Abstract
This cross-sectional study investigated the relationship between periodontal condition and ultrasound-diagnosed non-alcoholic fatty liver disease (NAFLD) in a Japanese oral health check population. A total of 1226 consecutive participant were enrolled in the study. Abdominal ultrasonography was applied to diagnose NAFLD. Of the study participants, 339 (27.7%) had ultrasonography-diagnosed NAFLD. The participants with NAFLD had a significantly higher prevalence of probing pocket depth (PPD) ≥ 4 mm (86.7%) than those without NAFLD (72.9%) (p < 0.05). After adjusting for gender, age, Brinkman index, regular exercise habits, body mass index, number of teeth present, presence of periodontitis, blood pressure, and serum parameters, there was a statistically significant difference in the adjusted odds ratios of having PPD ≥ 4 mm for NAFLD (Odds ratio = 1.881, 95% confidence interval 1.184–2.987, p < 0.01). Having PPD ≥ 4 mm may be a risk factor for ultrasound-diagnosed NAFLD in this cross-sectional study of a Japanese oral health check population.
Similar content being viewed by others
Introduction
Non-alcoholic fatty liver disease (NAFLD) is increasing recognized as one of the most common chronic liver diseases1. NAFLD encompasses a spectrum of diseases from simple hepatic steatosis to non-alcoholic steatohepatitis. While simple steatosis represents a relatively small health issue, steatohepatitis is of significant concern as it can potentially progress to liver cirrhosis and hepatocellular carcinoma2. The prevalence of NAFLD in Japanese adults is about 30%3,4. Although the mechanism of NAFLD is unknown, it can occur in association with metabolic diseases, such as obesity5, type 2 diabetes mellitus6, hypertension7 and hyperlipidemia8.
Periodontal disease is a chronic inflammatory disease of the supporting structures of the teeth. Increasing evidence indicates that periodontal disease is associated with many metabolic diseases, such as diabetes mellitus9 and cardiovascular disease10. Thus, since NAFLD is a metabolic disease, periodontal disease may be also associated with NAFLD. In animal studies, experimental periodontal disease induces increased blood levels of inflammatory molecules and oxidative stress, contributing to hepatic steatosis11. We also found that the improvement of periodontal inflammation reduced hepatic steatosis following periodontitis12. These observations support the hypothesis that the presence of periodontal disease may be a risk factor for NAFLD.
Clinical investigations have focused on the relationship between NAFLD and periodontal disease. It was reported that periodontal disease is more common in NAFLD patients with significant fibrosis compared to those with mild or no fibrosis13. It was also shown that relative to participants lacking clinical attachment level (CAL) ≥3 mm, the incidence of NAFLD was slightly elevated in participants with <30% of sites affected and moderately elevated in participants with ≥30% of sites affected, respectively14. However, since very little information is available about the relationship between NAFLD and periodontal disease in humans, additional clinical works are needed. In Japan, health check-ups in the hospital, including oral examinations, are popular. In addition, it is accepted that having probing pocket depth (PPD) ≥4 mm indicates that the individual has periodontal disease15. Therefore, the purpose of this cross-sectional study was to investigate the relationship between NAFLD and having PPD ≥4 mm in a Japanese oral health check population.
Results
Table 1 presents the characteristics of the participants. The overall prevalence of NAFLD was 27.7%. There were significant differences between the participants with and without NAFLD with respect to gender, age, body mass index (BMI), waist circumference (WC), Brinkman index, and having PPD ≥ 4 mm (p < 0.001). There were also significant differences between the participants with and without NAFLD with respect to serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), γ-glutamyl transferase (GGT), systolic blood pressure (SBP), diastolic blood pressure (DBP), hemoglobin A1c (HbA1c), triglyceride, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and C-reactive protein (CRP) concentrations (p < 0.001).
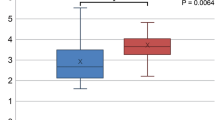
Comparative results of the participants with different severity of periodontal disease are shown in Fig. 1. In all participants and the female participants, the prevalence rate of NAFLD increased according to the severity of periodontal disease. There were significant differences between the participants with PPD ≤ 3 mm and PPD = 4–5 mm in the prevalence rate of NAFLD in male participants (p < 0.05). In all participants, there were significant differences between the participants with PPD ≤ 3 mm and PPD = 4–5 mm (p < 0.001), PPD ≤ 3 mm and PPD ≥ 6 mm (p < 0.001). In females, there were significant differences between the participants with PPD = 4–5 mm and PPD ≥ 6 mm (p < 0.001), PPD ≤ 3 mm and PPD ≥ 6 mm (p < 0.05).
Differences in prevalence rate of non-alcoholic fatty liver disease according to periodontal condition in all (A), male (B), and female (C) participants. NAFLD, non-alcoholic fatty liver disease and PPD, probing pocket depth. *p < 0.05, compared with the participants with PPD ≤ 3 mm, using the Kruskal Wallis test with post hoc Mann Whitney U test (corrected Bonferroni’s method).
Table 2 shows the results of the logistic regression analysis with prevalence of NAFLD as the dependent variable in all participants. The prevalence of NAFLD was related to gender (female, odds ratio (OR) = 0.583, p < 0.05), age (OR = 1.024, p < 0.05), BMI (OR = 1.470, p < 0.001), having PPD ≥ 4 mm (OR = 1.881, p < 0.01), HbA1c level (OR = 1.594, p < 0.05), total cholesterol concentration (OR = 0.968, p < 0.05), LDL cholesterol concentration (OR = 1.040, p < 0.01), triglyceride concentration (OR = 1.011, p < 0.001), DBP (OR = 1.050, p < 0.01), and CRP concentration (OR = 3.208, p < 0.01) after adjusting for gender, age, Brinkman index, regular exercise habits, BMI, number of present teeth, having PPD ≥ 4 mm, HbA1c level, total cholesterol concentration, HDL cholesterol concentration, LDL cholesterol concentration, triglyceride concentration, SBP, DBP, and CRP concentration.
Table 3 presents the results of the logistic regression analysis with prevalence of NAFLD as the dependent variable in male and female participants. In male participants, the prevalence of NAFLD was related to BMI (OR = 1.476, p < 0.001), triglyceride concentration (OR = 1.010, p < 0.01), SBP (OR = 0.969, p < 0.05), DBP (OR = 1.061, p < 0.01), and CRP concentration (OR = 2.757, p < 0.01). In female participants, the prevalence of NAFLD was related to age (OR = 1.067, p < 0.05), BMI (OR = 1.472, p < 0.001), having PPD ≥ 4 mm (OR = 2.972, p < 0.05), total cholesterol concentration (OR = 0.902, p < 0.01), triglyceride concentration (OR = 1.025, p < 0.01), HDL cholesterol concentration (OR = 1.103, p < 0.05), and CRP concentration (OR = 8.736, p < 0.05).
There were significant differences in serum HbA1c level between the participants with PPD ≤ 3 mm and PPD ≥ 6 mm (p < 0.01) and those with PPD = 4–5 mm and PPD ≥ 6 mm (p < 0.05) (Table 4). There were also significant differences in serum CRP concentration between the participants with PPD ≤ 3 mm and PPD ≥ 6 mm (p < 0.01) and those with PPD = 4–5 mm and PPD ≥ 6 mm (p < 0.05).
Discussion
This cross-sectional study assessed the relationship between NAFLD and periodontal condition in a Japanese oral health check population. We found that the group with NAFLD had a higher prevalence of having PPD ≥ 4 mm than that without NAFLD. In addition, the group with PPD ≥ 4 mm had higher risk of NAFLD than the group without PPD ≥ 4 mm after adjusting for gender, age, Brinkman index, regular exercise habits, BMI, number of teeth present, presence of having PPD ≥ 4 mm, HbA1c level, total cholesterol concentration, triglyceride concentration, HDL cholesterol concentration, LDL cholesterol concentration, SBP, DBP, and CRP concentration. This indicates that the presence of periodontal disease may increase the risk of NAFLD in the present population.
Our logistic regression analysis also showed that the presence of periodontitis was associated with NAFLD in female participants, but not in male participants. This suggests that there is a sex difference in the association between NAFLD and periodontal disease. In this study, the prevalence rate of having PPD ≥ 4 mm was 82.3% in male participants and 67.4% in female participants. Most of the male participants had PPD ≥ 4 mm, which may represent a bias that reduced the influence of periodontal disease on NAFLD.
In the present study, the increases in BMI, DBP and serum parameters, including triglyceride, LDL cholesterol, and HbA1c, were associated with NAFLD risk. These observations suggest that obesity, hypertension, hyperlipidemia, and type 2 diabetes mellitus could increase the risk for NAFLD. These are consistent with previous reports that demonstrated a positive relationship between NAFLD and other metabolic diseases16,17,18. In addition, we found that the increase in serum CRP concentration was also associated with NAFLD risk. This is in agreement with the previous findings, which showed that a 1 mg/dL increase in high sensitivity CRP level increased the risk of developing NAFLD by 1.7 fold as compared to control16.
In our findings, the serum HbA1c level was higher in the participants with PPD ≥ 6 mm than those with PPD ≤ 3 mm. This indicates that periodontitis induced an elevation in serum HbA1c level. Investigators have reported that increased serum HbA1c level is an independent risk factor for NAFLD17,18. It is feasible that periodontal disease could be detrimental to hepatic health through increased serum HbA1c level.
Animal studies have suggested that the increased serum level of inflammatory cytokines following periodontal disease contributed to NAFLD progression11. A clinical study also showed that the relationship between NAFLD and periodontal disease was modified by serum CRP concentration19. In the present study, the results showed that serum CRP concentration tended to increase according to the severity of periodontal disease. In particular, serum CRP concentration was significantly higher in the participants with PPD ≥ 6 mm than those with PPD ≤ 3 mm. This suggests that circulating inflammatory molecules play a crucial role in the association between NAFLD and periodontal disease. However, not only periodontal inflammation but also the inflammation of NAFLD could contribute to the elevation of serum CRP concentration. Additional studies are needed to clarify this point.
In our findings, the prevalence rate of NAFLD in male was higher in the participants with PPD 4–5 mm than those with PPD ≤ 3 mm, while that in female was higher in the participants with PPD ≥ 6 mm than those with PPD ≤ 3 mm or PPD 4–5 mm. The results indicate gender differences in the association between NAFLD and periodontal condition. This is consistent with the previous study, which revealed that gender differences seem to exist in the association between periodontal disease and metabolic syndrome20. It is known that sex hormones play an important role in the process of both periodontal inflammation21 and NAFLD22. The reason for the gender differences in the association between NAFLD and periodontal condition may appear due to sex hormones.
The gold standard diagnostic test for NAFLD is liver biopsy. However, since it is not reasonable to use the highly invasive liver biopsy as a diagnostic test in a health-check population, ultrasonography was used to detect NAFLD in this study. A meta-analysis shows that the overall sensitivity and specificity of ultrasound for detection of moderate-severe fatty liver compared to histology (the gold standard) were 84.8% and 93.6%, respectively23. This meta-analysis also revealed that the summary area under the receiver operating characteristics curve was 0.93. Therefore, it is suggested that ultrasound is an accurate, reliable imaging technique for the detection of NAFLD.
An epidemiological study demonstrated that periodontitis was significantly more common in patients with biopsy-proven non-alcoholic steatohepatitis and any fibrosis than without non-alcoholic steatohepatitis13. Another clinical study suggested that infection with the periodontal pathogenic bacteria Aggregatibacter actinomycetemcomitans affects NAFLD by altering the gut microbiota and glucose metabolism24. Furthermore, a cohort investigation clarified that relative to participants lacking CAL ≥3 mm, NAFLD incidence was elevated slightly in participants with <30% of CAL sites affected and moderately in participants with ≥30% of CAL sites affected14. These observations are consistent with the present concept that periodontal disease could increase the risk of NAFLD.
Increasing evidence has shown that periodontal disease may be associated with multiple metabolic diseases, such as diabetes mellitus 9, cardiovascular disease10, and atherosclerosis25. The present results have clarified that periodontal disease may be linked to NAFLD. In Japan, the Industrial Safety and Health Act stipulates that Japanese companies must offer annual health examinations for all employees in order to prevent metabolic diseases. However, the oral health examination is optional. The present and previous studies indicate the importance of periodontal examination in order to assess the risk of metabolic diseases in the health-check population.
This study has some limitations. First, all participants were recruited at the Asahi University Hospital. This may limit the ability to extrapolate our findings to the general population. Additionally, the present study was a cross-sectional study, and hence cannot demonstrate a causal relationship. Additional longitudinal studies are needed to investigate the relationship between NAFLD and having PPD ≥ 4 mm. Furthermore, it might be important to confirm the severity of NAFLD by liver biopsy, because the severity of NAFLD itself would affect the relationship between periodontal condition and NAFLD. On the other hand, the strength of this study is the sufficient sample size needed to assess the prevalence of NAFLD in participants with PPD ≥ 4 mm.
In conclusion, there appears to be a positive association between ultrasound-diagnosed NAFLD and having PPD ≥ 4 mm in a cross-sectional study in Japan.
Methods
Study population
The participants of this study consisted of 1280 Japanese who underwent oral health check-ups from Jan 2016 through Dec 2016 at the Asahi University Hospital in Gifu, Japan. Because the present study involves completing a survey, it was not necessary to perform sample size calculations. We excluded 37 participants with insufficient data. In addition, participants who had chronic hepatitis C infection (n = 6) and chronic hepatitis B infection (n = 11) were also excluded. In addition, because no participants reported alcohol intake of ≥20 g/day, we did not exclude participants who consumed alcohol26. Furthermore, there was no participant with the autoimmune hepatic disease. Accordingly, 1226 participants (772 men, 454 women) were eligible for this study. The study protocol was approved by the Ethics Committee of Asahi University (No. 27010). The study was performed in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to study participation.
Diagnosis of fatty liver
NAFLD was defined as fatty liver detected by ultrasonography (ProSound Alpha 7, Hitati Aloka Medical, Tokyo, Japan) in the absence of other causes of chronic liver disease (i.e., hepatitis C antibody-negative, hepatitis B surface antigen-negative, alcohol consumption <20 g/day)26. An ultrasonographical diagnosis of fatty liver was defined as a bright liver, increased liver echotexture compared with kidneys, vascular blurring, and deep attenuation of the liver. This diagnosis was performed by two specialists in internal medicine.
Measurement of biochemical markers
Venous blood samples were collected after an overnight fast. Chemiluminescence immunoassay (ARCHITECT HBsAg QT / ARCHITECT HCV, ABBOTT JAPAN, Tokyo, Japan) was used to test serum HBV surface antigen and antibody to HCV. The simultaneous multi-item automatic analyser (Dimension Vista 1500, Siemens Healthineers Japan, Toyko, Japan) was utilized to determine serum biochemical markers, including AST, ALT, GGT, total cholesterol, triglyceride, HDL cholesterol, LDL cholesterol, and CRP. In addition, the diabetes item automatic analyser (DM-JACK, Kyowa Medex, Tokyo, Japan) was utilized to determine HbA1c.
Assessment of body composition
An automatic height scale with body composition meter (TBF-110/TBF-210/DC-250, TANITA, Tokyo, Japan) was used to measure participants’ height and body weight. WC was measured by a nurse. BMI was computed as weight in kilograms divided by the square of height in meters.
Measurement of blood pressure
An automatic blood pressure monitor (HBP-9021/HBP-9020/BP-230RV3, OMRON HEALTHCARE, Kyoto, Japan) was used to measure SBP and DBP.
Oral examination
Three dentists examined the oral health status of the study participants. The number of teeth in the mouth was counted. PPD was assessed using a periodontal probe (YDM, Tokyo, Japan) at six sites (mesio-buccal, mid-buccal, disto-buccal, disto-lingual, mid-lingual and mesio-lingual) per tooth. The presence or absence of teeth exhibiting bleeding on probing (BOP) was recorded. Good intra- and inter-examiner agreement was achieved for repeated PPD measurements (Kappa statistic, >0.8).
Questionnaire
Participants were asked to complete a questionnaire regarding their health behaviors. The questionnaire included the following items: age, sex, presence or absence of regular exercise habit, alcohol habit, history of hepatic disease and smoking status (Brinkman index).
Statistical analysis
In this study, one or more teeth with ≥4 mm PPD was defined as the presence of periodontitis27. A chi-square test and the Mann-Whitney U test were used to assess significant differences in selected characteristics between study participants with and without NAFLD. The Kruskal-Wallis test with the post hoc Mann-Whitney U test (corrected Bonferroni’s method) was used for three group comparisons with different severity of periodontal disease (all teeth with PPD ≤ 3 mm, one or more teeth with PPD 4–5 mm, or one or more teeth with PPD ≥ 6 mm). Logistic regression analyses were also performed with the presence or absence of NAFLD as dependent variables. Independent variables were selected when the p value was <0.20 for the chi-square test and the Mann-Whitney U test in each variable, since previous studies have suggested that potential confounders should be eliminated only if p > 0.20, in order to prevent residual confounding28.
Analyses were performed using a statistical package (IBM SPSS statistics version 24, IBM Japan, Tokyo, Japan). All reported p values were considered statistically significant if less than 0.05.
References
Vernon, G., Baranova, A. & Younossi, Z. M. Systematic review: the epidemiology and natural history of non-alcoholic fatty liver disease and non-alcoholic steatohepatitis in adults. Aliment Pharmacol Ther. 34, 274–285 (2011).
Perumpail, B. J. et al. Clinical epidemiology and disease burden of nonalcoholic fatty liver disease. World J Gastroenterol. 23, 8263–8276 (2017).
Jimba, S. et al. Prevalence of non-alcoholic fatty liver disease and its association with impaired glucose metabolism in Japanese adults. Diabet Med. 22, 1141–1145 (2005).
Eguchi, Y. et al. Prevalence and associated metabolic factors of nonalcoholic fatty liver disease in the general population from 2009 to 2010 in Japan: a multicenter large retrospective study. J Gastroenterol. 47, 586–595 (2012).
Younossi, Z. M. et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64, 73–84 (2016).
Stepanova, M., Rafiq, N. & Younossi, Z. M. Components of metabolic syndrome are independent predictors of mortality in patients with chronic liver disease: a population-based study. Gut. 59, 1410–1415 (2010).
Lonardo, A., Nascimbeni, F., Mantovani, A. & Targher, G. Hypertension, diabetes, atherosclerosis and NASH: Cause or consequence? J Hepatol. 68, 335–352 (2018).
Sonmez, A. et al. Low- and high-density lipoprotein subclasses in subjects with nonalcoholic fatty liver disease. J Clin Lipidol. 9, 576–582 (2015).
Sanz, M. et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the International Diabetes Federation and the European Federation of Periodontology. J Clin Periodontol. 45, 138–149 (2018).
Holmlund, A., Lampa, E. & Lind, L. Poor response to periodontal treatment may predict future cardiovascular disease. J Dent Res. 96, 768–773 (2017).
Tomofuji, T. et al. Chronic administration of lipopolysaccharide and proteases induces periodontal inflammation and hepatic steatosis in rats. J Periodontol. 78, 1999–2006 (2007).
Tomofuji, T. et al. Effects of improvement in periodontal inflammation by toothbrushing on serum lipopolysaccharide concentration and liver injury in rats. Acta Odontol Scand. 67, 200–205 (2009).
Alazawi, W. et al. Periodontitis is associated with significant hepatic fibrosis in patients with non-alcoholic fatty liver disease. PLoS One 12, e0185902 (2017).
Akinkugbe, A. A. et al. Periodontitis and non-alcoholic fatty liver disease, a population-based cohort investigation in the study of health in Pomerania. J Clin Periodontol. 44, 1077–1087 (2017).
Lee, W. et al. Relationship between long working hours and periodontitis among the Korean workers. Sci Rep. 7, 7967 (2017).
Nigam, P. et al. Non-alcoholic fatty liver disease is closely associated with sub-clinical inflammation: a case-control study on Asian Indians in North India. PLoS One. 8, e49286 (2013).
Bae, J. C. et al. Impact of nonalcoholic fatty liver disease on insulin resistance in relation to HbA1c levels in nondiabetic subjects. Am J Gastroenterol. 105, 2389–2395 (2010).
Ma, H. et al. Independent association of HbA1c and nonalcoholic fatty liver disease in an elderly Chinese population. BMC Gastroenterol. 13, 3 (2013).
Akinkugbe, A. A. et al. Do genetic markers of inflammation modify the relationship between periodontitis and nonalcoholic fatty liver disease? Findings from the SHIP study. J Dent Res. 96, 1392–1399 (2017).
Hernaez, R. et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 54, 1082–1090 (2011).
Furuta, M. et al. Gender differences in the association between metabolic syndrome and periodontal disease: the Hisayama Study. J Clin Periodontol. 40, 743–752 (2013).
Steffens, J. P. et al. Associations between sex hormone levels and periodontitis in men: results from NHANES III. J Periodontol. 86, 1116–1125 (2015).
Ballestri, S. et al. NAFLD as a sexual dimorphic disease: role of gender and reproductive status in the development and progression of nonalcoholic fatty liver disease and inherent cardiovascular risk. Adv Ther. 34, 1291–1326 (2017).
Komazaki, R. et al. Periodontal pathogenic bacteria, Aggregatibacter actinomycetemcomitans affect non-alcoholic fatty liver disease by altering gut microbiota and glucose metabolism. Sci Rep. 24, 13950 (2017).
Hayashida, H. et al. Association of periodontitis with carotid artery intima–media thickness and arterial stiffness in community-dwelling people in Japan: The Nagasaki Islands study. Atherosclerosis. 229, 186–191 (2013).
Imaizumi, H. et al. The association between sleep duration and non-alcoholic fatty liver disease among Japanese men and women. Obesity Facts. 8, 234–242 (2015).
Morita, I. et al. Five-year incidence of periodontal disease is related to body mass index. J Dent Res. 90, 199–202 (2011).
Maldonado, G. & Greenland, S. Interpreting model coefficients when the true model form is unknown. Epidemiology. 4, 310–318 (1993).
Acknowledgements
The study was self-funded by the authors and their institution.
Author information
Authors and Affiliations
Contributions
T.I., A.H., T.A. and T.T. conceived and planned the project. A.O. and F.D. performed the diagnosis of NAFLD. T. I., K.W., T.K. and A.H. performed data entry. T.I. and T.T. wrote the main manuscript. T.I. and T.A. conducted statistical analysis. T.T. organized and supervised this study. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Iwasaki, T., Hirose, A., Azuma, T. et al. Correlation between ultrasound-diagnosed non-alcoholic fatty liver and periodontal condition in a cross-sectional study in Japan. Sci Rep 8, 7496 (2018). https://doi.org/10.1038/s41598-018-25857-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-018-25857-z
This article is cited by
-
The Association Between Periodontal Disease and Non-Alcoholic Fatty Liver Disease is Linked to Metabolic Disorders
Current Oral Health Reports (2023)
-
Oral microbiota in human systematic diseases
International Journal of Oral Science (2022)
-
Periodontal Disease and Nonalcoholic Fatty Liver Disease: New Microbiome-Targeted Therapy Based on the Oral–Gut–Liver Axis Concept
Current Oral Health Reports (2022)
-
Periodontal disease could be a potential risk factor for non-alcoholic fatty liver disease: An 11-year retrospective follow-up study
Clinical Oral Investigations (2022)
-
Infections at the nexus of metabolic-associated fatty liver disease
Archives of Toxicology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.