Abstract
HTLV-1 is transmitted primarily either through sexual intercourse or from mother to child. The mother/child pairs were classified as seroconcordant or serodiscordant. We analyzed mother to child transmission (MTCT) according to sociodemographic, clinical and epidemiological characteristics of the mother, child’s gender and duration of breastfeeding. Between June 2006 and August 2016 we followed 192 mothers with HTLV-1 infection (mean age 41 years old), resulting in 499 exposed offspring, 288 (57.7%) of whom were tested for HTLV-1, making up the final sample for the study, along with their 134 respective mothers. Among the tested mother/child pairs, 41 (14.2%) were HTLV-1 positive, highlighted that seven of 134 family clusters concentrated 48.8% of positive cases. Variables associated with a positive child: breastfeeding duration ≥12 months, maternal PVL ≥100 copies/104 PBMC, mother’s age at delivery >26 years old, and HTLV-1 in more than one child of the same mother. In a multiple logistic regression, breastfeeding ≥12 months, higher maternal PVL and ≥2 previous HTLV-1-infected children remained independently associated with the outcome. Thus, high maternal PVL and breastfeeding beyond 12 months were independently associated with MTCT of the HTLV-1 infection. Our results reinforce the need for both prenatal HTLV screening in endemic areas and for advising mothers on breastfeeding.
Similar content being viewed by others
Introduction
Human T-cell lymphotropic virus type 1 (HTLV-1), a virus infecting humans since ancient times, is the causative agent of a lymphoproliferative malignancy named Adult T-cell Leukemia/Lymphoma (ATLL), as well as of the HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP); HTLV-1 has also been associated with uveitis, infective dermatitis, and other inflammatory disorders1,2,3,4. This infection is endemic in many parts of the world, including southwestern Japan, South America, some of the Caribbean islands, western and central Africa, Australo-Melanesia and some areas of Middle-East, such as regions of Iran5. There are at least an estimated five to 10 million HTLV-1 carriers worldwide3, but screening of the population for the presence of the virus is performed for only one third of the endemic areas. Brazil is most likely the country with the highest absolute number of seropositive individuals, with almost one million infected people6.
HTLV-1 transmission primarily occurs through the following routes: 1) vertically (from mother to child), including a long time of breastfeeding (8); 2) sexual contact, primarily from men to women; and 3) parenterally through the transfusion of contaminated blood and blood components or through contaminated needles7. Concerning mother-to-child-transmission (MTCT) transmission, a review8 pointed out that in infants breastfed, the MTCT occurs at rates varying from 7.4%9 to 32%10, compared with a rate of less than 5% among bottle-fed children, some of them tested during adulthood9,11,12,13,14. Although prolonged breastfeeding seems to play a central role in the spread and maintenance of endemicity9,12,14, the relative importance of HTLV-1 main routes of transmission in Brazil is still a matter of discussion15,16. Preventive measures, such as prenatal screening and abstinence from breastfeeding by carrier mothers might reduce the prevalence of HTLV carriers, such as in Nagasaki population, in which a decline from 20–25% to 4% in the prevalence of HTLV carriers was observed. In Brazil, unfortunately, prenatal screening for HTLV has not yet been implemented throughout the country. In addition, there are no specific studies establishing the rate of MTCT transmission of the virus in the country, or the role of the HTLV-1 maternal proviral load as a risk factor for mother-to-child transmission. Analyses accounting simultaneously for possible epidemiological, clinical and biomolecular markers for vertical transmission are thus needed to assess any independent effect and may increase our understanding of the determinants of MTCT.
Material and Methods
Study Design and Study Population
A cross-sectional study involving mothers with HTLV-1 infection at the Institute of Infectology “Emílio Ribas” in São Paulo from June 2006 to August 2016 and their exposed child tested for HTLV infection. Mothers were excluded if they did not have any child tested or if their documented seroconversion occurred after the birth of their children. We classified the mother-child pairs into two groups: a) seropositive mother and her seropositive child tested and b) seropositive mother with her seronegative child tested.
Diagnostic algorithm
The serological tests were carried out in the clinical laboratory at the Instituto de Infectologia Emilio Ribas, at its immunology section, employing the GOLD ELISA HTLV-1/2 (Immunoenzyme Test) and Western Blot Test (WB) (MP Diagnostics (MPD) HTLV Blot 2.4), for HTLV screening and confirmation, respectively. All WB positive cases were also submitted to a qualitative polymerase chain reaction (Nested-PCR) test17.
HTLV-1 proviral load (PVL)
The HTLV-1 proviral load was quantified by real-time PCR, using primers and probes targeting the pol gene: SK110 (5′-CCCTACAATCCAACCAGCTCAG-3′, HTLV-1 nucleotide 4758–4779 (GenBank accession No. J02029), and SK111 (5′-GTGGTGAAGCTGCCATCGGGTTTT-3′, HTLV-1 nucleotide 4943–4920). The internal HTLV-1 TaqMan probe (5′-CTTTACTGACAAACCCGACCTACCCATGGA-3′) was selected using the Oligo (version 4, National Biosciences, Plymouth, MI, USA) and Primer Express (Perkin-Elmer Applied Biosystems, Boston, MA, USA) software programs and checked through a search of GenBank. The probe was located between positions 4829 and 4858 of the HTLV-1 genome and carried a 5′ reporter dye FAM (6-carboxy fluorescein) and a 3′ quencher dye TAMRA (6-carboxy tetramethylrhodamine). For quantification of the human albumin gene, the primers Alb-S (5′-GCTGTCATCTCTTGTGGGCTGT-3′) and Alb-AS (5′-AAACTCATGGGAGCTGCTGGTT-3′) and the albumin TaqMan probe (5′-FAMCCTGTCATGCCCACACAAATCTCTCCTAMRA-3′) were used as described previously18,19. Albumin DNA was quantified jointly in all samples in order to determine the amount of DNA used as an endogenous reference to normalize variations due to differences in PBMC counts or DNA extraction. The 25-μl PCR mixture for HTLV-1 or albumin DNA amplifications consisted of 5 μl DNA extract, primers SK110 and SK11 or Alb-S and Alb-AS (10 nM of each), 10 nM HTLV-1 or albumin TaqMan probe, TaqMan® Universal Master Mix II (Applied Biosystems®, Foster City, CA). For both the HTLV-1 and albumin DNA amplifications, after one cycle at 50 °C for 2 min and one cycle at 95 °C for 10 min, a two-step PAC procedure was used consisting in 15 s at 95 °C and 1 min at 60 °C for 45 cycles. Amplifications were carried out using the ABI 7300Fast Real-Time PCR System (Applied Biosystems®, Foster City, CA). The HTLV-1 copy number in each clinical sample was estimated by interpolation from the plasmid regression curve. To determine the proviral load, the HTLV-1 DNA copy number was normalized to the amount of the cellular albumin of the clinical sample, which was quantified in parallel. All samples were run in duplicates. Results were expressed as HTLV-1 DNA copies/104 PBMCs, as described elsewhere13. Based on the median of asymptomatic individuals, 200 copies/104 PBMCs of PVL was the value used as a cut off to discriminate from HAM/TSP subjects20.
Statistical analysis
The categorical variables included in the univariate analysis were “having HTLV-1 infected mother or sibling”, HAM/TSP, ATL, race/ethnicity, place of birth, hepatitis C virus infection (HCV) status, history of blood transfusion and educational level. We transformed numerical variables into categorical variables for the analysis of mother-to-child transmission, with their mean or median serving as reference; for PVL, ≥100 copies/104 PBMC were considered high; for breastfeeding duration, which averaged 11 months, the cut-off point adopted was 12 months.
Statistical analysis was conducted using Student’s t-test for parametric data, and the chi-square test for proportions. Possible differences in patient characteristics or laboratory values among the groups were evaluated with two-way Mann-Whitney’s test. Bivariate logistic analysis was performed to identify independent variables associated with HAM/TSP. Variables associated with the outcome at a significance level of p < 0.20 (HAM/TSP) in the bivariate analysis were included in a multiple logistic regression model; the only exception to this procedure was the inclusion of the variable gender, which was included regardless of its statistical significance in the bivariate model. Logistic analysis was performed with the aid of Stata 10 software (StataCorp. 2009. Stata: Release 10. Statistical Software. College Station, TX). We extracted all analyzed variables from our REDCap database (Valderbilt University, US) and used the statistical software Stata/IC 13.1 for the statistical analysis. P values < 0.05 were considered statistically significant. Variables reaching a significance level of p < 0.20 in the univariate analysis were included in a multiple logistic regression analysis, which we used to determine those that were independently and significantly associated with the outcome.
Ethics
The Research Ethics Committee of the Faculty of Medicine of the University of São Paulo approved the current study under the number 407/12. All patients signed an informed consent form. The performance of tests was conducted according with their norms and regulations.
Results
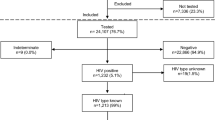
Among 192 mothers with HTLV-1 infection and their 499 respective exposed children, only 288 (57.7%) attended our invitation and were tested for HTLV-1, 41 of whom were positive for the virus, resulting in a transmission rate of 14.2%. Mothers’ age at delivery ranged from 14 to 48 years, with an average of 26.1 (±6.4) years. Maternal PVL ranged from 0 to 1221 copies/104 PBMC, with a mean of 128 copies/104 PBMC (±262); the number of children by mother ranged from 1 to 10, with a mean of 3.4 (±1.5) (Table 1).
From 288 tested children, 253 had been breastfed, and the duration of breastfeeding ranged from less than two weeks to 60 months (mean 10.7 months). Co-infections were detected in 19 mothers. We identified a tendency to familial aggregation of cases, where among 134 tested mother/child pairs, 41 (14.2%) were HTLV-1 positive, highlighted that seven of 134 family clusters concentrated 48.8% of positive cases. Table 2 present the prevalence and Odds Ratio (OR) for HTLV-1 seropositivity among 288 children exposed to vertical transmission according to the child’s gender, duration of breastfeeding, and maternal clinical and epidemiological characteristics.
Variables associated with an HTLV-1-seropositive offspring were: breastfeeding for 12 months or more (p < 0.001), having two or more HTLV-1 positive children(p < 0.001), mother’s PVL (≥100 copies/104 PBMC) (p = 0.014), mother with an epidemiological history suggestive of virus acquisition by mother-to-child transmission (her mother and/or sibling with HTLV-1 infection) (p = 0.016), age (p = 0.005), Asian maternal race (p = 0.02) and divorced marital status (p = 0.016). The odds ratio of possible protection conferred by cesarean delivery did not reach statistical significance (Table 1). Likewise, the mother’s schooling was inversely proportional to the child’s risk of seropositivity, but this association was not statistically significant (Table 2). Variables associated with the outcome at a significance level of p < 0.20 (MTCT) in the univariate analysis were included in a multivariate logistic model. On that model, variables independently associated with child seropositivity were: maternal proviral load ≥100 copies/104 PBMC (p = 0.005), breastfeeding for 12 months or more (p < 0.001), and having two or more HTLV-1-infected children (p < 0.001) (Table 3).
Discussion
For the first time in Brazil, in this study we simultaneously analyzed epidemiological, clinical markers, and maternal proviral load, for mother-to-child HTLV-1 transmission. The prevalence of concordant transmisson to the child was 14.2%, which was lower than found in countries such as Jamaica (18% to 22%)21,22, Peru (18%)23, Iran (16.6%)24, Gabon (17.5%)25, Martinique (27%)26, and Japan between 1986 to 199127 before implementing screening policies among pregnant women. In contrast, it was higher than in French Guiana (9.7%)28, and in Japan by the end of the 1990s (3.9%)27. This variability of transmission rates to the offspring between different populations, and the lower rates in countries that have implemented control measures, strongly suggest the predominant role of variables related to breastfeeding in HTLV-1 transmission27,28.
We addressed several risk factors for mother/child positive pairs, including maternal load of circulating HTLV-1. The risk of this kind of transmission increases exponentially in the presence of higher PVLs21, and in the present study women with PVL >100 copies/104 PBMC presented a higher transmission risk.
In agreement with our results a similar study done in Jamaica showed an association between prolonged breastfeeding and MTCT. Breastfeeding over 12 months was also associated with a risk of 32% transmission compared with only 9% for a shorter duration of breastfeeding10. In those children, the median time of HTLV-1 infection was estimated at 12 months29. Among children of Peruvian women with HTLV-1 the risk was 15.1 times greater for those who were breastfed during 12 to 24 months compared to less than six months30. One possible explanation would be that passive transfer of maternal antibodies during pregnancy may inhibit HTLV-1. However, those antibodies decline and disappear six to 12 months after birth31.
Overall, the mother/child positivity rate was 14.2%, reaching 50% for infected Asian-descendant mothers. However, the positive association between Asian ethnicity and seropositive child on the univariate analysis was not confirmed on the multivariate analysis. This higher transmission probably occurred because of the longer breastfeeding time in this group (24–36 months). The only one of the six positive children who had been breastfed for less than 24 months (six months) had a mother with a proviral load of 488 copies/104 PBMC. In fact, both the high mother PVL and prolonged breastfeeding were independently associated with transmission to the offspring. Divorced marital status was another possible confounding factor, which also did not remain in the multivariate analysis. Unfortunately, the small sample of this specific subgroup is not strong evidence and deserves further investigation of divorce as a risk for vertical transmission.
Mother’s age (>26 years) at the time of delivery was associated with seropositivity in the child, and one of the explanations could be the increase in the prevalence of HTLV-1 infection with increasing age, observed mainly among women32, for whom sexual transmission is more efficient33. This can also be due to the time between the beginning of the relationship with an infected partner and seroconversion. The seropositivity of the husband apparently did not influence child transmission, but most of the studied women were diagnosed after becoming mothers and information on the partner or ex-partner serological status was unknown for 40% of them. Thus, this possibility could not be totally ruled out in this study. In turn, the higher number of children per mother, which could be related to age, did not influence the transmission rate.
Mother’s schooling, as a prediction for mother income, tended to correlate inversely with the risk of seropositivity of the child, but probably the sample size was not large enough to identify this possible protecting factor. In fact, several studies reported that indicators of lower socioeconomic status, such as lower educational level and low income, are associated with HTLV-1 transmission2 and to MTCT2,34,35. Transmission occurred regardless of the sex of the child, but this data is controversial, since some studies have associated a higher risk of transmission to males23 or females22 exposed children, whereas others found a similar transmission risk for boys and girls24,27,28,30,36,37. However, the small sample size is a limitation to influence the child infection from other factors, such as history of blood transfusion, cesarean delivery or maternal coinfection with HIV, HTLV-2, HBV or an association of HIV/HCV.
The main limitation of this study was that most of the participants were 15 years old or older when tested. Therefore, we cannot assure that all our patients were infected through the vertical route. However, it is unlikely that all patients were infected by the sexual route at such young age. This possibility may not invalidate the major findings of our study. On the other hand, we cannot prospectively test such hypothesis due to obvious ethical issues.
It is important to highlight some independent risk factors identified in this cohort, such as a higher maternal proviral load (>100 copies/104 PBMC), prolonged breastfeeding, and familial aggregation with more than one infected child in the same offspring. In fact, there is a genetic susceptibility for HTLV-1 infection38, but this possibility must be addressed further in another study. Thus, PVL was a predictive marker for a positive child, even when performed several years after the putative transmission from the mother. Finally, screening for HTLV-1 among pregnant women and a non-breastfeeding policy by infected mothers continue to be the main measures to prevent MTCT where this infection is endemic.
References
Willems, L. et al. Reducing the global burden of HTLV-1 infection: An agenda for research and action. Antiviral Res. 137, 41–48 (2017).
Proietti, F. A., Carneiro-Proietti, A. B., Catalan-Soares, B. C. & Murphy, E. L. Global epidemiology of HTLV-I infection and associated diseases. Oncogene 24, 6058–6068 (2005).
Gessain, A. & Cassar, O. Epidemiological Aspects and World Distribution of HTLV-1 Infection. Front Microbiol 3, 388 (2012).
de Thé, G. & Bomford, R. An HTLV-I vaccine: why, how, for whom? AIDS Res. Hum. Retroviruses 9, 381–6 (1993).
Hedayati-Moghaddam, M. R., Tehranian, F. & Bayati, M. Human T-Lymphotropic virus type I (HTLV-1) infection among Iranian blood donors: First case-control study on the risk factors. Viruses 7, 5736–5745 (2015).
Catalan-Soares, B., Carneiro-Proietti, A. B. D. F. & Proietti, F. A. Heterogeneous geographic distribution of human T-cell lymphotropic viruses I and II (HTLV-I/II): serological screening prevalence rates in blood donors from large urban areas in Brazil. Cad. Saude Publica 21, 926–31 (2005).
Paiva, A. & Casseb, J. Sexual transmission of human T-cell lymphotropic virus type 1. Rev. Soc. Bras. Med. Trop. 47, 265–274
Percher, F. et al. Mother-to-child transmission of HTLV-1 epidemiological aspects, mechanisms and determinants of mother-to-child transmission. Viruses 8, 1–9 (2016).
Hino, S. Establishment of the milk-borne transmission as a key factor for the peculiar endemicity of human T-lymphotropic virus type 1 (HTLV-1): the ATL Prevention Program Nagasaki. Proc. Jpn. Acad. Ser. B. Phys. Biol. Sci. 87, 152–66 (2011).
Wiktor, S. Z. et al. Mother-to-child transmission of human T-cell lymphotropic virus type I associated with prolonged breast-feeding. J. Hum. Virol. 1, 37–44 (1997).
Bittencourt, A. L., Sabino, E. C., Costa, M. C., Pedroso, C. & Moreira, L. No evidence of vertical transmission of HTLV-I in bottle-fed children. Rev. Inst. Med. Trop. Sao Paulo 44, 63–65 (2002).
Moriuchi, H., Masuzaki, H., Doi, H. & Katamine, S. Mother-to-child Transmission of Human T-cell Lymphotropic Virus Type 1. Pediatr. Infect. Dis. J. 32, 175–177 (2013).
Ribeiro, M. A. et al. Blocking Vertical Transmission of Human T Cell Lymphotropic Virus Type 1 and 2 Through Breastfeeding Interruption. Pediatr. Infect. Dis. J. 31, 1139–1143 (2012).
Yamada, T. et al. Prevalence of human T-lymphotropic virus type 1 carriers among pregnant women in Hokkaido, Japan. Microbiol Immunol 58, 427–431 (2014).
Costa, C. A. D et al. Familial Transmission of Human T-cell Lymphotrophic Virus: Silent Dissemination of an Emerging but NeglectedInfection. PLoS Negl. Trop. Dis. 7 (2013).
Nunes, D. et al. HTLV-1 is predominantly sexually transmitted in Salvador, the city with the highest HTLV-1 prevalence in Brazil. PLoS One 12, e0171303 (2017).
Novoa, P. et al. Molecular Characterization of Human T-Cell Lymphotropic Virus Type 2 (HTLV-II) From People Living in Urban Areas of Sao Paulo City: Evidence of Multiple Subtypes Circulation. J. Med. Virol. 79, 182–187 (2007).
Dehée, A. et al. Quantitation of HTLV-I proviral load by a TaqMan real-time PCR assay. J. Virol. Methods 102, 37–51 (2002).
Montanheiro, P. et al. Low DNA HTLV-2 proviral load among women in São Paulo City. Virus Res. 135, 22–25 (2008).
Montanheiro, P. et al. Human T-cell lymphotropic virus type I (HTLV-I) proviral DNA viral load among asymptomatic patients and patients with HTLV-I-associated myelopathy/tropical spastic paraparesis. Braz J Med Biol Res 38, 1643–1647 (2005).
Hisada, M. et al. Virus markers associated with vertical transmission of human T lymphotropic virus type 1 in Jamaica. Clin. Infect. Dis. 34, 1551–1557 (2002).
Li, H.-C., Biggar, R. J. & Miley, W. J. Provirus load in breast milk and risk of mother-to-child transmission of human T lymphotropic virus type I. J. Infect. Dis. 191, 1780; author reply 1781 (2004).
Montano, S. M. et al. Human T cell lymphotropic virus type 1 infection and early neurologic development: a pilot study of 48 children. Clin. Infect. Dis. 39, 1079–82 (2004).
Hamedi, A. et al. The Prevalence of Human T-Cell lymphotropic Virus Type 1 in Pregnant Women and Their Newborns. ISRN Obstet. Gynecol. 2012, 975135 (2012).
Nyambi, P. N. et al. Mother-to-child transmission of human T-cell lymphotropic virus types I and II (HTLV-I/II) in Gabon: a prospective follow-up of 4 years. J. Acquir. Immune Defic. Syndr. Hum. Retrovirology 12, 187–192 (1996).
Monplaisir, N. et al. HTLV-I Maternal Transmission in Martinique, Using Serology and Polymerase Chain Reaction. AIDS Res. Hum. Retroviruses 9, 869–874 (1993).
Kashiwagi, K. et al. A decrease in mother-to-child transmission of human T lymphotropic virus type I (HTLV-I) in Okinawa, Japan. Am. J. Trop. Med. Hyg. 70, 158–63 (2004).
Ureta-Vidal, A. et al. Mother-to-child transmission of human T-cell-leukemia/lymphoma virus type I: Implication of high antiviral antibody titer and high proviral load in carrier mothers. Int. J. Cancer 82, 832–836 (1999).
Furnia, A. et al. Estimating the time of HTLV-I infection following mother-to-child transmission in a breast-feeding population in Jamaica. J. Med. Virol. 59, 541–546 (1999).
Gotuzzo, E. et al. Frequent HTLV-1 infection in the offspring of Peruvian women with HTLV-1-associated myelopathy/tropical spastic paraparesis or strongyloidiasis. Rev. Panam. Salud Pública 22, 223–230 (2007).
Takahashi, K. et al. Inhibitory Effect of Maternal Antibody on Mother-To-Child. 677, 673–677 (1991).
Van Tienen, C. et al. HTLV-1 in rural Guinea-Bissau: prevalence, incidence and a continued association with HIV between 1990 and 2007. Retrovirology 7, 50 (2010).
Kajiyama, W. et al. Intrafamilial transmission of adult T cell leukemia virus. J Infect Dis 154, 851–857 (1986).
Mello, M. A. et al. HTLV-1 in pregnant women from the Southern Bahia, Brazil: a neglected condition despite the high prevalence. Virol J 11, 28 (2014).
Villaverde, J. A., Romaní, F. R., Montano Torres, S. & Zunt, J. R. Vertical transmission of HTLV-1 in Peru. Rev. Peru. Med. Exp. y salud pública 28, 101–8 (2011).
Arango, C. et al. Risk Factors for HTLV-I Mother to Child Transmission: Influence of Genetic Markers. Braz. J. Infect. Dis. 2, 135–142 (1998).
Van Tienen, C. et al. Maternal proviral load and vertical transmission of human T cell lymphotropic virus type 1 in Guinea-Bissau. AIDS Res Hum Retroviruses 28, 584–590 (2012).
Plancoulaine, S. et al. Detection of a major gene predisposing to human T lymphotropic virus type I infection in children among an endemic population of African origin. J. Infect. Dis. 182, 405–12 (2000).
Acknowledgements
Maira Pedreschi for performing the HTLV-1 proviral load quantification. Support: FAPESP 2014/22827-7; and 2016/03025-2.
Author information
Authors and Affiliations
Contributions
A.M.P., L.A.M. and J.C. wrote the main manuscript text; O.L., L.A.M. and J.C. made the statistical analysis; T.A., and J.S. prepared tables, A.C.P.O. helped with the discussion. All authors reviewed the final version of this manuscript.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Paiva, A.M., Assone, T., Haziot, M.E.J. et al. Risk factors associated with HTLV-1 vertical transmission in Brazil: longer breastfeeding, higher maternal proviral load and previous HTLV-1-infected offspring. Sci Rep 8, 7742 (2018). https://doi.org/10.1038/s41598-018-25939-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25939-y
This article is cited by
-
The challenge of describing the epidemiology of HTLV in the Amazon region of Brazil
Retrovirology (2020)
-
Molecular detection of human T cell lymphotropic virus type 1 in pregnant women from Maranhão state, Brazil
Brazilian Journal of Microbiology (2020)
-
Human T cell leukemia virus type 1 and Zika virus: tale of two reemerging viruses with neuropathological sequelae of public health concern
Journal of NeuroVirology (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.