side) correlations observed for all relevant clinical scores.
Discussion
Digital transformation has the potential to deliver quantitative tools and personalised solutions to satisfy unmet societal health care needs. High-frequency, easily-accessible monitoring means can allow for availability of behavioural and biometric data that in turn, when analysed properly and combined with standard medical practices, can pave the way for the prognosis of diseases in early stages, with consequent benefits for patients. That is the case of PD, i.e., a disorder that is usually left undiagnosed for years due to the mildness of many early symptoms, while early treatment can significantly improve patients’ quality of life over the course of the disease. For this reason, PD detection and monitoring based on objective data arising from user-mobile interaction has been in the spotlight during recent years. The ability of unobtrusive capturing of such data further enhances their objective character as the information reflects the user’s natural behaviour and in addition, helps overcome issues of adherence that plague approaches requiring users’ active participation. Our method attempted to amalgamate all these desired parameters towards our aspiration to develop a tool for early PD screening in daily life. Results are rather promising, as classification performance denotes satisfactory discrimination between early PD patients and controls, based on features with plausible correlations with clinical scores of relevant symptoms.
In this study, data from a PD patients’ and a healthy controls’ group, matched in terms of demographics (see Table 3), were acquired and analysed. Data acquisition was based on a protocol emulating real-life conditions that included fragmentary typing of short text excerpts, which is common in daily user-mobile interaction, rather than continuous typing of longer duration (\( > 5\) min) that was employed in similar studies26,27. Resting intervals between short typing sessions also contributed towards the minimisation of fatigue effects on typing kinetics that could be otherwise amplified due to a continuous typing effort. Regarding the 18 early PD patients involved (Table 3), four of them have never received PD medication (de-novo) and all of them were at early stages (Hoehn-Yahr stages I or II, mean UPDRS Part III score/std 16.9/7.8) and recently diagnosed (mean disease onset yrs./std 2.5/1.6). These clinical characteristics of the study cohort strengthen the significance of the study outcomes, as the proposed method satisfactorily differentiates healthy controls from PD patients even at early stages of the disease.
Furthermore, this work explored for the first time the combined discriminative potential of enriched keystroke variables (associated with both timing and pressure), unlike previous research efforts that focused on a sole dimension of traditional keystroke dynamics, i.e., the HT26 or FT27. Based on this combinatory approach, the best classification performance achieved here (0.92 AUC, 0.82/0.81 sensitivity/specificity) suggests an improvement over benchmark methods27 applied on our dataset (Fig. 3(a)), as well as on single keystroke variable-based variants of our method (Supplementary Material Table S.3). Moreover, the adopted classification pipeline brought to light statistical features of keystroke dynamics (HT \(\overline{\mu i}\), HT \(\overline{\sigma i}\), NFT \(\overline{{S}_{i}}\), NP \(\overline{\mu i}\)) that differ significantly between subject groups and are in addition, consistently selected during LOSO training (Table 1). The discriminative power of these features is complemented by their explainable significant correlations with clinical scores (UPDRS Part III items) of PD motor symptoms (Table 2) that may affect typing kinetics, i.e., muscle rigidity and bradykinesia - hypokinesia. We further discuss these results in detail below as they provide evidence on the internal validity of our approach.
Overall, as all study participants were right-handed and they mainly used their dominant hand (alone or in combination with the left hand) to type during the experiment, correlations obtained for right-hand UPDRS Part III scores, where applicable, were consistently higher than those produced for the left extremity. Probing further, all discriminant features significantly differ between the two subject groups (see Fig. 4) and exhibit significant correlations (0.51 ≤ |r s | ≤ 0.62) with the total UPDRS Part III score that reflects the subject’s overall motor status. However, as the compound score includes evaluations of motor symptoms that are unlikely to affect typing kinetics, e.g., speech and facial expression, focusing on correlations with scores of more relevant symptoms (finger tapping, rigidity and action tremor of upper extremities, and body bradykinesia/hypokinesia) would provide more solid conclusions. For instance, and according to our results, action tremor appears to have moderate to low impact on typing kinetics, as correlation values of all discriminant features with the associated UPDRS Part III single-item score were generally low (|r s | ≤ 0.4) and in certain cases, insignificant (\(p > 0.05\)). This was the case for correlations of left hand action tremor with all features, except for the NP \(\overline{\mu i}\), which may denote a minor effect of this symptom on pressure values.
On the other hand, as derived from distributions of the most discriminant HT features (HT \(\overline{\mu i}\), HT \(\overline{\sigma i}\)), PD patients produced significantly longer (HT \(\overline{\mu i}\) mean/std: PD 0.15/0.06 vs. controls 0.09/0.03) and more variant HTs (HT \(\overline{\sigma i}\) mean/std: PD 0.03/0.01 vs. controls 0.02/0.00) compared to controls. These results might constitute a projection of the effects of rigidity (muscle stiffness) and bradykinesia (slowness of movement), as denoted by the moderate to high positive correlations (0.50 ≤ r s ≤ 0.71) of these HT features with corresponding UPDRS Part III single-item scores (bradykinesia/hypokinesia and right-hand finger tapping, rigidity). These motor symptoms may slow down finger reflexes causing PD patients to hold down keys for longer and inconsistent time intervals. Rigidity, and especially bradykinesia, may also have an impact on the latency between keystrokes, as derived by significant negative correlations of the skewness-related FT feature (NFT \(\overline{{S}_{i}}\)) with corresponding UPDRS Part III single-item scores, i.e., bradykinesia/hypokinesia (r s = −0.58) and right/left-hand rigidity (r s = −0.51/r s = −0.45). PD patients exhibited on average lower values of NFT distributions skewness across typing sessions as compared to controls (NFT \(\overline{{S}_{i}}\) mean/std: PD 0.90/0.53 vs. controls 1.51/0.61). Provided that the NFT distributions of both subject groups are of zero-mean, due to normalisation of FT values, lower skewness denotes a shift of the mass of patients’ NFT distributions towards higher values (see also Fig. 2 for indicative examples); this indicates the existence of longer latencies between keystrokes during typing, possibly due to slower movements. In our case, PD patients and controls also exhibit significant differences in terms of pressure applied to initiate keystrokes, as denoted by the NP feature distributions (NP \(\overline{\mu i}\) mean/std: PD 0.51/0.06 vs. controls 0.60/0.08). Significant negative correlations of this feature with right/left-hand finger tapping (r s = −0.60/r s = −0.56) and body bradykinesia/hypokinesia (r s = −0.62) scores indicate that, as the severity of the associated symptom increases, pressure applied on touchscreen keys decreases on average. The latter could be attributed to inadequately-scaled movements (in terms of speed and amplitude) that constitute manifestations of hypokinesia that is present in PD23. On a side note, unilateral upper extremities rigidity (r s = −0.55/r s = −0.52 right/left hand) and action tremor (r s = −0.40/r s = −0.31 right/left hand) appear to have moderate to low projection on average pressure values, respectively.
One possible limitation of our study is that patients under dopaminergic therapy were asked to refrain from taking their medication at least eight hours before their morning visit to participate in the experiment. This time interval might not have been sufficient enough for certain patients to be in the “practically off” condition, a transition that usually requires 12 hours after the last dose30. The latter, combined with potential effects of long-duration response to Levodopa31,32, may have improved the psychomotor state of these patients and consequently, their typing cadence, leading to a reduced discrimination performance across classification methods tested. From an overall perspective however, the best configuration of our approach exhibits a very promising potential in correctly classifying early PD patients and controls (average AUC = 0.92), despite any “echoing” effects of dopaminergic therapy on certain study participants’ fine motor skills.
Since the proposed approach is based on the analysis of quantitative data that can be collected in an unobtrusive fashion during an activity of daily living, privacy issues should be considered. In fact, our method is privacy-aware, as it is based on timing and pressure variables of keystrokes, without requiring the actual content of the typed text. Nevertheless, results of this work, as well as of similar research efforts, indicate that this source of information can provide insights into users’ health status and therefore, it belongs, by definition, to the category of sensitive personal data. The ability to unobtrusively integrate the recording of typing patterns with routine activities highlights even more the need to ensure that users are aware in advance of what data will be recorded, when and to which end, before using relevant applications. In this context, future commercial applications exploiting such patterns towards the recognition of psycho-motor impairments must comply with ethical guidelines and data protection regulations, as per standard practices in similar cases involving recordings of biometrics and health data.
In summary, our work provided evidence on a machine-learning method that satisfactorily detected early-stage PD in a relatively small cohort, based on the projection of fine-motor skills decline on keystroke dynamics variables during natural typing. Correlation results indicate the potential of evolving the binary classification problem into a regression analysis for estimating the severity of individual PD motor symptoms based on relevant statistical features of keystroke variables. A future deployment of the latter could assist physicians to gain objective, granular and explainable insights into the subject’s condition against PD. Beyond this study, our vision is to extend and validate the developed method on longitudinal data, unobtrusively captured from a large pool of subjects and their day-to-day typing interaction with their smartphone. This vision is consequent to the overarching aim to develop accessible, non-intrusive tools for early PD screening in daily life of the European Horizon 2020 project “i-PROGNOSIS” (www.i-prognosis.eu), within the framework of which the present work was realised.
Methods
A feature extraction and classification pipeline is presented for classifying subjects as PD patients or healthy controls based on data derived from sporadic typing sessions on a touchscreen smartphone. The data of interest comprise sequences of hold times (HT) (the time between pressing and releasing a key) and flight times (FT) (the time between releasing a key and pressing the next one), produced from captured time stamps, as well as normalised pressure (NP) sequences. Each typing session is characterised via three independent feature vectors, each composed of lower-order (mean, standard deviation) and higher-order (kurtosis, skewness, and covariance) statistics representing the HT, FT and NP sequences, respectively. For each typing session, a two-stage multi-model classification scheme is adopted with the first stage yielding classification probabilities based on each of the feature vectors of HT, FT, and NP sequences and the second stage fusing these probabilities to produce a final prediction probability for the particular session (belonging to a PD patient or a healthy control). The mean of the probability distribution formed by classifying all typing sessions of a subject is used to reach the final decision on her/his condition (PD patient or healthy control).
Study procedures
The study protocol was approved by the Aristotle University of Thessaloniki, Greece (Bioethics Committee of Medical School, approval no. 359/3.4.17). Informed consent was obtained from all subjects prior to their participation in the study. Subjects held the right to withdraw from the procedure at any time, without providing any justification. Recruitment and study procedures were carried out according to institutional and international guidelines on research involving adult human beings.
Study cohort
The study cohort comprised two groups, i.e., the early PD patients’ group that consisted of 18 subjects (Hoehn-Yahr stages I or II) with a confirmed diagnosis of less than five years and the controls’ group that included 15 healthy subjects without any sign of Parkinsonism. The two groups were matched in terms of gender, age, education level, and years of experience with smartphones. All subjects were native Greek speakers, 40 years of age or older, and right-handed. Demographic and clinical characteristics of the study cohort are tabulated in Table 3. Only participants who self-reported that they used a touchscreen-equipped smartphone for at least a year were considered eligible. Subjects with other diagnosed psycho-motor impairments (including drug abuse and sleep disorders), cognitive dysfunctions, upper limb functional limitations (including recoveries from recent surgery) or uncorrected vision problems were excluded from the study. Subjects were recruited from two neurological clinics in Thessaloniki (Greece) and the Aristotle University of Thessaloniki (Greece). The study protocol (typing experiment and clinical evaluation) was conducted during a single morning visit of each subject at one of the clinics. Patients receiving symptomatic relief medication for PD were asked to refrain from taking it for at least eight hours before their visit, i.e., they practically underwent all study procedures in the “off-state”, before their morning dose.
Dataset acquisition
Each visit included a typing experiment and a clinical evaluation. During the typing experiment, subjects were asked to transcribe 11 text excerpts of the famous fairy-tale “The Little Prince” in Greek, using an Android smartphone (LG Nexus 5X with a screen of 5.2 inches in diagonal and a resolution of 1080 × 1920 pixels, running native Android 7.0) and a custom mobile application with an editable text placeholder. The text excerpts were presented on a 17-inch laptop in front of the subject. The first excerpt was 200 characters-long and it was the same for all subjects, in order to familiarise themselves with the software keyboard and the mobile device. Data acquired during this session were not included in the analysis. Following this, each subject typed 10 short text excerpts (46–115 characters-long), pseudo-randomly and uniformly drawn from the fairy-tale, with an one minute-interval between two consecutive excerpts. Subjects were instructed to sit comfortably and to type using their own typing style (one or both hands) and capital letters only. There were no time constraints for typing the excerpts and participants were at liberty to adjust their posture and to correct typing errors or not. The goal was to simulate real-life, natural typing interaction with mobile devices, i.e., sporadic short typing sessions during the day, so as to acquire a dataset as ecologically-valid as possible in a laboratory environment. A custom software keyboard was developed for the Android Operating System (OS) to capture the raw data of interest in the background, while typing. These included the raw time stamps of the press and release touch events for each key tapped (in milliseconds) and the normalised pressure (0.000–1.000) applied on each key tap, as outputted by native functions of the OS; raw pressure values are not exposed by the OS. The captured time stamps and pressure values were recorded in a separate .txt file for each typing session, stored on the device internal storage memory. The filename of each file included the coded ID assigned to the subject at the beginning of the experiment. The keyboard did not support any “long press” actions that would constitute noise artefacts. Nevertheless, in a more advanced keyboard that supports such actions, relevant key presses can be easily flagged and excluded from a subsequent analysis.
Regarding clinical evaluation, after completing the typing experiment, each participant was subjected once to evaluation in terms of the motor section of the Unified Parkinson’s Disease Rating Scale (UPDRS-Part III)10 that was performed by a specialised neurologist. UPDRS-Part III scores were afterwards logged, along with demographic data, in a spreadsheet file and mapped to the subjects’ coded IDs by the neurologist.
Feature vector extraction
Let \({t}_{n}^{p}\) and \({t}_{n}^{r}\) be monotonically increasing sequences of time stamps (ms) corresponding to the press (p) and release (r) of key n = 1, 2, …, N, respectively, where N is the total number of keys pressed during a typing session. A lower bound of 20 characters per minute is set for the typing rate per typing session; sessions which did not meet this bound were omitted from the subsequent analysis. The sequences of HTs and FTs are defined as \(H{T}_{n}={t}_{n}^{r}-{t}_{n}^{p}\), n = 1, 2, …, N, and \(F{T}_{n}={t}_{n+1}^{p}-{t}_{n}^{r}\), n = 1, 2, …, N-1, respectively, and the normalised pressure sequence as \(N{P}_{n}=NP({t}_{n}^{p})\), n = 1, 2, …, N, where NP(t) is the varying normalised pressure applied during the pressing of each key.
To further minimize the effects of varying typing dexterity amongst subjects and remove outlier values (due to subjects stalling key presses because of reading the text to be transcribed on the laptop screen), conditional filtering is applied to the FT sequence which is affected the most by these factors. The filtering approach27 of Arroyo-Gallego et al. was adopted here for the analysis results to be comparable. FT values exceeding a 3 s-threshold are removed from the sequence, FT n sequences are further detrended and values outside the 99% interval of a real-life data distribution [−1.27,1.7] (s) are filtered out. The latter real-life data distribution was estimated by leveraging an external dataset of ten healthy participants who used our Android custom keyboard for typing in their daily life for more than three weeks. Thus, the conditionally-filtered FT n sequence for each typing session, hereby referred to as the normalised flight time sequence NFT n , can be written as:
On the other hand, HT values (usually in the range of 100 milliseconds), being the relatively short timing outcome of a finger reflex - the finger presses down (to initiate the action) and releases the key (upon success of the intended action) after visual or haptic feedback that the key was registered - are not expected to be affected by the aforementioned collateral factors. For example, HT, unlike FT, is almost unaffected by interruptions during typing. Non-pathological factors that affect HT values, such as the type of medium (hardware with key travel or touch-based virtual keyboard), deliberate long-presses or the type of key feedback, were mitigated by the experiment protocol as no long-presses were supported and the virtual keyboard and key feedback (visual) was common among all subjects. In this context, no conditional filtering/normalisation is applied on the HT sequence, on par with works that exploited features of raw HTs for psycho-motor impairment detection22,26.
Thereafter, we define subsequences of HT n , NFT n , NP n based on a time window w with duration T (T = 15 s in our implementation as in Arroyo-Gallego et al.27) as:
where i denotes the increasing index of the window and k = 1, 2, ..., n w − n1 + 1 the index of the elements in the window. A minimum of five elements per subsequence (n w − n1 >4) is considered in order to extract meaningful statistical features; subsequences not meeting this criterion were omitted from further analysis. For simplicity, we denote any of the valid subsequences as \({a}_{k}^{i}\). Statistical features extracted to represent the i-th subsequence are: the mean \({\mu }_{i}={\sum }_{k}{a}_{k}^{i}/({n}_{w}-{n}_{1}+\mathrm{1)}\), standard deviation \({\sigma }_{i}=\sqrt{\sum k({a}_{k}^{i}-{\mu }_{i})/({n}_{w}-{n}_{1})}\), skewness \({S}_{i}={\sum }_{k}{(\frac{{a}_{k}^{i}-{\mu }_{i}}{{\sigma }_{i}})}^{3}/({n}_{w}-{n}_{1}+\mathrm{1)}\) and kurtosis \({K}_{i}={\sum }_{k}{(\frac{{a}_{k}^{i}-{\mu }_{i}}{{\sigma }_{i}})}^{4}/({n}_{w}-{n}_{1}+\mathrm{1)}\) of the samples of the subsequence. Moreover, an approximation of the probability density function (PDF) f i(X) of the i-th subsequence is estimated by employing Kernel Density Estimation (KDE)22 with a Gaussian kernel. In the present implementation, ten quantization levels (L = 10) are used and the bandwidth parameter (b) of the Gaussian kernels is calculated by applying the Sheather-Jones method33 on the aforementioned external dataset, resulting in b = 0.0060, 0.0289, and 0.0300 for HT, NFT, and NP data respectively. For each typing session, we construct three feature vectors, each representing a type of sequence (HT n , FT n or NP n ), by aggregating the statistical representations of all subsequences as:
where a∈{HT, NFT, NP} and \(\mathop{\bullet }\limits^{\mbox{--}},{\sigma }_{\cdot }\) denote the mean and the standard deviation of the corresponding values, respectively. C U is the upper triangle of the covariance matrix formed by the values of sample covariance between the estimated PDFs f i(X) of all subsequences, i.e., \({C}_{i,j}=\frac{1}{L-1}{\sum }_{X}[\,{f}^{i}(X)-\overline{{f}^{i}(X)}\,][{f}^{j}(X)-\overline{{f}^{j}(X)}]\), and \(\sum |{C}_{U}|\) is the sum of absolute values of C U . In the case of flight time, \(\overline{{\mu }_{i}}\) and \({\sigma }_{{\mu }_{i}}\) are omitted as NFT n is of zero mean due to the aforementioned conditional filtering. After applying the feature vector extraction approach on all typing sessions from all subjects, three feature vector sets are produced, i.e., I HT = {v HT }, I NFT = {v NFT }, and I NP = {v NP }.
Classification methodology
A two-stage classification pipeline is adopted for classifying subjects as having PD or not. In the first stage, three models (feature selection and classifier combination) are employed, with each one learning and predicting based on the representations of HT (v HT ), NFT (v NFT ), and NP (v NP ) of each typing session, respectively, and the labels inherited from the corresponding subject’s condition (PD patient or healthy control). This approach was conceptualised after observing differences in each of these keystroke dynamics variables amongst PD patients (see Fig. 2). In the second stage, prediction probabilities produced by each of the three first-stage models are combined into a single vector (v p ) and serve as input to a single classifier that yields a fused prediction probability on whether or not the particular typing session belongs to a PD patient or a healthy control. A leave-one-subject-out (LOSO) scheme with inner k-fold cross-validations is employed to evaluate the discrimination potential of the proposed approach. The training and testing procedures of the classification approach are illustrated in Figs. 5 and 1(b), respectively.
Training procedure of each leave-one-subject-out loop. (1) The computed I a feature vector sets, a∈{HT,NFT,NP}, with each feature vector representing a typing session, are split in two parts, i.e., I′ a and \({I^{\prime} }_{a}^{C}\) (the left-out subject feature vector sets). The I′ a sets are further split in two subsets, i.e., the I′a,TRN (80%) and the I′a,TST (20%). (2) The I′a,TRN subsets are used for feature selection, hyper parameter optimisation using an inner 4-fold cross-validation, and training of three classifiers, resulting in three independent models M a , each one dedicated to a specific keystroke dynamics variable (HT, NFT and NP). (3) The I′a,TST subsets are then used to test the three M a models, resulting in three classification probabilities sets {P a }, a∈{HT,NFT,NP}. (4) Sets {P HT }, {P NFT }, and {P NP } are fused in single feature vectors, forming a set {v P } that serves to optimise (as in (2)) and train a Logistic Regression classifier C LR . The end product of the training procedure is a configuration of three optimised/trained models (first stage) that independently classify each typing session according to each keystroke dynamics variable, and an optimised/trained Logistic Regression classifier (second stage) that aggregates the latter decisions to reach a final verdict on whether the typing session belongs to a PD patient or not. The latter two-stage configuration is tested using the left-out subject’s \({I^{\prime} }_{a}^{C}\) sets (see also Fig. 1(b)).
For the training step of each LOSO loop, each of the I′ HT , I′ NFT , I′ NP feature vector sets, derived from the complete I HT , I NFT , I NP sets by leaving out a subject’s typing sessions, is randomly split to a training I′a,TRN (80%) and a testing I′a,TST subset (20%), a∈{HT, NFT, NP}. Training subsets I′a,TRN are subjected to a recursive feature elimination procedure that updates feature vectors by selecting the most discriminant features. An upper limit of five selected features is set to avoid the “curse of dimensionality”34. The updated I′a,TRN serve to train each of the first-stage classifiers. For each classifier, grid search is performed at first, using the updated I′a,TRN and an inner 4-fold cross-validation for hyper parameter optimization. The outcomes of this process are three optimised models in terms of discriminant features and classifier parameters. Following this, the testing subsets I′a,TST are used to test the optimised models. Testing probabilities P a , a∈{HT, NFT, NP}, outputted from the three first-stage models, are afterwards grouped into three-element vectors v p = [P HT , P NFT , P NP ], which populate the training subset I′ P for the second-stage classifier. The latter is again optimised in terms of parameters based on the I′ P and a 4-fold cross-validation. The output of the optimised classifier is the final probability P f that denotes whether a typing session belongs to a PD patient or a healthy control.
Once the training step completes, the left-out subject’s feature vector sets \({I^{\prime} }_{a}^{C}={I}_{a}\backslash {I^{\prime} }_{a}\), where C denotes the complement set and a∈{HT, NFT, NP}, are fed to the classification pipeline to test it under the optimised scenario (selected features and trained classifiers with optimised hyper parameters). The output of the pipeline is a set of prediction probabilities {P f } formed by classifying all typing sessions of the subject. Eventually, the left-out subject is classified as having PD or not by taking the mean of the set {P f }. The LOSO scheme is completed when all subjects are left out and afterwards evaluated against PD. For the first stage of the proposed methodology, combinations of four types of classifiers, i.e., Linear Support-Vector Machine35, Logistic Regression36, Random Forest29, and k-Nearest Neighbours, with three feature ranking-selection methods, i.e., Lasso, Ridge28, and Gini Impurity37, were examined in terms of classification performance. A Logistic Regression classifier was used in the second stage of the methodology, in all cases.
Classification performance evaluation
Different classification pipeline configurations (different combinations of classifiers and feature selection methods) conceived here, as well as existing classification methods described in literature, are evaluated using the receiver operating characteristic (ROC) analysis. ROC analysis is an iterative process of varying the discrimination threshold of a binary classifier and outputting the (Sensitivity, Specificity) pair for each threshold. The ROC curve is then formed by plotting the output pairs of (1 − Specificity, Sensitivity). The analysis provides reliable insights into the performance of a classification model even when datasets are not completely balanced (45.5% healthy controls, 54.5% early PD patients in our case). To assess the statistical significance of classification results, sampling with replacement (1,000 bootstraps) is further used here to define a ROC curve distribution. The average value and the confidence intervals of the area under the ROC curve (AUC) over 1,000 bootstraps are used to evaluate the performance of each binary (PD patient vs. control) classification approach. Where reported, sensitivity/specificity values correspond to the optimal threshold for equal cost of misclassifying PD patients and healthy controls.
Data Availability
All data generated and analysed during the current study are available from the corresponding author on a reasonable request.
References
Shulman, J., De Jager, P. & Feany, M. Parkinson’s disease: genetics and pathogenesis. Annual Review of Pathology: Mechanisms of Disease 6, 193–222 (2011).
Hoehn, M. & Yahr, M. Parkinsonism onset, progression, and mortality. Neurology 17, 427 (1967).
Kalia, L. & Lang, A. Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nature reviews Neurology 12, 65–66 (2016).
Chaudhuri, K., Healy, D. & Schapira, A. Non-motor symptoms of Parkinson’s disease: diagnosis and management. The Lancet Neurology 5, 235–245 (2006).
Todorova, A., Jenner, P. & Chaudhuri, K. Non-motor Parkinson’s: integral to motor Parkinson’s, yet often neglected. Practical neurology 14, 310–322 (2014).
Titova, N., Qamar, A. & Chaudhuri, K. The Nonmotor Features of Parkinson’s Disease. International review of neurobiology 132, 33–54 (2017).
Jellinger, K. Neuropathology of Nonmotor Symptoms of Parkinson’s Disease. International review of neurobiology 133, 13–62 (2017).
Weingarten, C., Sundman, M., Hickey, P. & Chen, N. Neuroimaging of Parkinson’s disease: Expanding views. Neuroscience & Biobehavioral Reviews 59, 16–52 (2015).
Schrag, A. et al. Prediagnostic presentations of Parkinson’s disease in primary care: a case-control study. The Lancet Neurology 14, 57–64 (2015).
Goetz, C. et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): Scale presentation and clinimetric testing results. Movement Disorders 23, 2129–2170 (2008).
Pagan, F. Improving outcomes through early diagnosis of Parkinson’s disease. The American journal of managed care 18, S176–82 (2012).
Murman, D. Early treatment of Parkinson’s disease: opportunities for managed care. The American journal of managed care 18, S183–8 (2012).
Tsanas, A., Little, M., McSharry, P., Spielman, J. & Ramig, L. Novel speech signal processing algorithms for high-accuracy classification of Parkinson’s disease. Transactions on Biomedical Engineering 59, 1264–1271 (2012).
Kostikis, N., Hristu-Varsakelis, D., Arnaoutoglou, M. & Kotsavasiloglou, C. A Smartphone-Based Tool for Assessing Parkinsonian Hand Tremor. IEEE Journal of Biomedical and Health Informatics 19, 1835–1842 (2015).
Ahlrichs, C. et al. Detecting freezing of gait with a tri-axial accelerometer in Parkinson’s disease patients. Medical & biological engineering & computing 54, 223–233 (2015).
Sánchez-Ferro, Á. et al. New methods for the assessment of Parkinson’s disease (2005 to 2015): A systematic review. Movement Disorders 31, 1283–1292 (2016).
Sarwar, M. & Soomro, T. Impact of smartphone’s on society. European journal of scientific research 98, 216–226 (2013).
Bot, B. et al. The mPower study, Parkinson disease mobile data collected using ResearchKit. Scientific Data 3, 160011 (2016).
Monahan, T. & Jill, F. Benefits of â’observer effects’: lessons from the field. Qualitative Research 10, 357–376 (2010).
Monrose, F. & Rubin, A. Keystroke dynamics as a biometric for authentication. Future Generation computer systems 16, 351–359 (2000).
Van Waes, L., Leijten, M., Mariën, P. & Engelborghs, S. Typing competencies in Alzheimer’s disease: An exploration of copy tasks. Computers in Human behaviour 73, 311–319 (2017).
Giancardo, L., Sánchez-Ferro, A., Butterworth, I., Mendoza, C. & Hooker, J. Psychomotor impairment detection via finger interactions with a computer keyboard during natural typing. Scientific reports 5 (2015).
Mazzoni, P., Shabbott, B. & Cortés, J. Motor control abnormalities in Parkinson’s disease. Cold Spring Harbor perspectives in medicine 2, a009282 (2012).
Konczak, J., Ackermann, H., Hertrich, I., Spieker, S. & Dichgans, J. Control of repetitive lip and finger movements in Parkinson’s disease: Influence of external timing signals and simultaneous execution on motor performance. Movement Disorders 12, 665–676 (1997).
Teulings, H., Contreras-Vidal, J., Stelmach, G. & Adler, C. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Experimental neurology 146, 159–170 (1997).
Giancardo, L. et al. Computer keyboard interaction as an indicator of early Parkinson’s disease. Scientific reports 6 (2016).
Arroyo-Gallego, T. et al. Detection of Motor Impairment in Parkinson’s Disease via Mobile Touchscreen Typing. IEEE Transactions on Biomedical Engineering. 64, 1994–2002 (2017).
Ng, A. Feature selection, L1 vs. L2 regularization, and rotational invariance. In Proceedings of the twenty-first international conference on Machine learning (ACM 2004).
Liaw, A. & Wiener, M. Classification and regression by randomForest. R news 2, 18–22 (2002).
Langston, W. et al. Core assessment program for intracerebral transplantations (CAPIT). Movement Disorders 7, 2–13 (1992).
Zappia, M. et al. Long-duration response to levodopa influences the pharmacodynamics of short-duration response in Parkinson’s disease. Annals of neurology 42, 245–248 (1997).
Zappia, M. et al. Loss of long-duration response to levodopa over time in PD Implications for wearing-off. Neurology 52, 763–763 (1999).
Sheather, J. & Jones, M. C. A Reliable Data-Based Bandwidth Selection Method for Kernel Density Estimation. Journal of the Royal Statistical Society. Series B (Methodological) 53, 683–690 (1991).
Bishop, C. Neural networks for pattern recognition. (Oxford university press 1995).
Cristianini, N. & Shawe-Taylor, J. An introduction to support vector machines. (Cambridge University Press, 2002).
Walker, S. & Duncan, D. Estimation of the probability of an event as a function of several independent variables. Biometrika 54, 167 (1967).
Quinlan, J. Induction of decision trees. Machine learning 1, 81–106 (1986).
Acknowledgements
The research leading to these results has received funding from the European Union’s Horizon 2020 research and innovation programme under grant agreement No 690494 - i-PROGNOSIS: Intelligent Parkinson early detection guiding novel supportive interventions.
Author information
Authors and Affiliations
Contributions
S.H., V.C., S.B. and Z.K. conceived the study protocol; D.I., S.H. and V.C. developed the keyboard and the algorithms; D.I., S.H. and V.C. conducted the typing experiment; S.B. and Z.K. conducted the clinical evaluations; D.I., S.H., L.H. and V.C. analysed the data. All authors discussed the results and contributed to the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iakovakis, D., Hadjidimitriou, S., Charisis, V. et al. Touchscreen typing-pattern analysis for detecting fine motor skills decline in early-stage Parkinson’s disease. Sci Rep 8, 7663 (2018). https://doi.org/10.1038/s41598-018-25999-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25999-0
This article is cited by
-
Disease severity classification using passively collected smartphone-based keystroke dynamics within multiple sclerosis
Scientific Reports (2023)
-
A scoping review of neurodegenerative manifestations in explainable digital phenotyping
npj Parkinson's Disease (2023)
-
Diagnostic accuracy of keystroke dynamics as digital biomarkers for fine motor decline in neuropsychiatric disorders: a systematic review and meta-analysis
Scientific Reports (2022)
-
Parkinson’s disease severity clustering based on tapping activity on mobile device
Scientific Reports (2022)
-
Assessment of real life eating difficulties in Parkinson’s disease patients by measuring plate to mouth movement elongation with inertial sensors
Scientific Reports (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.