Abstract
Canine circoviruses (CanineCV’s), belonging to the genus Circovirus of the Circoviridae family, were detected by next generation sequencing in samples from Thai dogs with respiratory symptoms. Genetic characterization and phylogenetic analysis of nearly complete CanineCV genomes suggested that natural recombination had occurred among different lineages of CanineCV’s. Similarity plot and bootscaning analyses indicated that American and Chinese viruses had served as major and minor parental viruses, respectively. Positions of recombination breakpoints were estimated using maximum-likelihood frameworks with statistical significant testing. The putative recombination event was located in the Replicase gene, intersecting with open reading frame-3. Analysis of nucleotide changes confirmed the origin of the recombination event. This is the first description of naturally occurring recombinant CanineCV’s that have resulted in the circulation of newly emerging CanineCV lineages.
Similar content being viewed by others
Introduction
Circoviruses are non-enveloped DNA viruses belonging to the genus Circovirus of the family Circoviridae, and contain a small circular, single stranded ~2 kb DNA genome1. This genus harbors viruses that infect domestic and wildlife animal species, including porcine circoviruses (PCV-1 and -2), canary circovirus (CaCV) and beak and feather disease virus (BFDV) of birds2,3,4,5. Next generation sequencing (NGS) has recently allowed the discovery of additional mammalian circoviruses including PCV-3 in pigs6 and canine circovirus-1 (CanineCV-1) in dogs1. Infection with CanineCV-1, recently re-classified as ‘CanineCV’7, has been associated with several disease entities accompanied by manifestations like vasculitis, hemorrhages, thrombocytopenia, neutropenia and diarrhea2,7,8,9, although it is also detected in healthy dogs1,8,10. Interestingly, dogs infected with CanineCV are often co-infected with other enteric or respiratory pathogens2,7,10,11. Therefore, the pathogenic role of CanineCV is not clear. To date, CanineCV-infected dogs have been documented in several countries including the USA1,8,11, Italy9, Germany7, China (GenBank accession number: KT946839) and Taiwan2.
Besides genetic mutation, recombination is a driver of circovirus evolution, as several previous studies have shown genetic recombination within the Circoviridae family. These include BFDV12, Torque teno virus13 and PCV-214,15,16,17, and have contributed to genetic diversity of these viruses. Types of recombination mechanisms include homologous recombination in which homologous sequences are exchanged, non-homologous recombination in which genome regions are rearranged, deleted, duplicated or inserted into the host genome and reassortment in which whole genome components of the virus get exchanged between strains and species18. In homologous recombination, any interruption during replication, such as the encounter of strand break sites or clashes between replication and transcription complexes, can create temporary detachment of the replication enzyme. The enzyme can then use another similar template to reinitiate replication, thus generating a recombinant genome18. Thus far, genetic recombination of CanineCV genomes has not been documented. In the present study, we have molecularly characterized CanineCV strains from Thai dogs by next generation sequencing with special emphasis on the occurrence of genetic recombination.
Results
Routine virological investigations of Thai dogs
Samples from three autopsied Thai dogs that had exhibited respiratory symptoms and had been subjected to routine diagnostics were selected for further investigation. This included multiplex PCR screening for a panel of common canine respiratory viruses, which showed the presence of canine influenza virus (CIV) and canine respiratory coronavirus (CRCoV) in the nasal swab of dog no. 14P105D and canine parainfluenza (CPIV) in the oral swab of dog no. 14P112N (Table 1). No viruses had been detected using the multiplex PCR screen in the lungs of these two dogs or in samples from dog no. 15P061D. In addition, no viruses had been detected upon screening of respiratory swabs collected from twenty other dogs with respiratory problems of varying severity, using multiplex PCR or pan-PCRs for corona-, paramyxo- and herpesviruses (Table 1). In addition, no indications for the presence of canine parvovirus (CPV) or canine coronavirus (CCV) were found in intestinal swabs taken from the three autopsied dogs (Nos 14P105D, 14P112N, 15P061D) using an antigen detection kit.
Detection of CanineCV by NGS, conventional PCRs and in situ hybridization
To identify other viral pathogens associated with respiratory disease, lung samples of dog no.14P105D and 14P112N were processed for NGS. Over 900,000 trimmed reads were obtained from individual samples, of which approximately 853 (64x coverage) and 18 (0.7x coverage) reads were detected with highest homology to CanineCV in samples originating from dog no. 14P105D and dog no. 14P112N, respectively. Only partial CanineCV sequences were obtained by NGS analysis. Additional sequences were obtained using conventional PCR strategies based on primers designed from the NGS output sequences. The resulting consensus sequences represented ~92% of the complete CanineCV genome and confirmed the detection of this virus in both dogs, with the two strains named CanineCV_14P105D/TH2016 (accession no. MG737378) and CanineCV_14P112N/TH2016 (accession no. MG737386), respectively. Moreover, conventional PCR specific for CanineCV showed positive results in six out of the 20 nasal swabs, namely those coming from CP_28, CP_134, CP_144, CP_182, CP_188 and CP_191, and lung sample of dog no. 15P061D. The genomes of CanineCV in the swab samples were named CanineCV_CP28/TH2016 (MG737380), CanineCV_CP134/TH2016 (MG737381), CanineCV_CP144/TH2016 (MG737382), CanineCV_CP182/TH2016 (MG737383), CanineCV_CP188/TH2016 (MG737384), CanineCV_CP191/TH2016 (MG737385) and in the additional lung tissue sample, CanineCV_15P061D/TH2016 (MG737379).
To investigate the organ distribution of CanineCV, CanineCV-specific PCR successfully detected the virus genome in lung, liver and tracheobronchial lymph node in the three dogs from which tissue samples were collected (Table 1). CanineCV nucleic acid was also detected in hyperplastic tonsils of dog nos. 14P015D and 14P112N, whereas it was detected in kidneys of dog 15P061D. Brain tissues of all dogs were negative (Table 1). In situ hybridization revealed single weakly positive cells in a lymph node and strongly positive cells within the follicle center of the tonsils from one dog (14P105D) (Fig. 1A–C). All other tissue samples including lung, liver, spleen, intestine and kidney from all three animals were negative for CanineCV by in situ hybridization.
Histology of hyperplasia tonsillitis (A) and in situ hybridization (B,C) of CanineCV specific probe of dog no 14P105D TH/2016 showing strong positive signals in lymphoid follicles of the tonsil (B), located in the cytoplasm of lymphoid cells (C). The lymphoid follicle shown at higher magnification in (C) is indicated by a box in (B). Bar: A,B = 200 µm; C = 50 µm.
Genome organization, phylogenetic and recombination analyses of Thai CanineCV’s
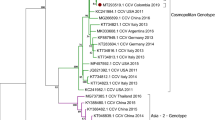
As expected, the coding sequence of all CanineCV’s TH/2016 contained 2 main open reading frames (ORFs), which encode a putative replication associated (Rep) and capsid (Cap) proteins. The Rep gene, nt 1-912, encoded 303 amino acids and the Cap gene, nt 1,116–1,928, encoded 270 amino acids. The origin of replication site (ori) was located at nt sites 2013–2020 (AGTATTAC). Moreover, one additional ORF, ORF3, located at nt 470–889 in the antisense of ORF1 was also identified. Pairwise distances revealed that the CanineCV’s TH/2016 differed by 10.5–16.2% from the USA-origin CanineCV’s UCD3-478 and Italy-origin AZ5586-13. Interestingly, most of the CanineCV’s detected in this study were most closely related to CanineCV_UCD3-478. However, CanineCV CP_191/TH2016 was found to be most closely related to the China-origin JZ98/2014 strain within another lineage (Supplementary Table S1). Phylogenetic analysis confirmed that the majority of the CanineCV’s TH/2016 were divergent to most CanineCV’s and were most closely related to CanineCV UCD3-478 (Fig. 2A). The individual Rep and Cap genes were used to construct additional phylogenetic trees, revealing that CanineCV’s detected in Thailand clustered with CanineCV UCD3-478 for both Cap and Rep genes. However, the phylogenetic trees obtained revealed discrepant patterns among genes by showing distinct and divergent homologous clade of the Rep gene, compared to the Cap gene (Fig. 2B,C).
Phylogenetic tree of CanineCVs shows that strains detected in Thailand (red triangles) were mostly clustered with CanineCV UCD3-478; however, the CanineCV CP191 TH/2016 was clustered with CanineCV JZ98/2014 (A). The phylogenetic tree of individual Rep (B) and Cap genes (C) of CanineCV genomes were also constructed and suggested the CanineCV circulating in Thailand were divergent and revealed discrepant patterns among genes by showing distinct and divergent homologous clade of Rep gene, compared to Cap gene.
All of the CanineCV’s-TH/2016, except CanineCV CP191_TH/2016, were identified as recombinants using the RDP4 software. The recombination events in these strains were supported by a number of statistical measurement recombination assays including RDP, GENECONV, BootScan, Maxchi, Chimaera, SiScan, 3Seq and LARD at average p-value = 1.42 × 10−4. Among these viruses, CanineCV’s UCD3-478 and JZ98/2014 were identified as major and minor parents, respectively. The results obtained from RDP analysis were further analyzed to identify potential recombination breakpoints using SIMPLOT software, which also indicated that CanineCV’s TH/2016 (except the CP191 strain) were recombinants. The similarity generated plot for CanineCV’s TH/2016 revealed that these strains had high nucleotide identity to a CanineCV UCD3-478 (pink line) in the initial Rep gene region and a region of the Cap gene. The CanineCV TH/2016 genomes also showed obvious nucleotide similarity to the CanineCV JZ98/2014 strain (blue line) in the middle portion of Rep gene (Fig. 3A). Furthermore, bootscan analysis confirmed the disparities observed in phylogenetic analyses of CanineCV’s TH/2016. The putative recombination breakpoint of the CanineCV’s TH/2016 that contained sequences derived from CanineCV JZ98/2014 was nt 380 to nt 820, within the Rep gene. CanineCV UCD3-478 served as a parental template for the Cap gene (Fig. 3B).
Similarity plot (A) and Bootscan analysis (B) of CanineCV Thai strain (query), compared with published CanineCV in various strains, showing potential recombination events locating in the Rep gene. The groups consisted of CanineCV strain UCD3-478 (UCD3-478, accession no. KC241983), three groups of reference sequences consisted of CanineCV-US, n = 3 (accession nos. KC241982, KC241984 and JQ821392), CanineCV-IT, n = 18 (accession nos. KT734812-KT734828 and KJ530972), CanineCV-DE, n = 2 (accession nos. KF887949 and KT283604) and CanineCV-CN, n = 1 (strain JZ98/2014, accession no. KT946839). Potential recombinants, CanineCV/TH2016, n = 8; CanineCV_14P105D/TH2016, 14P112N/TH2016, 15P061D/TH2016, CP28/TH2016, CP134/TH2016, CP144/TH2016, CP182/TH2016, CP188TH/2016 were used as a query.
Sequence comparison of recombinant CanineCV’s TH/2016 with parental strains
Further investigation of recombination events of CanineCV’s TH/2016 was achieved by differentiation of genetic marker positions between recombinants (CanineCV 14P105D) and their parental lineages (CanineCV_UCD3-478 and CanineCV_JZ98/2014) as shown in Table 2. The genetic markers were identified by nucleotide marker positions starting from nt 383 through nt 807 in the CanineCV 14P105D TH/2016 (green shading), which was derived from CanineCV JZ98/2014. This analysis also confirmed the predicted nucleotide recombination break points as shown in RDP and SIMPLOT analyses and was useful to corroborate the site of genetic recombination. Moreover, CanineCV CP191 TH/2016 was also analyzed and it was shown to be most likely recombined with CanineCV JZ98/2014. However, mutant nucleotides were detected in nt 333, which were thus far only observed previously in two Italian CanineCV strains (AZ4133/1, accession no. KT734815 and AZ4133/2, accession no. KT734827).
Discussion
In the present study samples from dogs with acute respiratory disease in Thailand, in which three respiratory viruses (CIV, CRCoV, CPIV) had been detected in routine screening, were subjected to additional analyses. Partial CanineCV genome sequences were detected by NGS and PCR of lung and swab samples respectively. However, the CanineCV specific ISH was positive only in macrophages present in tracheobronchial lymph nodes and tonsils of one dog. The demonstration of CanineCV in lungs and other organs by NGS and PCR, in the absence of CanineCV ISH signal outside the lymphoid tissues, was consistent with hematogenous spread of CanineCV’s TH/2016. Similar findings of PCR-positive but ISH-negative tests in CanineCV–infected dogs were also recently documented8. Full-length genome was obtained from liver tissue, however upon ISH analysis, only the lymphoid tissue had positive signals.
The detection of partial CanineCV genome sequences by several techniques prompted us to perform molecular analyses of CanineCV genomes generated from the positive dogs. It was shown that these clustered into two distinct lineages. The majority of CanineCV’s in Thailand represented a lineage that also contained the closely related CanineCV UCD3-478 from the USA8. However, CanineCV CP191/TH2016 was most closely related to CanineCV JZ98/2014, from China. Surprisingly, pairwise distance analysis of CanineCV’s from Thailand showed at least 10.5% genetic difference from a previously characterized and related virus even though they were all present in the same lineage, thus suggesting the detection of novel strains of CanineCV in this study. Preliminary analysis of CanineCV’s detected in Thailand revealed discordant phylogenetic relationship with other CanineCV’s. For instance, the CanineCV’s TH2016 clustered with CanineCV UCD3-478 when compared by analysis of complete genomes and Cap genes. However, they differentially clustered within a new lineage together with the CanineCV UCD3-478 strain when the phylogenetic tree was based on analysis of the Rep gene. Such paradoxical results indicate recombination events during CanineCV TH/2016 evolution. Therefore, we used boot scanning, phylogenetic incongruity, genetic marker identifications and additional sequence analyses to detect recombination events that may have occurred in circulating CanineCV’s, enabling the identification of recombination breakpoints occurring in putative recombinant CanineCV’s TH/2016. Here, we showed a recombination breakpoint at base positions 380–820, located in the Rep gene, which overlaps the putative ORF3 region. The Rep gene encoded by ORF1, plays a critical role in viral replication19,20,21, whereas ORF2 encodes the immunogenic icosahedral capsid protein22,23.
Studies of PCV2 genomes have shown that these viruses undergo homologous recombination events contributing to an increased genetic diversity and that those events are most commonly observed in the Rep gene15,16,17,24,25. However, intra- and inter-genotypic recombination events have also been documented to occur in the Cap gene of other circoviruses26,27. Mixed infections are a predisposing factor for homologous recombination. The recombination patterns that are generated can be influenced likewise by different mechanistic factors. These include the ori site, secondary structure features, sequence similarity and replication/transcription discongruities among other factors28,29. In the present study, the presence of a strain (CanineCV CP191/TH2016) most closely related to CanineCV JZ98/2014, indicates that the strain from which the nt region 380–820 of the recombinant viruses derives, circulates in Thailand. Therefore, we speculate that homologous recombination during a mixed infection has most likely generated the present recombinant strain. However, with the present data, it is not clear which mechanistic factor influenced the occurrence of the discussed recombination event. Moreover, a recent study on PCV2 revealed that the virus may have gradually evolved into several strains due to selective pressure such as vaccination by promoting a change of viral capsid protein away from the vaccine specific antigenic determinants30,31. Furthermore, in addition to a recombination breakpoint in CanineCV’s TH/2016, a point mutation (nt 333) in Rep gene (nt 333) was observed in CanineCV-CP191 TH/2016, a mutation observed previously in only two Italian CanineCVs AZ4133-1/13, KT734815 and AZ4133-2/13, KT734827). Although point mutations are a potential source of circovirus evolution as observed in PCV227, the effect of this mutation on CanineCV is unknown.
Analysis of genome sequences of the novel Thai CanineCV’s identified in this study, showed the presence of ORF3, a recently recognized overlapping anti-directional region of ORF1 gene that is dispensable for virus replication32 but has been linked to virus-induced apoptosis in PCV233. Recent studies showed that ORF3-deficient PCV2 were less pathogenic in mice in comparison to analogous infections with wild type strains34. However, the relevance in natural infections remains questionable as virus attenuation was not observed upon infection of pigs with ORF-3-deficit PCV232. Investigations into the function of the ORF-3 region in addition to phenotypic consequences of any mutations in this region of the Thai CanineCV’s should therefore be undertaken. The recombination breakpoint of CanineCV strains in our study occurred in the Rep gene, which also encompasses the ORF3 region. It is not clear if this recombination event impacts on the pathogenesis of CanineCV infection, calling for further investigation.
In summary we present the first comprehensive description of circulating CanineCVs in Thailand and document a recombination event that adds to our understanding of the genetic diversity of recently recognized CanineCV’s. Retrospective studies on historical samples taken from CanineCV-infected dogs may provide answers as to when such a recombinant virus first emerged. The identification of such genetic recombination events among CanineCV’s also supports whole genome sequencing as an alternative to phylogenetic analysis based on only single genome regions. Further applied studies should be undertaken into the functioning of CanineCV proteins, to better understand how mutations and/or recombination events alter CanineCV pathogenesis and transmission.
Materials and Methods
Animal description and sample collection
Fresh tissue samples were collected from brain, lung, liver, kidneys, tonsil and tracheobronchial lymph nodes of three dogs (14P105D, 14P112N, 15P061D) with respiratory symptoms in Bangkok, Thailand, that were also subjected to routine post-mortem gross and histopathological examination at Department of Pathology, Faculty of Veterinary Science, Chulalongkorn University, Bangkok, Thailand. In addition, twenty nasal or oral swabs were collected from other Thai dogs showing various degrees of respiratory problems (Table 1), according to approval of Chulalongkorn University Animal Care and Use Committee (No. 1431005). All methods were performed in accordance guidelines and regulations.
Routine virological testing
Extracted nucleic acids from the fresh tissues and swabs were subjected to routine laboratory investigations. Common viruses of canine infectious respiratory disease complex (CIRDC) including canine influenza virus (CIV), canine parainfluenza virus (CPIV), canine distemper virus, (CDV), canine respiratory coronavirus (CRCoV), canine adenovirus type 1 and 2 (CAdV1-2) and canine herpesvirus type 1 (CaHV-1) were screened for by multiplex PCRs35,36. Pan-PCRs using degenerate primers specific for paramyxoviruses, coronaviruses and herpesviruses were also performed on extracted nucleic acids as described previously37,38,39 with some modifications. A commercial rapid CPV/CCV Ag test kit (Bionote Inc., Suwon, South Korea), was used to test intestinal swabs taken from the three necropsied dogs, to test for the presence of canine parvovirus (CPV) and canine enteric coronavirus (CCV) infections.
Sequence independent single primer amplification and next-generation sequencing
Fresh lung samples of dogs No. 14P105D and No. 14P112N were prepared for NGS using a modified sequence-independent single-primer amplification (SISPA) protocol as previously described40. Briefly, after homogenization, RNA was extracted by QIAmp Viral RNA mini kit (Qiagen, Hilden, Germany), cDNA was constructed by using a mixture of random and non-ribosomal hexamers41,42 with SuperScript IV (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA), followed by Klenow reaction. Next, Taq polymerase (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA) was used to randomly amplify from cDNA. The PCR product was purified using Monarch® PCR and DNA clean up kit (New England Biolab, Frankfurt, Germany). Final DNA concentrations were measured by PicoGreen (Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). A DNA Library was then constructed following the Nextera XT protocol (Illumina, USA). Samples were then deep sequenced on an Illumina MiSeq system using MiSeq Reagent kit V3 (300 × 2 cycles). Raw reads were initially screened using an in-house metagenomics pipeline to identify interesting viral reads40. Reference assembly was performed with CLC Genomics Workbench 9.0. Phylogenetic analyses were carried out using MEGA 7.
Genome sequencing and CanineCV-specific PCR
PCRs with Sanger sequencing were performed to confirm the presence of specific viral sequences. CanineCV genomes were obtained by PCR amplification using degenerated primers designed from various strains of published CanineCV genome in GenBank (Supplementary Table S2). Briefly, PCR reactions comprised of a mixture of 1 U of Phusion DNA polymerase (New England Biolab, UK), 10 mM of dNTP in 5× Phusion HF Buffer, 10 µM final concentration of each primer and 2 µl of template. Cycler conditions consisted of initial denaturation for 98 °C for 30 sec, followed by 45 cycles of 98 °C, 20 sec, 50 °C, 30 sec, 72 °C, 1 min and 72 °C, 7 min for final extension. The PCR product was run on a 1% agarose gel and then purified using Monarch DNA Gel extraction kit (New England Biolab, Frankfurt, Germany). All the purified PCR products were subjected to Sanger sequencing in order to determine the complete nucleotide sequences of detected CanineCV. The resulting sequences of CanineCV were deposited in GenBank.
CanineCV in canine respiratory swab samples
The genetic diversity of CanineCV’s was investigated in the lung tissue sample from dog no. 15P061D and 20 respiratory swabs, using specific CanineCV PCR amplification and sequencing. Primers were designed based on CanineCV strains available in GenBank, targeting 517 bp of partial capsid gene (Supplementary Table S2). Subsequently, a panel of primers designed for amplification of the CanineCV genome was used to further analyze samples which were CanineCV positive (Supplementary Table S2). The PCR reaction consisted of 1 U of Phusion DNA polymerase (New England Biolab, UK), 10 mM of dNTP in 5x Phusion HF Buffer, 10 µM final concentration of each primer and 2 µl of cDNA. Cycler conditions consisted of initial denaturation for 98 °C for 30 sec, followed by 45 cycles of 98 °C, 20 sec, 50 °C, 30 sec, 72 °C, 1 min and 72 °C, 7 min for final extension. CanineCV positive PCR products were visualized on 1% Agarose gel and then purified by Monarch DNA Gel extraction kit (New England Biolab, Frankfurt, Germany) prior to Sanger sequencing (Eurofins Genomics).
Sequence analysis and genomic recombination
CanineCV sequences from all positive samples were aligned using MAFFT alignment version 7 (http://mafft.cbrc.jp/alignment/server/) and MEGA 7 based on nucleotide sequences of the nearly complete genome (nt 49 to 1949), replicase, and capsid genes. These alignments were used to construct phylogenetic trees using maximum likelihood method with TN93+G for complete genome, K2+G model for Rep gene and HKY+G for Cap gene as best-fit model of nucleotide substitution according to Bayesian Information Criterion40. All phylogenetic trees were tested by bootstrapping with 1000 replicates. Sequence pairwise distances of CanineCV genomes were calculated using Maximum Composite Likelihood model43. The evolutionary analyses were conducted in MEGA 744. In order to identify the possibly recombinant origin(s) of CanineCV TH/2016 strains, a recombination detection program 4 (RDP4) package v. Beta 4.94, an integrated software program45 was used. Briefly, the recombination events of CanineCV’s TH/2016 were detected by using a range of recombination detection methods including RDP, GeneConv, Bootscan, MaxChi, Chimera, SiScan and 3Seq with cut-off acceptable p-value at 0.01. Bonferroni correction was used as a general setting. The complete genomes of CanineCV’s available in GenBank were included in the RDP analyses. Similarity plot and bootscan analysis in SIMPLOT software package version 3.5.146 were also performed on CanineCV sequences. Briefly, a bootscaning analysis was performed on four groups of complete CanineCV genome sequences based on differential clustering in the phylogenetic tree. The bootscan analysis was tested under the Kimura-2 parameter (K2P) model for 100 replications, with a window size of 200 bp, step size of 20 bp and a transition/transversion ratio of 2. The similarity plot was performed under the same group and conditions with GapStrip model. Identified recombinant sequences were also allegorized with their potential parents by genetic marker positions to obtain further evidence of genetic recombination.
Molecular detection of CanineCV in canine tissues
Primers targeting the capsid gene of CanineCV were used to assess the distribution of this virus in the dogs. In addition to lung tissue, brain, liver, tracheobronchial lymph node, tonsil and kidneys were analyzed for the presence of CanineCV (Supplementary Table S2). Briefly, the extracted nucleic acids from each sample were used as template for cDNA synthesis as described above. The PCR reaction consisted of 5x OneTaq Buffer, 10 mM of dNTP, 1 U Hot start Taq polymerase (New England Biolab, Ipswich, MA, U.S.A.), 10 µM final concentration of each primer and 2 µl of cDNA. Cycler conditions consisted of initial denaturation for 94 °C for 5 min, followed by 45 cycles of 94 °C, 30 sec, 50 °C, 30 sec, 72 °C, 1 min and 72 °C, 7 min for final extension. CanineCV positive PCR products were visualized on 1% Agarose gel and then purified by Monarch DNA Gel extraction kit (New England Biolab, Frankfurt, Germany) prior to Sanger sequencing (Eurofins Genomics). To confirm the presence of CanineCV, in situ-hybridization (ISH) was performed on FFPE tissues, including lymph nodes, tonsils, thymus, spleen, pancreas, small intestine, kidney, urinary bladder, heart, trachea and lungs. ISH was performed as previously described with minor variations47. Probes were designed from alignment of published CanineCV genomes as follows: sense Probe: 5′-ACTACTGAAAGATAAAGGCCTCTCG-3′, antisense Probe: 5′CGAGAGGCCTTTATCTTTCAGTAGT-3′, and labelled with Digoxigenin (DIG) that covered 25 bp of the CanineCV (nt 905–929). Briefly, after de-parafinization of FFPE sections and hydration in graded ethanol, samples were washed in diethyl pyrocarbonate (DEPC)-treated water. Proteolytic digestion of samples was performed with 1 µg/ml proteinase K (Roche Diagnostics, Basel, Switzerland). An overnight incubation at 52 °C with 1000 ng/100 µl sense and antisense probe, respectively, was performed following post-fixation, acetylation and pre-hybridization. Detection was carried out using an anti-DIG antibody (diluted 1:200) conjugated with alkaline phosphatase (Roche Diagnostics, Basel, Switzerland) in combination with nitrobluetetrazoliumchloride (NBT, Sigma-Aldrich, St. Louis, MO, U.S.A.) and 5-bromo-4-chloro-3-indolyl phosphatate (BCIP, X-Phosphate, Sigma-Aldrich, St. Louis, MO, U.S.A.) as substrates. Distinct, purple precipitates, that were clearly cellular located, were considered positive.
References
Kapoor, A., Dubovi, E. J., Henriquez-Rivera, J. A. & Lipkin, W. I. Complete genome sequence of the first canine circovirus. J. Virol. 86, 7018, https://doi.org/10.1128/jvi.00791-12 (2012).
Hsu, H. S. et al. High detection rate of dog circovirus in diarrheal dogs. BMC Vet Res. 12, 116, https://doi.org/10.1186/s12917-016-0722-8 (2016).
Tischer, I., Mields, W., Wolff, D., Vagt, M. & Griem, W. Studies on epidemiology and pathogenicity of porcine circovirus. Arch. Virol. 91, 271–276, https://doi.org/10.1007/bf01314286 (1986).
Todd, D., Weston, J. H., Soike, D. & Smyth, J. A. Genome sequence determinations and analyses of novel circoviruses from goose and pigeon. Virology 286, 354–362, https://doi.org/10.1006/viro.2001.0985 (2001).
Todd, D. Avian circovirus diseases: lessons for the study of PMWS. Vet. Microbiol. 98, 169–174, https://doi.org/10.1016/j.vetmic.2003.10.010 (2004).
Palinski, R. et al. A Novel Porcine Circovirus Distantly Related to Known Circoviruses Is Associated with Porcine Dermatitis and Nephropathy Syndrome and Reproductive Failure. J. Virol. 91, https://doi.org/10.1128/jvi.01879-16 (2017).
Anderson, A. et al. Role of canine circovirus in dogs with acute haemorrhagic diarrhoea. Vet. Rec. 180, 542, https://doi.org/10.1136/vr.103926 (2017).
Li, L. et al. Circovirus in tissues of dogs with vasculitis and hemorrhage. Emerg. Infect. Dis. 19, 534–541, https://doi.org/10.3201/eid1904.121390 (2013).
Decaro, N. et al. Genomic characterization of a circovirus associated with fatal hemorrhagic enteritis in dog, Italy. PloS one 9, e105909, https://doi.org/10.1371/journal.pone.0105909 (2014).
Zaccaria, G. et al. Circovirus in domestic and wild carnivores: An important opportunistic agent? Virology 490, 69–74, https://doi.org/10.1016/j.virol.2016.01.007 (2016).
Thaiwong, T., Wise, A. G., Maes, R. K., Mullaney, T. & Kiupel, M. Canine circovirus 1 (CaCV-1) and canine parvovirus 2 (CPV-2): recurrent dual infections in a papillon breeding colony. Vet. Pathol. 53, 1204–1209, https://doi.org/10.1177/0300985816646430 (2016).
Heath, L. et al. Evidence of Unique Genotypes of Beak and Feather Disease Virus in Southern Africa. J. Virol. 78, 9277–9284, https://doi.org/10.1128/JVI.78.17.9277-9284.2004 (2004).
Manni, F., Rotola, A., Caselli, E., Bertorelle, G. & Di Luca, D. Detecting recombination in TT virus: a phylogenetic approach. J. Mol. Evol. 55, 563–572, https://doi.org/10.1007/s00239-002-2352-y (2002).
Csagola, A., Kecskemeti, S., Kardos, G., Kiss, I. & Tuboly, T. Genetic characterization of type 2 porcine circoviruses detected in Hungarian wild boars. Arch. Virol. 151, 495–507, https://doi.org/10.1007/s00705-005-0639-1 (2006).
Ma, C. M. et al. Evidence for recombination in natural populations of porcine circovirus type 2 in Hong Kong and mainland China. J. Gen. Virol. 88, 1733–1737, https://doi.org/10.1099/vir.0.82629-0 (2007).
Olvera, A., Cortey, M. & Segales, J. Molecular evolution of porcine circovirus type 2 genomes: phylogeny and clonality. Virology 357, 175–185, https://doi.org/10.1016/j.virol.2006.07.047 (2007).
Hesse, R., Kerrigan, M. & Rowland, R. R. Evidence for recombination between PCV2a and PCV2b in the field. Virus Res. 132, 201–207, https://doi.org/10.1016/j.virusres.2007.10.013 (2008).
Martin, D. P. et al. Recombination in eukaryotic single stranded DNA viruses. Viruses 3, 1699–1738, https://doi.org/10.3390/v3091699 (2011).
Mankertz, A. & Hillenbrand, B. Replication of porcine circovirus type 1 requires two proteins encoded by the viral rep gene. Virology 279, 429–438, https://doi.org/10.1006/viro.2000.0730 (2001).
Cheung, A. K. Regeneration of the replication-associated proteins tandem direct repeat recognition nucleotide sequence at the origin of DNA replication of porcine circovirus type 1. Virology 346, 32–39, https://doi.org/10.1016/j.virol.2005.10.004 (2006).
Finsterbusch, T., Steinfeldt, T., Caliskan, R. & Mankertz, A. Analysis of the subcellular localization of the proteins Rep, Rep’ and Cap of porcine circovirus type 1. Virology 343, 36–46, https://doi.org/10.1016/j.virol.2005.08.021 (2005).
Lekcharoensuk, P. et al. Epitope mapping of the major capsid protein of type 2 porcine circovirus (PCV2) by using chimeric PCV1 and PCV2. J. Virol. 78, 8135–8145, https://doi.org/10.1128/jvi.78.15.8135-8145.2004 (2004).
Nawagitgul, P. et al. Open reading frame 2 of porcine circovirus type 2 encodes a major capsid protein. J. Gen. Virol. 81, 2281–2287, https://doi.org/10.1099/0022-1317-81-9-2281 (2000).
Ramos, N., Mirazo, S., Castro, G. & Arbiza, J. Molecular analysis of Porcine Circovirus Type 2 strains from Uruguay: evidence for natural occurring recombination. Infect. Genet. Evol. 19, 23–31, https://doi.org/10.1016/j.meegid.2013.06.017 (2013).
Kim, H. K. et al. Phylogenetic and recombination analysis of genomic sequences of PCV2 isolated in Korea. Virus Genes 39, 352–358, https://doi.org/10.1007/s11262-009-0395-5 (2009).
Cadar, D. et al. Detection of natural inter- and intra-genotype recombination events revealed by cap gene analysis and decreasing prevalence of PCV2 in wild boars. Infect. Gen. Evol. 12, 420–427, https://doi.org/10.1016/j.meegid.2012.01.014 (2012).
Cai, L. et al. Identification of an emerging recombinant cluster in porcine circovirus type 2. Virus Res. 165, 95–102, https://doi.org/10.1016/j.virusres.2012.01.008 (2012).
Lefeuvre, P., Lett, J. M., Varsani, A. & Martin, D. P. Widely conserved recombination patterns among single-stranded DNA viruses. J. Virol. 83, 2697–2707, https://doi.org/10.1128/JVI.02152-08 (2009).
Cheung, A. K. Identification of an octanucleotide motif sequence essential for viral protein, DNA, and progeny virus biosynthesis at the origin of DNA replication of porcine circovirus type 2. Virology 324, 28–36, https://doi.org/10.1016/j.virol.2004.03.037 (2004).
Franzo, G., Tucciarone, C. M., Cecchinato, M. & Drigo, M. Porcine circovirus type 2 (PCV2) evolution before and after the vaccination introduction: A large scale epidemiological study. Sci. Rep. 6, 39458, https://doi.org/10.1038/srep39458 (2016).
Hu, J. et al. Identification of natural recombinants derived from PCV2a and PCV2b. Genet. Mol. Res. 14, 11780–11790, https://doi.org/10.4238/2015.October.2.12 (2015).
Juhan, N. M., LeRoith, T., Opriessnig, T. & Meng, X. J. The open reading frame 3 (ORF3) of porcine circovirus type 2 (PCV2) is dispensable for virus infection but evidence of reduced pathogenicity is limited in pigs infected by an ORF3-null PCV2 mutant. Virus Res. 147, 60–66, https://doi.org/10.1016/j.virusres.2009.10.007 (2010).
Liu, J., Chen, I. & Kwang, J. Characterization of a previously unidentified viral protein in porcine circovirus type 2-infected cells and its role in virus-induced apoptosis. J. Virol. 79, 8262–8274, https://doi.org/10.1128/jvi.79.13.8262-8274.2005 (2005).
Liu, J. et al. The ORF3 protein of porcine circovirus type 2 interacts with porcine ubiquitin E3 ligase Pirh2 and facilitates p53 expression in viral infection. J. Virol. 81, 9560–9567, https://doi.org/10.1128/jvi.00681-07 (2007).
Piewbang, C., Rungsipipat, A., Poovorawan, Y. & Techangamsuwan, S. In Acta Veterinaria Vol. 67 11–24 (2017).
Piewbang, C., Rungsipipat, A., Poovorawan, Y. & Techangamsuwan, S. Development and application of multiplex PCR assays for detection of virus-induced respiratory disease complex in dogs. J. Vet. Med. Sci. 78, 1847–1854, https://doi.org/10.1292/jvms.16-0342 (2017).
Tong, S., Chern, S. W., Li, Y., Pallansch, M. A. & Anderson, L. J. Sensitive and broadly reactive reverse transcription-PCR assays to detect novel paramyxoviruses. J. Clin. Microbiol. 46, 2652–2658, https://doi.org/10.1128/jcm.00192-08 (2008).
Ksiazek, T. G. et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Medicine 348, 1953–1966, https://doi.org/10.1056/NEJMoa030781 (2003).
VanDevanter, D. R. et al. Detection and analysis of diverse herpesviral species by consensus primer PCR. J. Clin. Microbiol. 34, 1666–1671 (1996).
Piewbang, C. et al. Canine bocavirus type 2 infection associated with intestinal lesions. Vet. Pathol. 300985818755253, https://doi.org/10.1177/0300985818755253 (2018).
Allander, T., Emerson, S. U., Engle, R. E., Purcell, R. H. & Bukh, J. A virus discovery method incorporating DNase treatment and its application to the identification of two bovine parvovirus species. Proc Natl Acad Sci USA 98, 11609–11614, https://doi.org/10.1073/pnas.211424698 (2001).
Endoh, D. et al. Species-independent detection of RNA virus by representational difference analysis using non-ribosomal hexanucleotides for reverse transcription. Nucleic Acids Res. 33, e65, https://doi.org/10.1093/nar/gni064 (2005).
Tamura, K., Nei, M. & Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proc Natl Acad Sci USA 101, 11030–11035, https://doi.org/10.1073/pnas.0404206101 (2004).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 33, 1870–1874, https://doi.org/10.1093/molbev/msw054 (2016).
Martin, D. P., Murrell, B., Golden, M., Khoosal, A. & Muhire, B. RDP4: Detection and analysis of recombination patterns in virus genomes. Virus Evol. 1, vev003, https://doi.org/10.1093/ve/vev003 (2015).
Lole, K. S. et al. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73, 152–160 (1999).
Bodewes, R. et al. Novel canine bocavirus strain associated with severe enteritis in a dog litter. Vet. Microbiol. 174, 1–8, https://doi.org/10.1016/j.vetmic.2014.08.025 (2014).
Acknowledgements
We acknowledge M. Schubert and Danuta Waschke for technical assistance. This study was in part supported by a grant from the Niedersachsen-Research Network on Neuroinfectiology (N-RENNT) from the Ministry of Science and Culture of Lower Saxony, Germany and supported the COMPARE project, which received funding from the European Union’s Horizon 2020 research and innovation program COMPARE (grant agreement no. 643476). C.P. was supported by the 100th Anniversary Chulalongkorn University Fund for Doctoral Scholarship and Oversea Research Experience Scholarship for Graduate Student. This work was partially funded by The Thailand Research Fund (S.T., grant number TRG5780250); Grant for Joint Funding of External Research Project, Ratchadaphiseksomphot Endowment Fund, Veterinary Science Research Fund, Chulalongkorn University (S.T., grant number RES_57_397_31_037); and The National Research Council of Thailand (S.T., grant number GRB_APS_38_59_31_02).
Author information
Authors and Affiliations
Contributions
S.T., W.B. and A.O. designed the study. C.Pi., S.K., W.J., C.Pu., J.K., M.L. and K.J. performed the experiments and analyses. C.Pi. wrote the first draft of the manuscript. M.P., E.V., W.J., Ch.P., A.R., S.T., W.B., M.L. and A.O. provided discussion. M.L. and A.O. finalized the manuscript. All authors approved the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Piewbang, C., Jo, W.K., Puff, C. et al. Novel canine circovirus strains from Thailand: Evidence for genetic recombination. Sci Rep 8, 7524 (2018). https://doi.org/10.1038/s41598-018-25936-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25936-1
This article is cited by
-
Recombinantly expressed virus-like particles (VLPs) of canine circovirus for development of an indirect ELISA
Veterinary Research Communications (2024)
-
Identification of small circular DNA viruses in coyote fecal samples from Arizona (USA)
Archives of Virology (2024)
-
Epidemiology, genetic diversity, and association of canine circovirus infection in dogs with respiratory disease
Scientific Reports (2022)
-
Canine circovirus and Canine adenovirus type 1 and 2 in dogs with parvoviral enteritis
Veterinary Research Communications (2022)
-
Genetic changes and evolutionary analysis of canine circovirus
Archives of Virology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.